Protection and Alleviated Inflammation Induced by Virus-like Particle Vaccines Containing Plasmodium berghei MSP-8, MSP-9 and RAP1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Experimental Reagents and Preparation of Animals, Cells, and Parasites
2.3. Generation of P. berghei VLPs
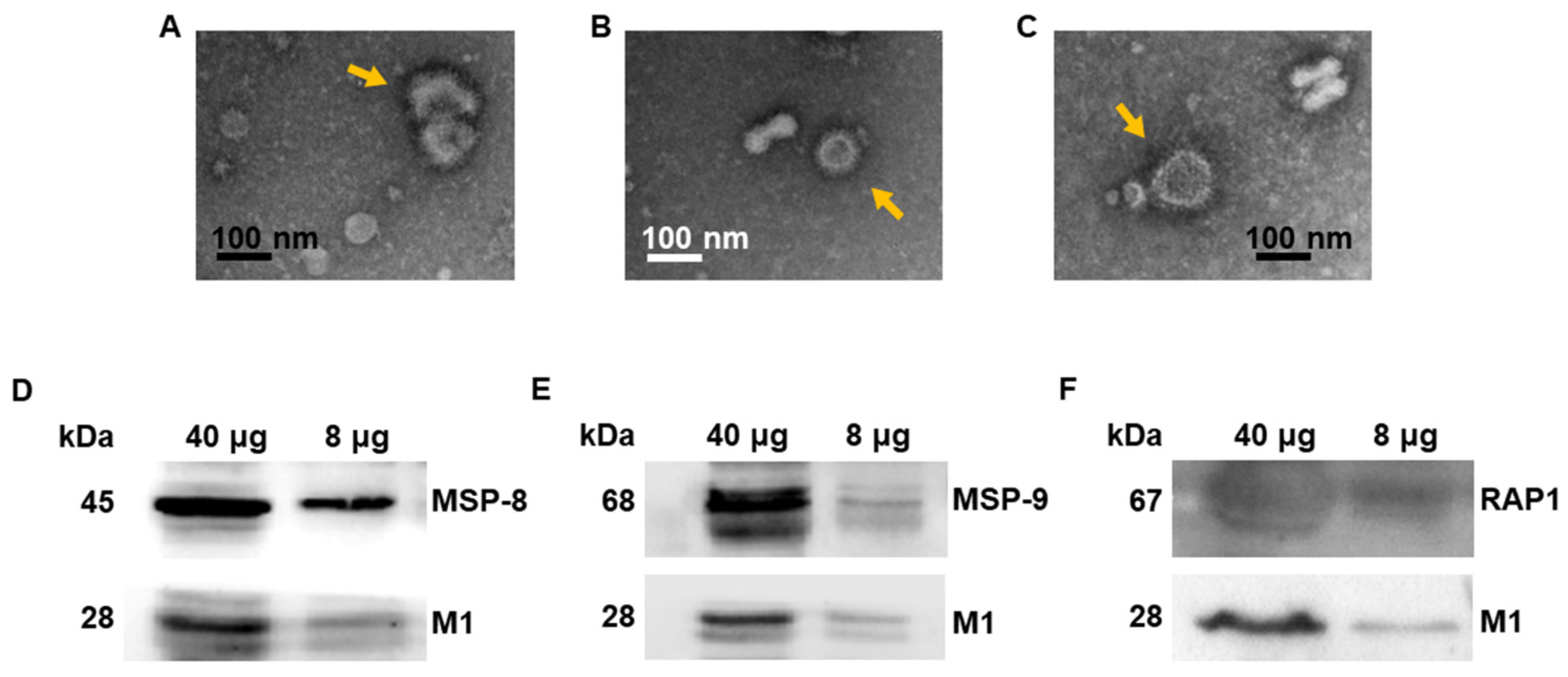
2.4. VLP Characterization via Western Blot and Transmission Electron Microscopy
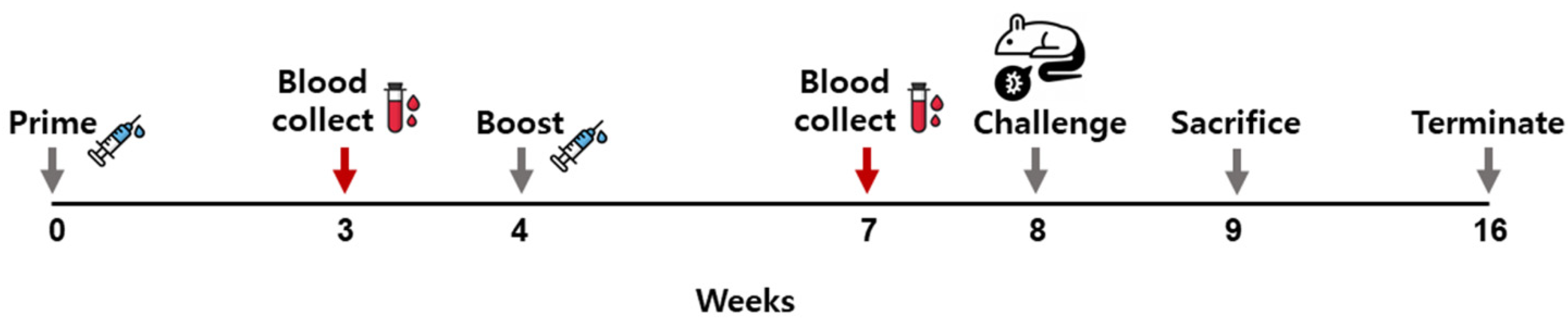
2.5. Immunizing Mice with the VLPs and P. berghei Challenge Infection
2.6. Immune Cell Responses
2.7. Inflammatory Cytokine Analysis
2.8. Peripheral Blood Parasitemia Measurement
2.9. Statistical Analysis
3. Results
3.1. Virus-like Particle Vaccines Were Generated
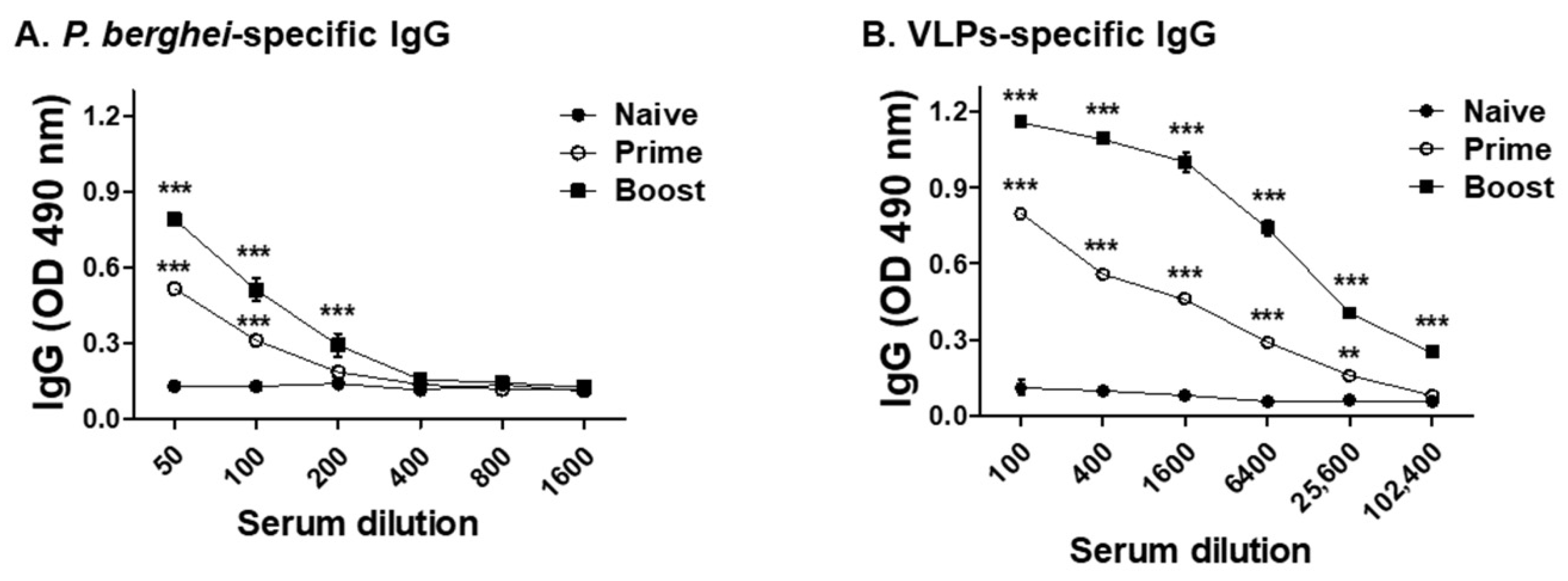
3.2. VLPs Vaccine Elicited Parasite-Specific IgG Antibody Response
3.3. Vaccination with VLPs Induced T Cells and Memory B Cell Responses
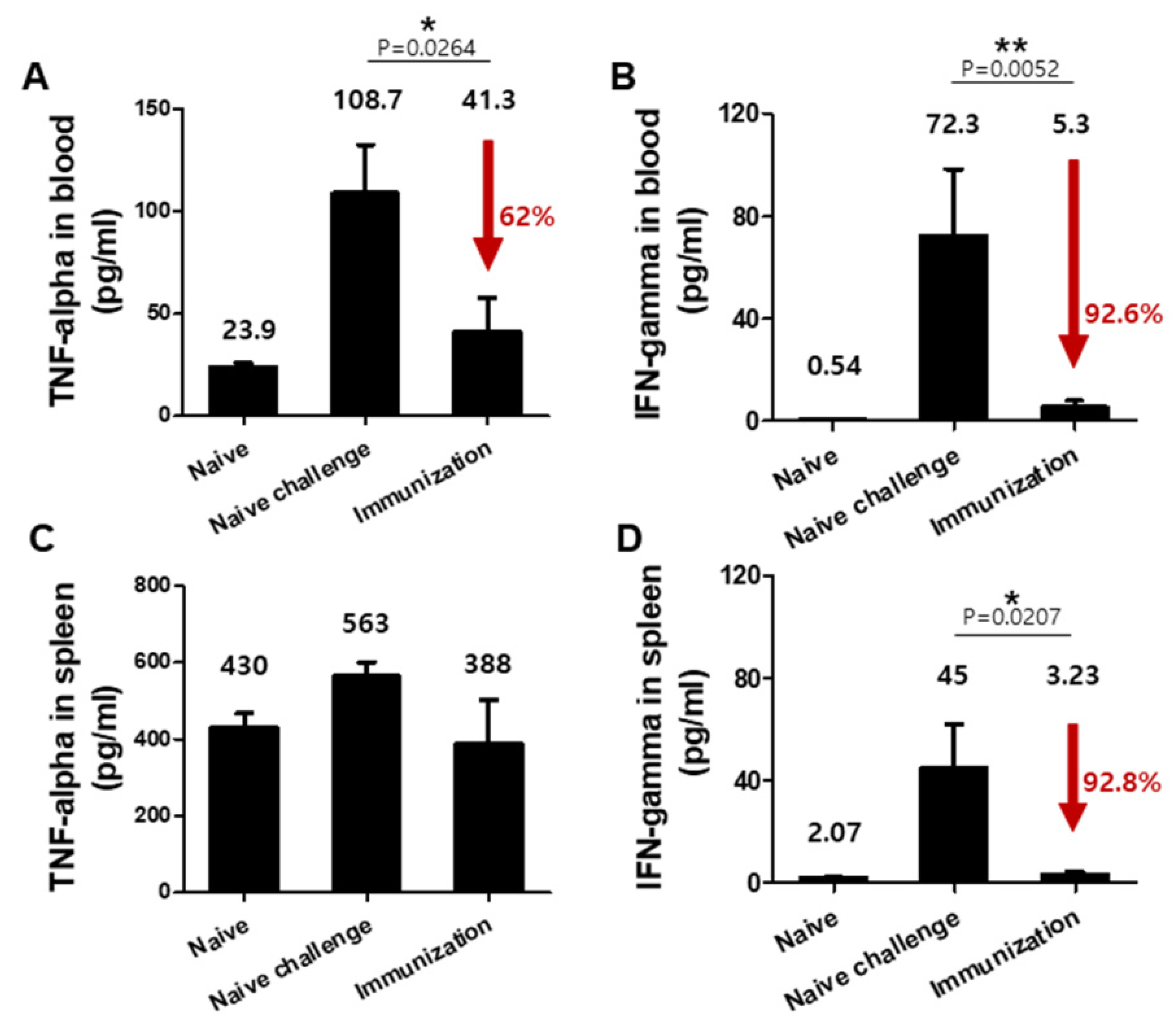
3.4. VLPs Vaccination Significantly Reduced Pro-Inflammatory Cytokine Responses upon Challenge Infection with P. berghei
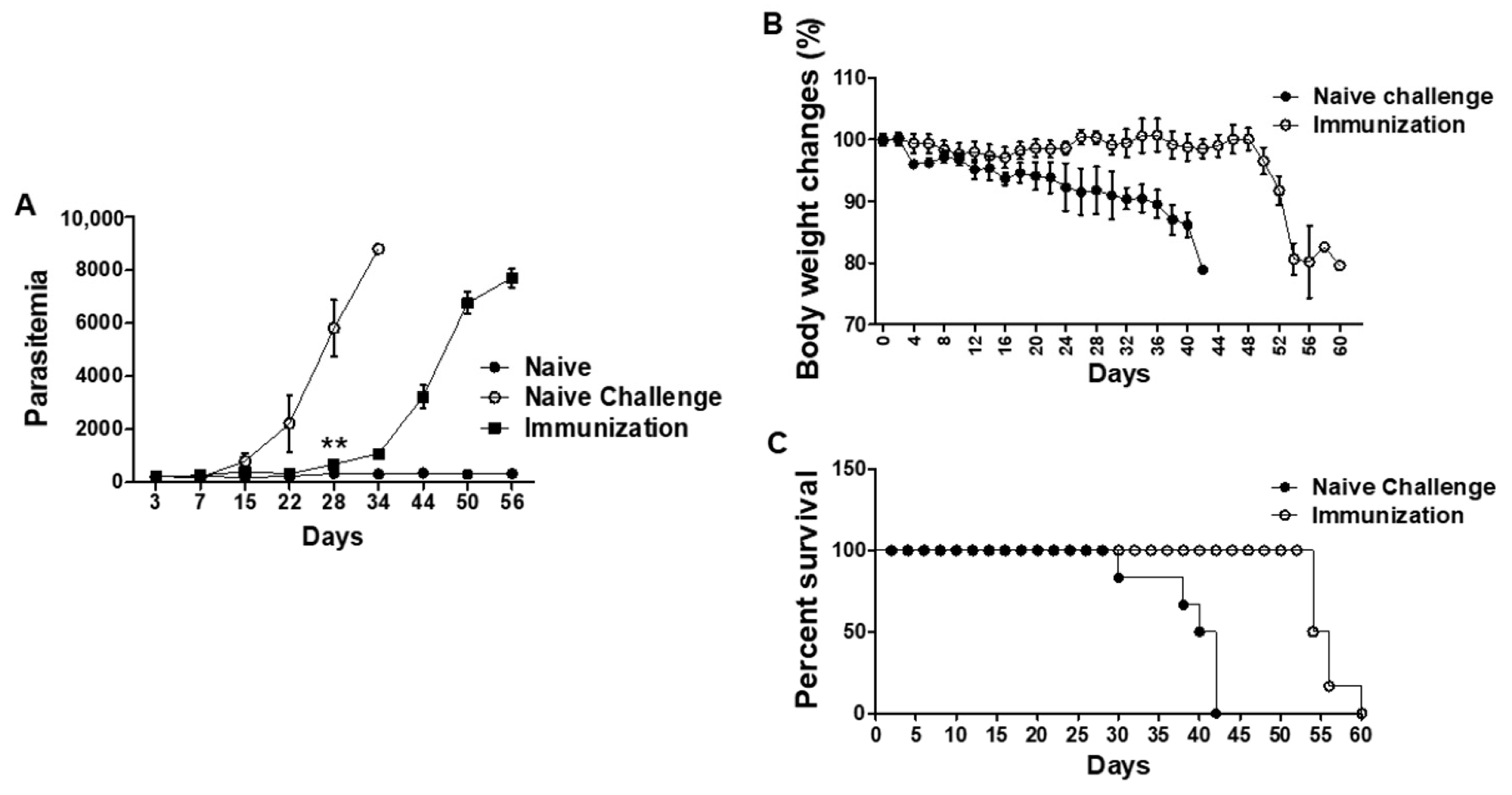
3.5. VLPs Vaccination Led to Significantly Reduced Parasitemia, Delayed Bodyweight Loss, and Prolonged Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghebreyesus, T. World Malaria Report 2017: Foreword; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- World Health Organization. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Alonso, P.L.; Brown, G.; Arevalo-Herrera, M.; Binka, F.; Chitnis, C.; Collins, F.; Doumbo, O.K.; Greenwood, B.; Hall, B.F.; Levine, M.M. A research agenda to underpin malaria eradication. PLoS Med. 2011, 8, e1000406. [Google Scholar] [CrossRef] [PubMed]
- Miura, K. Progress and prospects for blood-stage malaria vaccines. Expert Rev. Vaccines 2016, 15, 765–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wykes, M.N.; Good, M.F. What have we learnt from mouse models for the study of malaria? Eur. J. Immunol. 2009, 39, 2004–2007. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.; Diggs, C.L. Plasmodia of rodents. Parasit. Protozoa 1977, 3, 359–465. [Google Scholar]
- Amani, V.; Vigário, A.M.; Belnoue, E.; Marussig, M.; Fonseca, L.; Mazier, D.; Rénia, L. Involvement of IFN-γ receptor-mediated signaling in pathology and anti-malarial immunity induced by Plasmodium berghei infection. Eur. J. Immunol. 2000, 30, 1646–1655. [Google Scholar] [CrossRef]
- Grau, G.E.; Fajardo, L.F.; Piguet, P.F.; Allet, B.; Lambert, P.H.; Vassalli, P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science 1987, 237, 1210–1212. [Google Scholar] [CrossRef]
- Ayimba, E.; Hegewald, J.; Segbena, A.; Gantin, R.; Lechner, C.; Agosssou, A.; Banla, M.; Soboslay, P. Proinflammatory and regulatory cytokines and chemokines in infants with uncomplicated and severe Plasmodium falciparum malaria. Clin. Exp. Immunol. 2011, 166, 218–226. [Google Scholar] [CrossRef]
- Good, M.F. Towards a blood-stage vaccine for malaria: Are we following all the leads? Nat. Rev. Immunol. 2001, 1, 117. [Google Scholar] [CrossRef]
- Fowkes, F.J.; Richards, J.S.; Simpson, J.A.; Beeson, J.G. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: A systematic review and meta-analysis. PLoS Med. 2010, 7, e1000218. [Google Scholar] [CrossRef] [Green Version]
- Cowman, A.F.; Tonkin, C.J.; Tham, W.; Duraisingh, M.T. The molecular basis of erythrocyte invasion by malaria parasites. Cell Host Microbe 2017, 22, 232–245. [Google Scholar] [CrossRef]
- Tham, W.; Healer, J.; Cowman, A.F. Erythrocyte and reticulocyte binding-like proteins of Plasmodium falciparum. Trends Parasitol 2012, 28, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Burns, A.L.; Dans, M.G.; Balbin, J.M.; de Koning-Ward, T.F.; Gilson, P.R.; Beeson, J.G.; Boyle, M.J.; Wilson, D.W. Targeting malaria parasite invasion of red blood cells as an antimalarial strategy. FEMS Microbiol. Rev. 2019, 43, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kang, H.; Chu, K.; Basak, S.; Lee, D.; Moon, E.; Quan, F. Protective immunity induced by virus-like particle containing merozoite surface protein 9 of plasmodium berghei. Vaccines 2020, 8, 428. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chu, K.; Kang, H.; Basak, S.; Kim, M.; Park, H.; Jin, H.; Moon, E.; Quan, F. Virus-like particles expressing Plasmodium berghei MSP-8 induce protection against P. berghei infection. Parasite Immunol. 2020, 42, e12781. [Google Scholar] [CrossRef]
- Iyer, J.; Grüner, A.C.; Rénia, L.; Snounou, G.; Preiser, P.R. Invasion of host cells by malaria parasites: A tale of two protein families. Mol. Microbiol. 2007, 65, 231–249. [Google Scholar] [CrossRef]
- Baum, J.; Richard, D.; Riglar, D.T. Malaria parasite invasion: Achieving superb resolution. Cell Host Microbe 2017, 21, 294–296. [Google Scholar] [CrossRef] [Green Version]
- Tokunaga, N.; Nozaki, M.; Tachibana, M.; Baba, M.; Matsuoka, K.; Tsuboi, T.; Torii, M.; Ishino, T. Expression and localization profiles of rhoptry proteins in Plasmodium berghei sporozoites. Front. Cell. Infect. Microbiol. 2019, 9, 316. [Google Scholar] [CrossRef]
- Baldi, D.L.; Andrews, K.T.; Waller, R.F.; Roos, D.S.; Howard, R.F.; Crabb, B.S.; Cowman, A.F. RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum. EMBO J. 2000, 19, 2435–2443. [Google Scholar] [CrossRef] [Green Version]
- Counihan, N.A.; Kalanon, M.; Coppel, R.L.; de Koning-Ward, T.F. Plasmodium rhoptry proteins: Why order is important. Trends Parasitol. 2013, 29, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Niikura, M.; Inoue, S.; Fukutomi, T.; Yamagishi, J.; Asahi, H.; Kobayashi, F. Comparative genomics and proteomic analyses between lethal and nonlethal strains of Plasmodium berghei. Exp. Parasitol. 2018, 185, 1–9. [Google Scholar] [CrossRef]
- Fairlie-Clarke, K.J.; Lamb, T.J.; Langhorne, J.; Graham, A.L.; Allen, J.E. Antibody isotype analysis of malaria-nematode co-infection: Problems and solutions associated with cross-reactivity. BMC Immunol. 2010, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, D.; Pan, W. Construction of transgenic Plasmodium berghei as a model for evaluation of blood-stage vaccine candidate of Plasmodium falciparum chimeric protein 2.9. PLoS ONE 2009, 4, e6894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, S.; Lee, D.; Kim, A.; Quan, F. Evaluation of protective efficacy induced by virus-like particles containing a Trichinella spiralis excretory-secretory (ES) protein in mice. Parasites & Vectors 2016, 9, 384. [Google Scholar]
- Lee, S.; Kim, A.; Lee, D.; Rubino, I.; Choi, H.; Quan, F. Protection induced by virus-like particles containing Toxoplasma gondii microneme protein 8 against highly virulent RH strain of Toxoplasma gondii infection. PLoS ONE 2017, 12, e0175644. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, S.H.; Kim, A.R.; Quan, F.S. Virus-Like Nanoparticle Vaccine Confers Protection against Toxoplasma gondii. PLoS ONE 2016, 11, e0161231. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Yoo, D.; Bondy, B.J.; Quan, F.; Compans, R.W.; Kang, S.; Prausnitz, M.R. Stability of influenza vaccine coated onto microneedles. Biomaterials 2012, 33, 3756–3769. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Chu, K.; Kang, H.; Lee, S.; Chopra, M.; Choi, H.; Moon, E.; Inn, K.; Quan, F. Protection induced by malaria virus-like particles containing codon-optimized AMA-1 of Plasmodium berghei. Malar. J. 2019, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Waki, S.; Uehara, S.; Kanbe, K.; Ono, K.; Suzuki, M.; Nariuchi, H. The role of T cells in pathogenesis and protective immunity to murine malaria. Immunology 1992, 75, 646. [Google Scholar]
- Mbengue, B.; Niang, B.; Niang, M.S.; Varela, M.L.; Fall, B.; Fall, M.M.; Diallo, R.N.; Diatta, B.; Gowda, D.C.; Dieye, A. Inflammatory cytokine and humoral responses to Plasmodium falciparum glycosylphosphatidylinositols correlates with malaria immunity and pathogenesis. Immun. Inflamm. Dis. 2016, 4, 24–34. [Google Scholar] [CrossRef]
- Raza, A.; Ghanchi, N.K.; Sarwar Zubairi, A.B.; Raheem, A.; Nizami, S.; Beg, M.A. Tumor necrosis factor-α, interleukin-10, intercellular and vascular adhesion molecules are possible biomarkers of disease severity in complicated Plasmodium vivax isolates from Pakistan. PLoS ONE 2013, 8, e81363. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kang, H.; Lee, D.; Quan, F. Protective Immunity Induced by Incorporating Multiple Antigenic Proteins of Toxoplasma gondii Into Influenza Virus-like Particles. Front. Immunol. 2018, 9, 3073. [Google Scholar] [CrossRef] [PubMed]
- de Souza, J.B.; Hafalla, J.C.; Riley, E.M.; Couper, K.N. Cerebral malaria: Why experimental murine models are required to understand the pathogenesis of disease. Parasitology 2010, 137, 755–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, L. The spleen in malaria: The role of barrier cells. Immunol. Lett. 1990, 25, 165–172. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-H.; Chu, K.-B.; Kang, H.-J.; Quan, F.-S. Protection and Alleviated Inflammation Induced by Virus-like Particle Vaccines Containing Plasmodium berghei MSP-8, MSP-9 and RAP1. Vaccines 2022, 10, 203. https://doi.org/10.3390/vaccines10020203
Lee S-H, Chu K-B, Kang H-J, Quan F-S. Protection and Alleviated Inflammation Induced by Virus-like Particle Vaccines Containing Plasmodium berghei MSP-8, MSP-9 and RAP1. Vaccines. 2022; 10(2):203. https://doi.org/10.3390/vaccines10020203
Chicago/Turabian StyleLee, Su-Hwa, Ki-Back Chu, Hae-Ji Kang, and Fu-Shi Quan. 2022. "Protection and Alleviated Inflammation Induced by Virus-like Particle Vaccines Containing Plasmodium berghei MSP-8, MSP-9 and RAP1" Vaccines 10, no. 2: 203. https://doi.org/10.3390/vaccines10020203
APA StyleLee, S.-H., Chu, K.-B., Kang, H.-J., & Quan, F.-S. (2022). Protection and Alleviated Inflammation Induced by Virus-like Particle Vaccines Containing Plasmodium berghei MSP-8, MSP-9 and RAP1. Vaccines, 10(2), 203. https://doi.org/10.3390/vaccines10020203





