A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, and Animals
2.2. Gene Construction
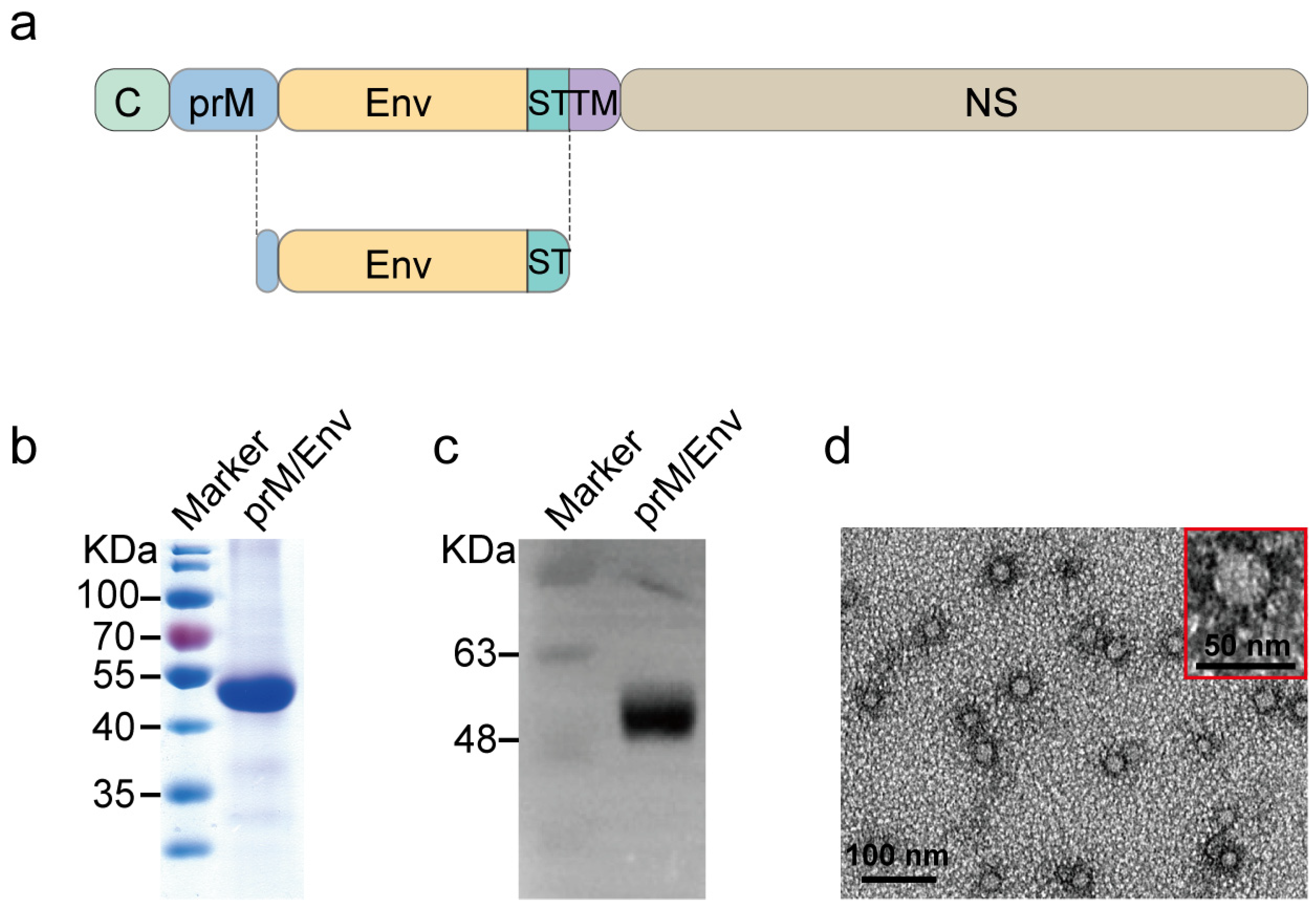
2.3. Generation of JEV VLP Vaccine Candidate
2.4. Western Blotting
2.5. Immunization
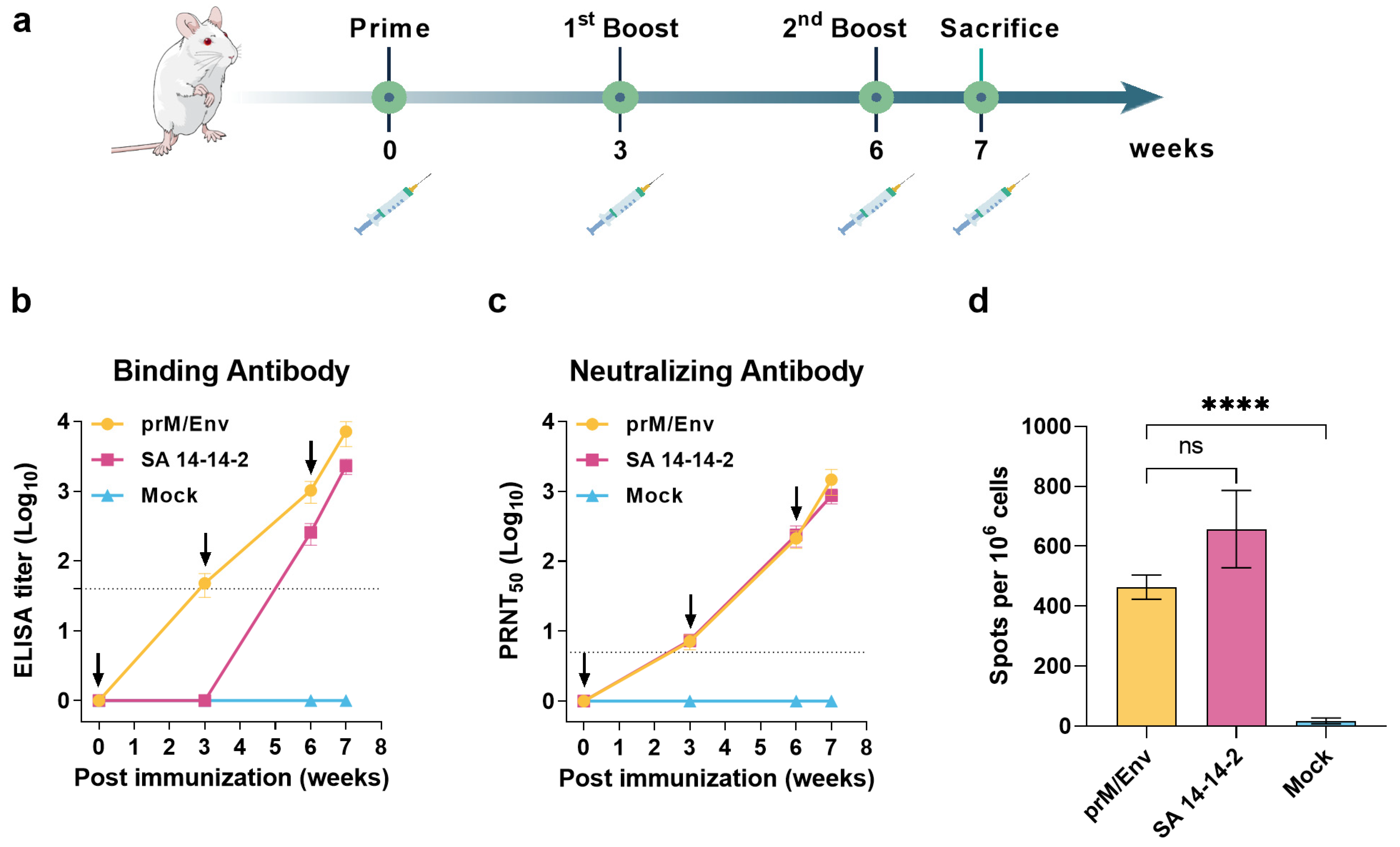
2.5.1. BALB/c Mice
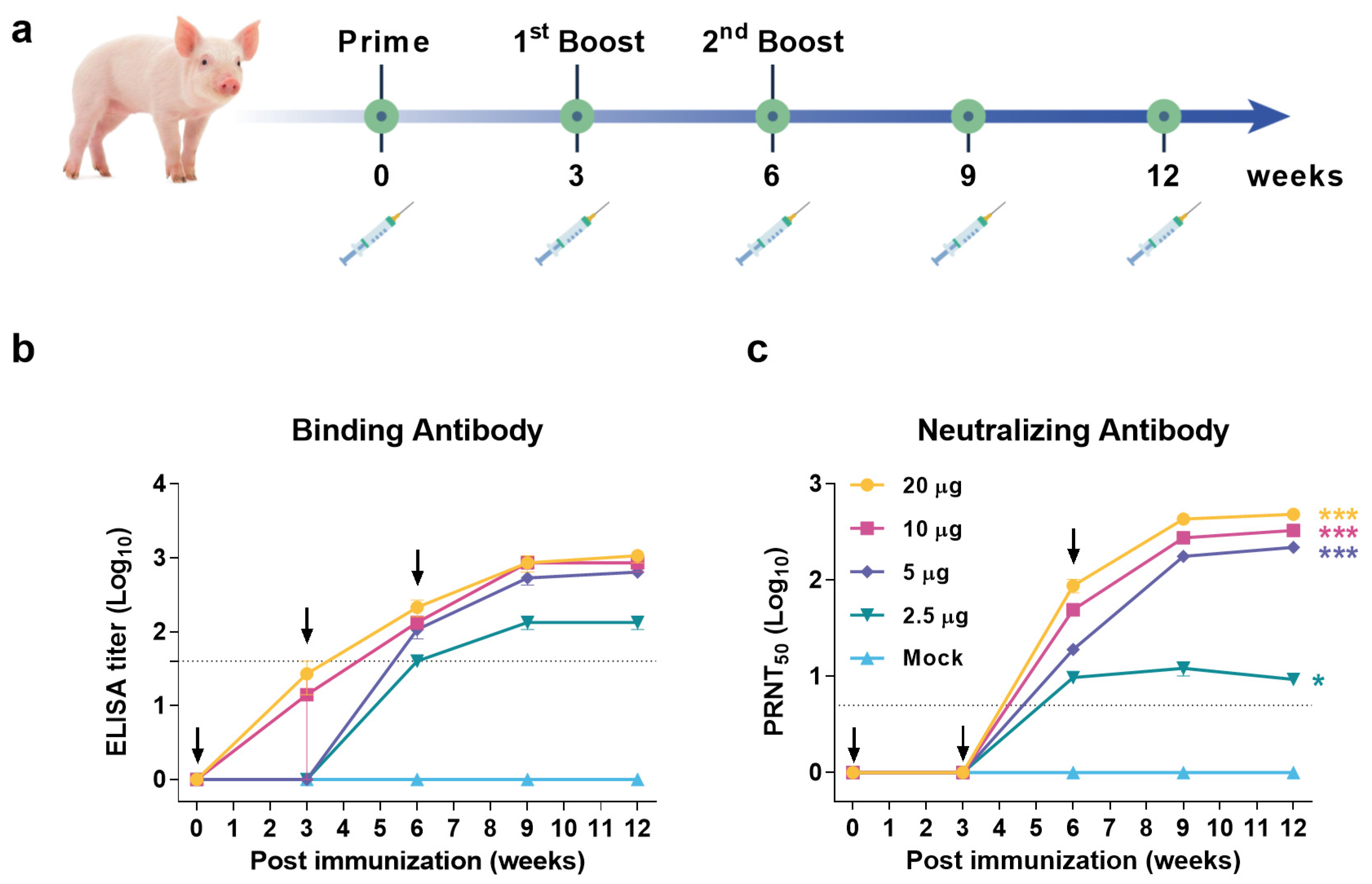
2.5.2. Pigs
2.6. ELISA
2.7. Neutralization Assay
2.8. ELISPOT
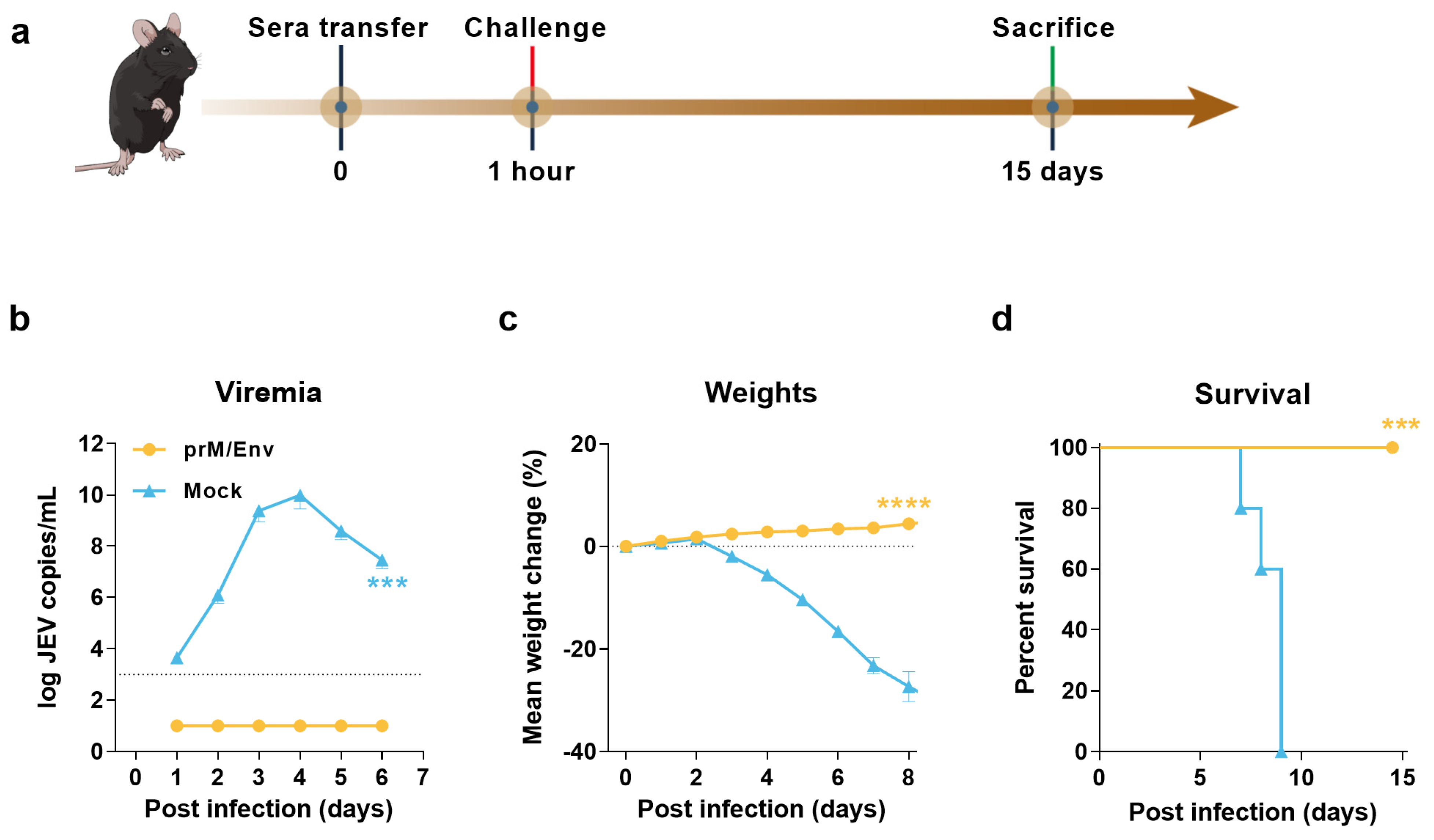
2.9. Adoptive Transfer Experiment
2.10. qRT-PCR
2.11. Statistics
3. Results
3.1. Construction and Characteristics of JEV VLP
3.2. JEV VLP Elicited Robust Humoral and T Cell Immune Responses in BALB/c Mice
3.3. Protection of Immunocompromised Mice through Passive Transfer of Anti-VLP Sera
3.4. JEV VLP Elicited Robust Humoral Immune Response in Pigs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calisher, C.H.; Gould, E.A. Taxonomy of the virus family Flaviviridae. Adv. Virus Res. 2003, 59, 1–19. [Google Scholar] [PubMed]
- Weaver, S.C.; Barrett, A.D. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2004, 2, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.I.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccines Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.L.; Hills, S.L.; Fischer, M.; Jacobson, J.A.; Hoke, C.H.; Hombach, J.M.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systematic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef]
- Halstead, S.B.; Thomas, S.J. Japanese encephalitis: New options for active immunization. Clin. Infect. Dis. 2010, 50, 1155–1164. [Google Scholar] [CrossRef]
- Mansfield, K.L.; Hernandez-Triana, L.M.; Banyard, A.C.; Fooks, A.R.; Johnson, N. Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet. Microbiol. 2017, 201, 85–92. [Google Scholar] [CrossRef]
- Amicizia, D.; Zangrillo, F.; Lai, P.L.; Iovine, M.; Panatto, D. Overview of Japanese encephalitis disease and its prevention. Focus on IC51 vaccine (IXIARO((R))). J. Prev. Med. Hyg. 2018, 59, E99–E107. [Google Scholar]
- Zheng, X.; Yu, X.; Wang, Y.; Turtle, L.; Cui, M.; Wang, R.; Yin, C. Complete protection for mice conferred by a DNA vaccine based on the Japanese encephalitis virus P3 strain used to prepare the inactivated vaccine in China. Virol. J. 2020, 17, 126. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, X.; Wang, Y.; Cui, M.; Wang, R.; Yin, C. Immune responses and protective effects against Japanese encephalitis induced by a DNA vaccine encoding the prM/E proteins of the attenuated SA14-14-2 strain. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 85, 104443. [Google Scholar] [CrossRef]
- Wu, C.J.; Li, T.L.; Huang, H.W.; Tao, M.H.; Chan, Y.L. Development of an effective Japanese encephalitis virus-specific DNA vaccine. Microbes Infect. 2006, 8, 2578–2586. [Google Scholar] [CrossRef]
- Garg, H.; Mehmetoglu-Gurbuz, T.; Joshi, A. Virus Like Particles (VLP) as multivalent vaccine candidate against Chikungunya, Japanese Encephalitis, Yellow Fever and Zika Virus. Sci. Rep. 2020, 10, 4017. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Barman, A.; Deb, B. Japanese encephalitis virus: A multi-epitope loaded peptide vaccine formulation using reverse vaccinology approach. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 78, 104106. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Nerome, R.; Maegawa, K.; Kotaki, A.; Sugita, S.; Kawasaki, K.; Kuroda, K.; Yamaguchi, R.; Takasaki, T.; Nerome, K. Development of a Japanese encephalitis virus-like particle vaccine in silkworms using codon-optimised prM and envelope genes. Heliyon 2017, 3, e00286. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Z.R.; Zhang, Y.N.; Liu, J.; Deng, C.L.; Shi, P.Y.; Yuan, Z.M.; Ye, H.Q.; Zhang, B. A replication-defective Japanese encephalitis virus (JEV) vaccine candidate with NS1 deletion confers dual protection against JEV and West Nile virus in mice. NPJ Vaccines 2020, 5, 73. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J.C.; Jing, J.; Liu, H.; Zhang, H.; Li, Z.H.; Jin, N.Y.; Lu, H.J. Construction and Immunogenicity of Recombinant Vaccinia Virus Vaccine Against Japanese Encephalitis and Chikungunya Viruses Infection in Mice. Vector Borne Zoonotic Dis. 2020, 20, 788–796. [Google Scholar] [CrossRef]
- Kwon, H.J.; Lee, S.Y.; Kim, K.H.; Kim, D.S.; Cha, S.H.; Jo, D.S.; Kang, J.H. The Immunogenicity and Safety of the Live-attenuated SA 14-14-2 Japanese Encephalitis Vaccine Given with a Two-dose Primary Schedule in Children. J. Korean Med. Sci. 2015, 30, 612–616. [Google Scholar] [CrossRef]
- Yang, L.; Tian, D.; Han, J.B.; Fan, W.; Zhang, Y.; Li, Y.; Sun, W.; Wei, Y.; Tian, X.; Yu, D.D.; et al. A recombinant receptor-binding domain in trimeric form generates protective immunity against SARS-CoV-2 infection in nonhuman primates. Innovation 2021, 2, 100140. [Google Scholar] [CrossRef]
- Hombach, J.; Solomon, T.; Kurane, I.; Jacobson, J.; Wood, D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September 2004. Vaccine 2005, 23, 5205–5211. [Google Scholar] [CrossRef]
- Sugawara, K.; Nishiyama, K.; Ishikawa, Y.; Abe, M.; Sonoda, K.; Komatsu, K.; Horikawa, Y.; Takeda, K.; Honda, T.; Kuzuhara, S.; et al. Development of Vero cell-derived inactivated Japanese encephalitis vaccine. Biologicals 2002, 30, 303–314. [Google Scholar] [CrossRef]
- Erra, E.O.; Kantele, A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev. Vaccines 2015, 14, 1167–1179. [Google Scholar] [CrossRef]
- Fujisaki, Y.; Sugimori, T.; Morimoto, T.; Miura, Y. Development of an attenuated strain for Japanese encephalitis live virus vaccine for porcine use. Natl. Inst. Anim. Health Q. 1975, 15, 15–23. [Google Scholar]
- Appaiahgari, M.B.; Vrati, S. Clinical development of IMOJEV (R)—A recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin. Biol. Ther. 2012, 12, 1251–1263. [Google Scholar] [CrossRef] [PubMed]
- Fujita, H.; Sumiyoshi, H.; Mori, C.; Manabe, S.; Takagi, M.; Yoshida, I.; Morita, K.; Fuke, I.; Fukai, K.; Igarashi, A. Studies in the development of Japanese encephalitis vaccine: Expression of virus envelope glycoprotein V3 (E) gene in yeast. Bull. World Health Organ. 1987, 65, 303–308. [Google Scholar] [PubMed]
- Roldao, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, P.; Medina, A.; Rutter, W.J.; Ammerer, G.; Hall, B.D. Synthesis and assembly of hepatitis B virus surface antigen particles in yeast. Nature 1982, 298, 347–350. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, X.Y.; Stenzel, D.J.; Frazer, I.H. Expression of Vaccinia Recombinant HPV 16 Ll and L2 ORF Proteins in Epithelial Cells Is Suffcient for Assembly of HPV Virion-like Particle. Virology 1991, 185, 251–257. [Google Scholar] [CrossRef]
- Taylor, T.J.; Diaz, F.; Colgrove, R.C.; Bernard, K.A.; DeLuca, N.A.; Whelan, S.P.J.; Knipe, D.M. Production of immunogenic West Nile virus-like particles using a herpes simplex virus 1 recombinant vector. Virology 2016, 496, 186–193. [Google Scholar] [CrossRef]
- Akahata, W.; Yang, Z.Y.; Andersen, H.; Sun, S.; Holdaway, H.A.; Kong, W.P.; Lewis, M.G.; Higgs, S.; Rossmann, M.G.; Rao, S.; et al. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 2010, 16, 334–338. [Google Scholar] [CrossRef]
- Urakami, A.; Ngwe Tun, M.M.; Moi, M.L.; Sakurai, A.; Ishikawa, M.; Kuno, S.; Ueno, R.; Morita, K.; Akahata, W. An Envelope-Modified Tetravalent Dengue Virus-Like-Particle Vaccine Has Implications for Flavivirus Vaccine Design. J. Virol. 2017, 91, e01181-17. [Google Scholar] [CrossRef]
- Zeltins, A. Construction and characterization of virus-like particles: A review. Mol. Biotechnol. 2013, 53, 92–107. [Google Scholar] [CrossRef]
- Emini, E.A.; Ellis, R.W.; Miller, W.J.; McAleer, W.J.; Scolnick, E.M.; Gerety, R.J. Production and immunological analysis of recombinant hepatitis B vaccine. J. Infect. 1986, 13 (Suppl. A), 3–9. [Google Scholar] [CrossRef]
- Wu, H.L.; Chen, P.J.; Mu, J.J.; Chi, W.K.; Kao, T.L.; Hwang, L.H.; Chen, D.S. Assembly of hepatitis delta virus-like empty particles in yeast. Virology 1997, 236, 374–381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buonamassa, D.T.; Greer, C.E.; Capo, S.; Yen, T.S.; Galeotti, C.L.; Bensi, G. Yeast coexpression of human papillomavirus types 6 and 16 capsid proteins. Virology 2002, 293, 335–344. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, C.; Yao, Y.; Yang, X.; Bai, H.; Huang, W.; Xia, Y.; Ma, Y. Production of Recombinant Human Papillomavirus Type 52 L1 Protein in Hansenula polymorpha Formed Virus-Like Particles. J. Microbiol. Biotechnol. 2015, 25, 936–940. [Google Scholar] [CrossRef]
- Hanumantha Rao, N.; Baji Babu, P.; Rajendra, L.; Sriraman, R.; Pang, Y.Y.; Schiller, J.T.; Srinivasan, V.A. Expression of codon optimized major capsid protein (L1) of human papillomavirus type 16 and 18 in Pichia pastoris; purification and characterization of the virus-like particles. Vaccine 2011, 29, 7326–7334. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, J.; Perlman, S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef]
- Yang, L.; Tian, D.; Liu, W. Strategies for vaccine development of COVID-19. Chin. J. Biotech. 2020, 36, 593–604. [Google Scholar]
- Calvert, A.E.; Dixon, K.L.; Delorey, M.J.; Blair, C.D.; Roehrig, J.T. Development of a small animal peripheral challenge model of Japanese encephalitis virus using interferon deficient AG129 mice and the SA14-14-2 vaccine virus strain. Vaccine 2014, 32, 258–264. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Xiao, A.; Wang, H.; Zhang, X.; Zhang, Y.; Li, Y.; Wei, Y.; Liu, W.; Chen, C. A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection. Vaccines 2022, 10, 197. https://doi.org/10.3390/vaccines10020197
Yang L, Xiao A, Wang H, Zhang X, Zhang Y, Li Y, Wei Y, Liu W, Chen C. A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection. Vaccines. 2022; 10(2):197. https://doi.org/10.3390/vaccines10020197
Chicago/Turabian StyleYang, Limin, Aibo Xiao, Hu Wang, Xiaojuan Zhang, Yuan Zhang, Yunlong Li, Yanqiu Wei, Wenjun Liu, and Chuangfu Chen. 2022. "A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection" Vaccines 10, no. 2: 197. https://doi.org/10.3390/vaccines10020197
APA StyleYang, L., Xiao, A., Wang, H., Zhang, X., Zhang, Y., Li, Y., Wei, Y., Liu, W., & Chen, C. (2022). A VLP-Based Vaccine Candidate Protects Mice against Japanese Encephalitis Virus Infection. Vaccines, 10(2), 197. https://doi.org/10.3390/vaccines10020197





