Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Fatigue and Symptom Severity Assessment, Outcomes
2.3. Laboratory Analysis and Assay
2.4. Ethical Approval
2.5. Statistics
3. Results
3.1. Characteristics
3.2. Fatigue Status and Antibody Level
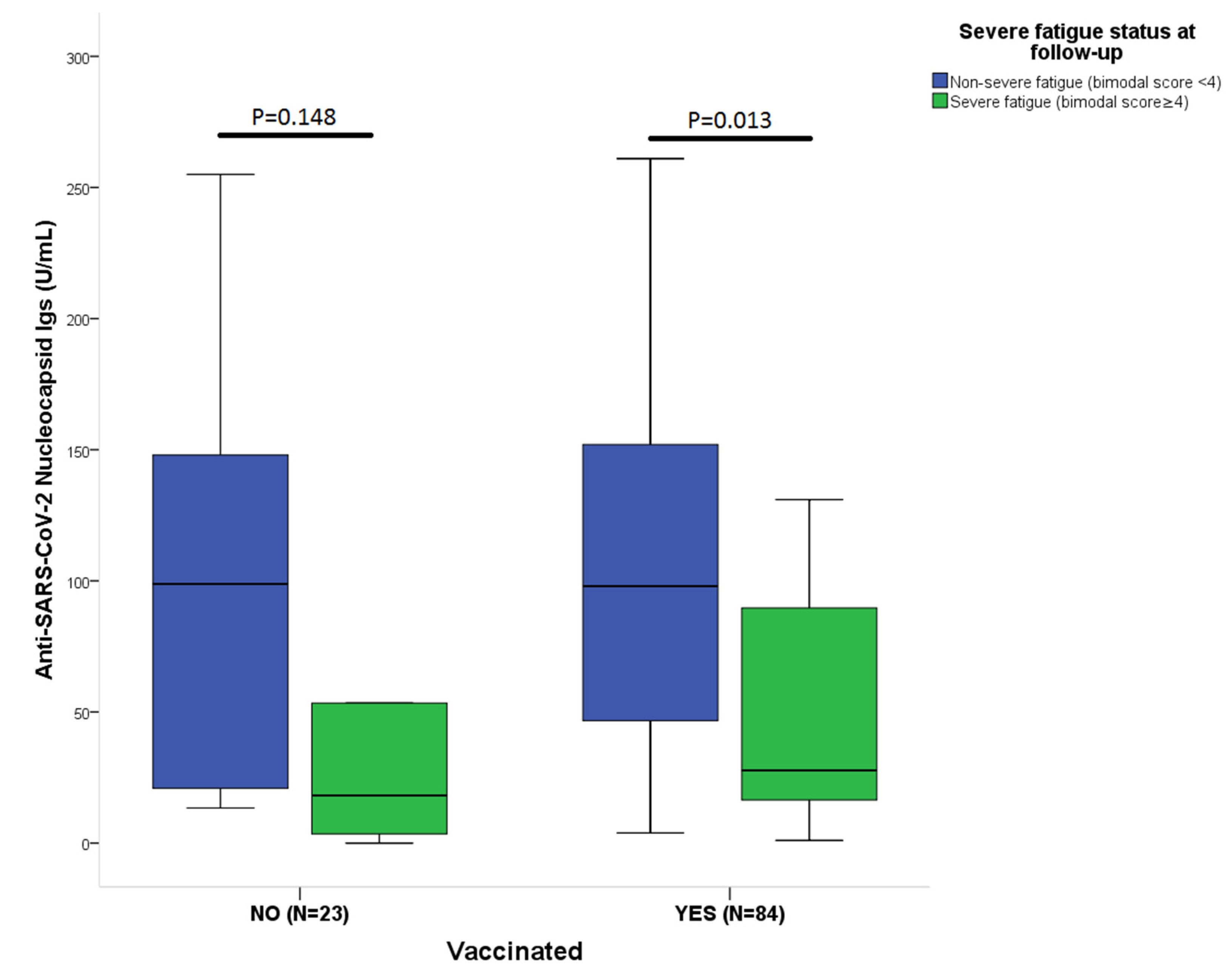
3.3. Symptoms Severity at Follow-Up and Antibody Level
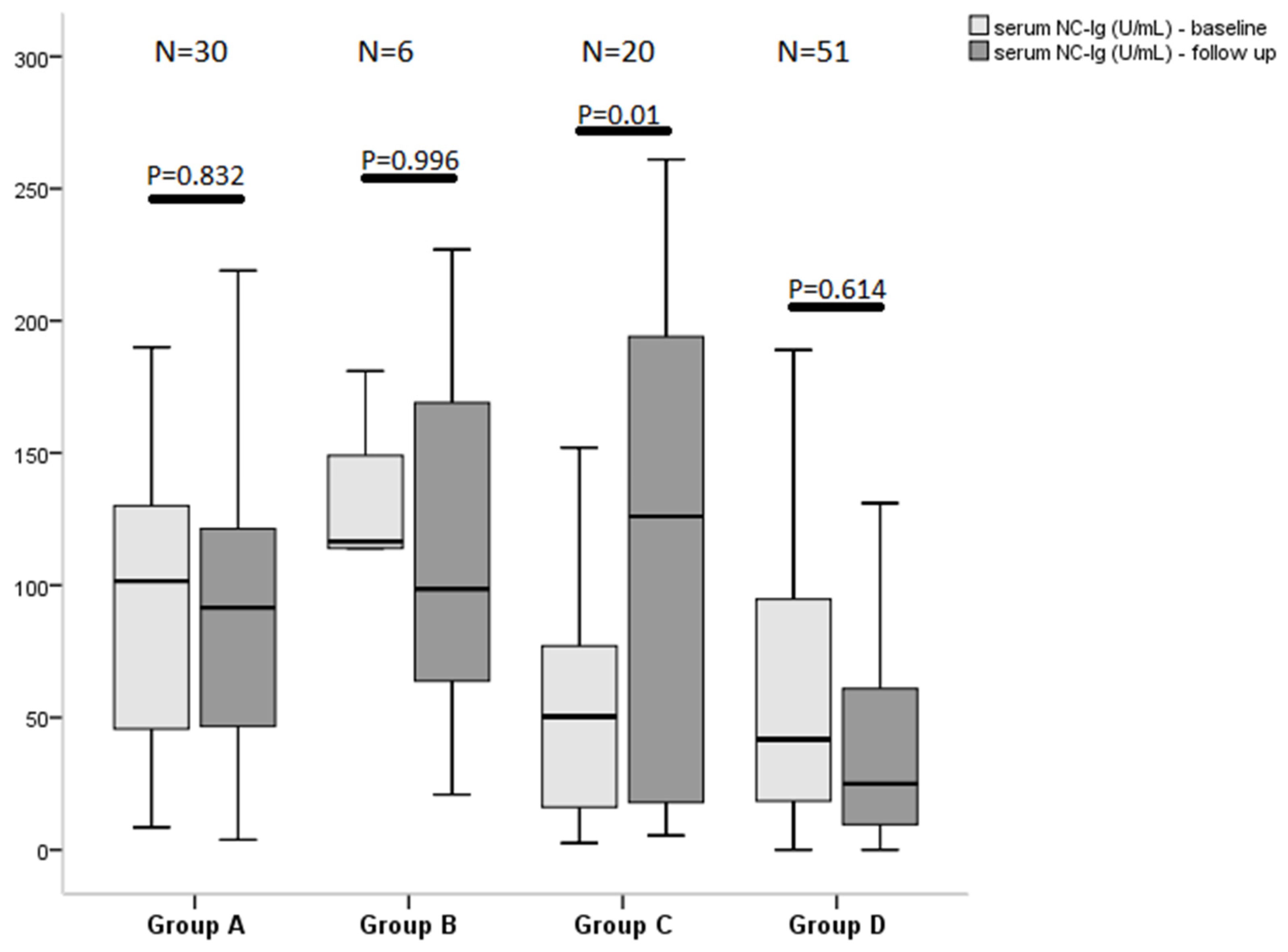
3.4. Effect of Vaccination on Antibody Levels and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2021. Available online: https://covid19.who.int/ (accessed on 8 December 2021).
- Huang, C.; Huang, L.; Wang, Y.; Li, X.; Ren, L.; Gu, X.; Kang, L.; Guo, L.; Liu, M.; Zhou, X.; et al. 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet 2021, 397, 220–232. [Google Scholar] [CrossRef]
- NICE COVID-19 rapid guidelines. Pharm. Outcomes News 2021, 877, 33. [CrossRef] [PubMed]
- Molnar, T.; Varnai, R.; Schranz, D.; Zavori, L.; Peterfi, Z.; Sipos, D.; Tőkés-Füzesi, M.; Illes, Z.; Buki, A.; Csecsei, P. Severe Fatigue and Memory Impairment Are Associated with Lower Serum Level of Anti-SARS-CoV-2 Antibodies in Patients with Post-COVID Symptoms. J. Clin. Med. 2021, 10, 4337. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of COVID-19 Vaccination on the Symptoms of Patients With Long COVID: A Target Trial Emulation Using Data from the ComPaRe e-Cohort in France. Available online: https://ssrn.com/abstract=3932953 (accessed on 8 December 2021).
- Chalder, T.; Berelowitz, G.; Pawlikowska, T.; Watts, L.; Wessely, S.; Wright, D.; Wallace, E.P. Development of a fatigue scale. J. Psychosom. Res. 1993, 37, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Jackson, C. The Chalder Fatigue Scale (CFQ 11). Occup. Med. 2016, 65, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morriss, R.K.; Wearden, A.J.; Mullis, R. Exploring the validity of the Chalder Fatigue scale in chronic fatigue syndrome. J. Psychosom Res. 1998, 45, 411–417. [Google Scholar] [CrossRef]
- LongCovidSOS. The Impact of COVID Vaccination on Symptoms of Long COVID. An International Survey of 900 People with Lived Experience. 2021. Available online: https://3ca26cd7-266e-4609-b25f-6f3d1497c4cf.filesusr.com/ugd/8bd4fe_a338597f76bf4279a851a7a4cb0e0a74.pdf (accessed on 8 December 2021).
- Li, K.; Huang, B.; Wu, M.; Zhong, A.; Li, L.; Cai, Y.; Wang, Z.; Wu, L.; Zhu, M.; Li, J.; et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020, 11, 6044. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Ulasli, M.; Schepers, H.; Mauthe, M.; V’kovski, P.; Kriegenburg, F.; Thiel, V.; de Haan, C.; Reggiori, F. Nucleocapsid Protein Recruitment to Replication-Transcription Complexes Plays a Crucial Role in Coronaviral Life Cycle. J. Virol. 2020, 94, e01925-19. [Google Scholar] [CrossRef] [Green Version]
- Ikegami, S.; Benirschke, R.; Flanagan, T.; Tanna, N.; Klein, T.; Elue, R.; Debosz, P.; Mallek, J.; Wright, G.; Guariglia, P.; et al. Persistence of SARS-CoV-2 nasopharyngeal swab PCR positivity in COVID-19 convalescent plasma donors. Transfusion 2020, 60, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Matchett, W.E.; Joag, V.; Stolley, J.M.; Shepherd, F.K.; Quarnstrom, C.F.; Mickelson, C.K.; Wijeyesinghe, S.; Soerens, A.G.; Becker, S.; Thiede, J.M.; et al. Cutting Edge: Nucleocapsid Vaccine Elicits Spike-Independent SARS-CoV-2 Protective Immunity. J. Immunol. 2021, 207, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Tso, F.Y.; Lidenge, S.J.; Peña, P.B.; Clegg, A.A.; Ngowi, J.R.; Mwaiselage, J.; Ngalamika, O.; Julius, P.; West, J.T.; Wood, C. High prevalence of pre-existing serological cross-reactivity against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in sub-Saharan Africa. Int. J. Infect. Dis. 2021, 102, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.S.; Bradley, A.S.; Bishop, K.N.; Kiani-Alikhan, S.; Ford, B. Chronic fatigue syndrome, the immune system and viral infection. Brain Behav. Immun. 2012, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. eBioMedicine 2021, 74. [Google Scholar] [CrossRef] [PubMed]
- Brenu, E.W.; van Driel, M.L.; Staines, D.R.; Ashton, K.J.; Ramos, S.B.; Keane, J.; Klimas, N.G.; Marshall-Gradisnik, S.M. Immunological abnormalities as potential biomarkers in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. J. Transl. Med. 2011, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic encephalomyelitis/chronic fatigue syndrome patients exhibit altered T cell metabolism and cytokine associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cliff, J.M.; King, E.C.; Lee, J.-S.; Sepulveda, N.; Wolf, A.-S.; Kingdon, C.; Bowman, E.; Dockrell, H.M.; Nacul, L.; Lacerda, E.; et al. Cellular Immune Function in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Front. Immunol. 2019, 10, 796. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.W.; Linderman, S.L.; Moodie, Z.; Czartoski, J.; Lai, L.; Mantus, G.; Norwood, C.; Nyhoff, L.E.; Edara, V.V.; Floyd, K.; et al. Longitudinal analysis shows durable and broad immune memory after SARS-CoV-2 infection with persisting antibody responses and memory B and T cells. medRxiv 2021, in press. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2021, 70, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
| Baseline (N = 107) | Follow-Up (N = 107) | ||||||
|---|---|---|---|---|---|---|---|
| Non-Severe Fatigue | Severe Fatigue | Non-Severe Fatigue | Severe Fatigue | ||||
| Characteristics | N = 36 (33.6%) | N = 71 (66.4%) | p Value | N = 50 (46.7%) | N = 57 (53.3%) | p Value | |
| Female | N, % | 16 (44) | 50 (70) | 0.009 | 27 (54) | 39 (68) | 0.126 |
| Age | Years (mean ± SD) | 53 ± 12 | 49 ± 11 | 0.035 | 50 ± 12 | 50 ± 12 | 1.000 |
| BMI | mean ± SD | 28.5 ± 8 | 26.5 ± 4 | 0.200 | 27.7 ± 7 | 26.7 ± 5 | 0.748 |
| Hospitalization | N, % | 14 (38.9) | 26 (36.6) | 0.819 | 25 (50) | 15 (26.3) | 0.012 |
| O2-supplementation | N, % | 5 (13.9) | 14 (19.7) | 0.456 | 12 (24) | 7 (12) | 0.113 |
| Antiviral medication | N, % | 13 (36.1) | 24 (33.8) | 0.813 | 25 (50) | 12 (21) | 0.002 |
| Vaccinated | N, % | N/A * | N/A * | N/A * | 41 (82) | 43 (75.4) | 0.410 |
| mRNA-based | N, % | N/A * | N/A * | N/A * | 30 (73) | 33 (77) | 0.658 |
| Vector-based | N, % | N/A * | N/A * | N/A * | 6 (15) | 8 (19) | 0.522 |
| Inactivated | N, % | N/A * | N/A * | N/A * | 5 (12) | 2 (4) | 0.08 |
| Time from vaccination (1.dose) to follow-up in days | days, mean ± SD | N/A * | N/A * | N/A * | 98 (56–134) | 90 (48–122) | 0.495 |
| Total CFQ-11 Score (Liekert Scoring) | mean ± SD | 11 (7–13) | 19 (17–22) | <0.001 | 8 (3–11) | 17 (15–21) | <0.001 |
| Physical Fatigue (CFQ-11 items 1–7) | mean ± SD | 7 (6–9) | 14 (13–16) | <0.001 | 6 (3–7) | 13 (11–14) | <0.001 |
| Psychological Fatigue (CFQ-11 items 8–11) | mean ± SD | 4 (1–4) | 5 (4–7) | <0.001 | 2 (0–4) | 5 (4–7) | <0.001 |
| Total CFQ-11 Score (Bimodal Scoring) | mean ± SD | 1 (0–3) | 7 (6–8) | <0.001 | 0 (0–2) | 6 (5–8) | <0.001 |
| anti-SARS-CoV-2 S-Ig | U/mL, median, IQR | 183 (106–696) | 113 (28–246) | 0.003 | 6949 (1430–12,500) | 3723 (911–10,932) | 0.155 |
| anti-SARS-CoV-2 NC-Ig | U/mL, median, IQR | 104 (48–131) | 45 (18–89) | <0.001 | 98 (29–152) | 27 (10–85) | 0.002 |
| Symptom onset to baseline | day, median, IQR | 74 (56–100) | 60 (40–99) | 0.126 | 65 (42–93) | 69 (46–103) | 0.359 |
| Symptom onset to follow-up | day, median, IQR | N/A * | N/A * | N/A * | 203 (179–233) | 208 (179–256) | 0.512 |
| Interval baseline to follow-up | day, median, IQR | N/A * | N/A * | N/A * | 142 (119–171) | 148 (119–168) | 0.837 |
| Value of NC-Ig at Follow-Up (U/mL, Median as the Cutoff) § | ||||
|---|---|---|---|---|
| Variables | B | Odds Ratio | 95% CI | p-Value |
| Total CFQ-11 at follow-up | 0.013 | 1.013 | 0.913–1.123 | 0.811 |
| Hospitalization | 0.441 | 1.555 | 0.114–21.152 | 0.740 |
| Antiviral medication | −0.679 | 0.507 | 0.035–7.296 | 0.618 |
| Interval between symptom onset and follow-up | −0.015 | 0.985 | 0.972–0.999 | 0.032 |
| Mean value of VAS at follow-up | −0.501 | 0.606 | 0.443–0.829 | 0.002 |
| Age | 0.058 | 1.060 | 1.005–1.117 | 0.032 |
| Total number of symptoms at follow-up | −0.053 | 0.949 | 0.767–1.172 | 0.625 |
| Gender | −0.602 | 0.547 | 0.163–1.840 | 0.330 |
| Complete remission at follow-up (bimodal score = 0, VAS score = 0) | ||||
| Variables | B | Odds ratio | 95% CI | p-Value |
| Hospitalization | −0.401 | 0.670 | 0.084–5.312 | 0.704 |
| Antiviral medication | −0.546 | 0.579 | 0.071–4.746 | 0.611 |
| NC median § (follow-up) | −1.089 | 0.337 | 0.120–0.946 | 0.039 |
| Gender | −0.356 | 0.701 | 0.265–1.849 | 0.472 |
| Age | −0.005 | 0.995 | 0.954–1.038 | 0.829 |
| Total VAS score at follow-up (median as the cutoff) § | ||||
| Variables | B | Odds ratio | 95% CI | p-Value |
| Age | 0.012 | 1.012 | 0.968–1.058 | 0.599 |
| Gender | 1.110 | 3.034 | 1.063–8.656 | 0.038 |
| NC-Ig (U/mL, follow-up) | −0.015 | 0.986 | 0.977–0.994 | 0.001 |
| Interval between symptom onset and follow-up | 0.007 | 1.007 | 0.996–1.019 | 0.210 |
| Antiviral medication | 1.224 | 3.401 | 0.392–29.492 | 0.267 |
| Hospitalization | −0.352 | 0.704 | 0.088–5.616 | 0.740 |
| S-Ig (U/mL, follow-up) | 0.000 | 1.000 | 1.000–1.000 | 0.882 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varnai, R.; Molnar, T.; Zavori, L.; Tőkés-Füzesi, M.; Illes, Z.; Kanizsai, A.; Csecsei, P. Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms. Vaccines 2022, 10, 165. https://doi.org/10.3390/vaccines10020165
Varnai R, Molnar T, Zavori L, Tőkés-Füzesi M, Illes Z, Kanizsai A, Csecsei P. Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms. Vaccines. 2022; 10(2):165. https://doi.org/10.3390/vaccines10020165
Chicago/Turabian StyleVarnai, Reka, Tihamer Molnar, Laszlo Zavori, Margit Tőkés-Füzesi, Zsolt Illes, Andrea Kanizsai, and Peter Csecsei. 2022. "Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms" Vaccines 10, no. 2: 165. https://doi.org/10.3390/vaccines10020165
APA StyleVarnai, R., Molnar, T., Zavori, L., Tőkés-Füzesi, M., Illes, Z., Kanizsai, A., & Csecsei, P. (2022). Serum Level of Anti-Nucleocapsid, but Not Anti-Spike Antibody, Is Associated with Improvement of Long COVID Symptoms. Vaccines, 10(2), 165. https://doi.org/10.3390/vaccines10020165







