Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Study Population and Sample
2.4. Data Sources
- National Maternity Collection for women using publicly funded maternity/newborn services.
- National Health Index (NHI) number for demographic information.
- National Immunisation Register (NIR) for National Immunisation Schedule vaccinations administered.
- Proclaim for general practice and pharmacy claims for vaccination events.
- DHB Maternity Clinic Data for vaccination events within the hospital.
- Pharmaceutical Collection for community pharmacy dispensing of prescribed medicines.
- Waikato Pharmacy Claims Data for Tdap vaccination of pregnant women.
2.5. Outcomes
2.6. Co-Variates
2.7. Statistical Analysis
3. Results
3.1. Sample
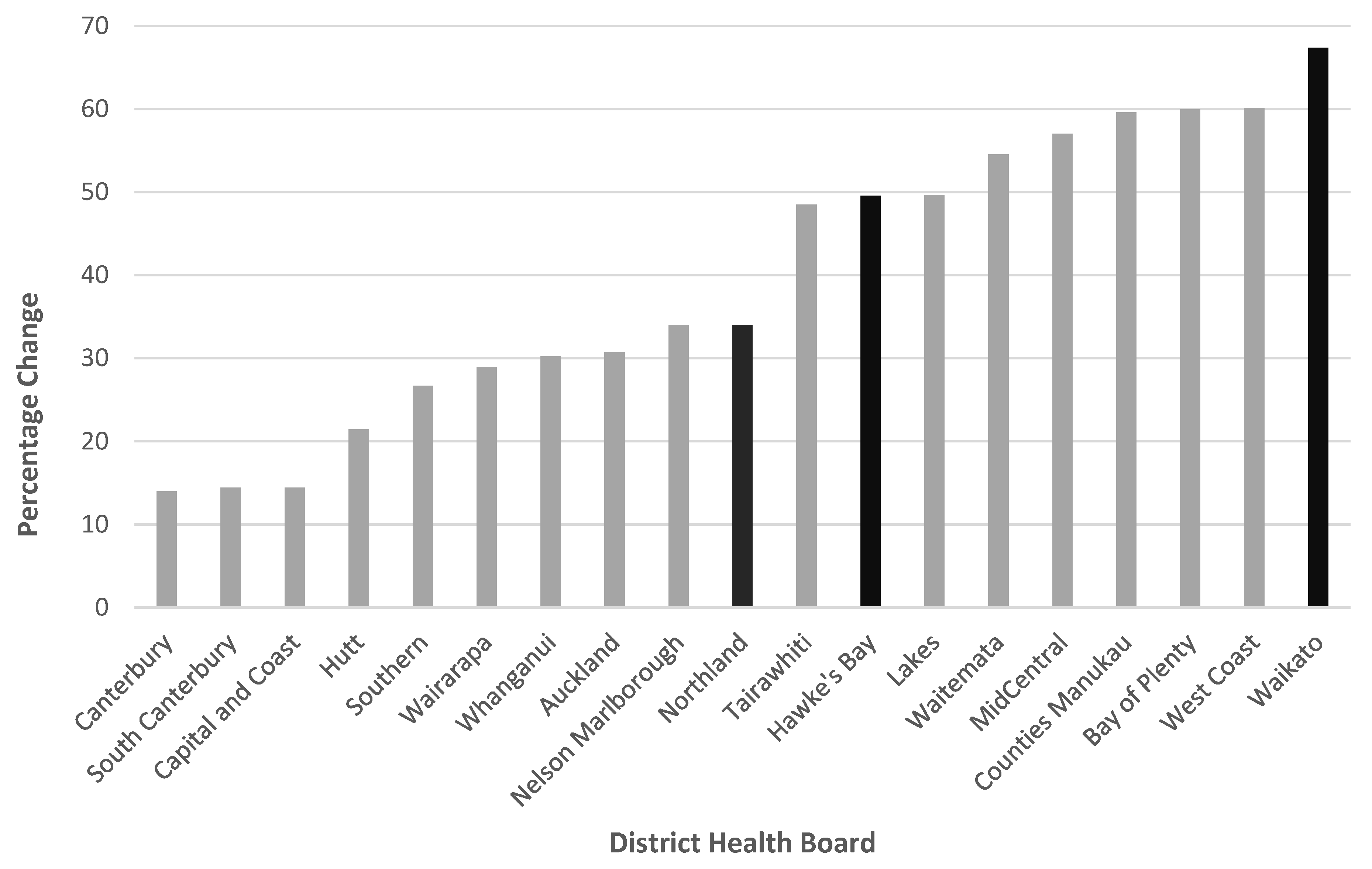
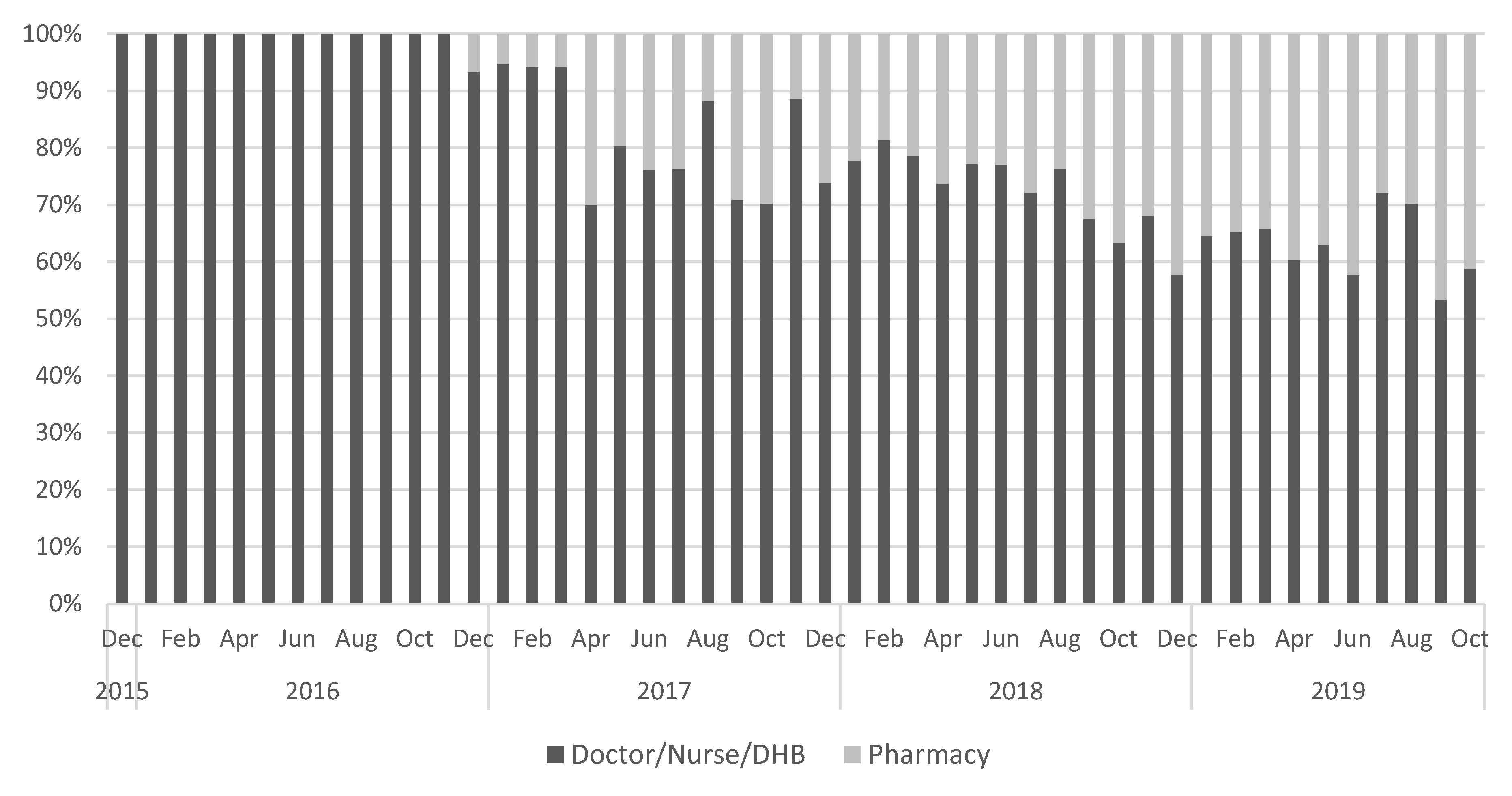
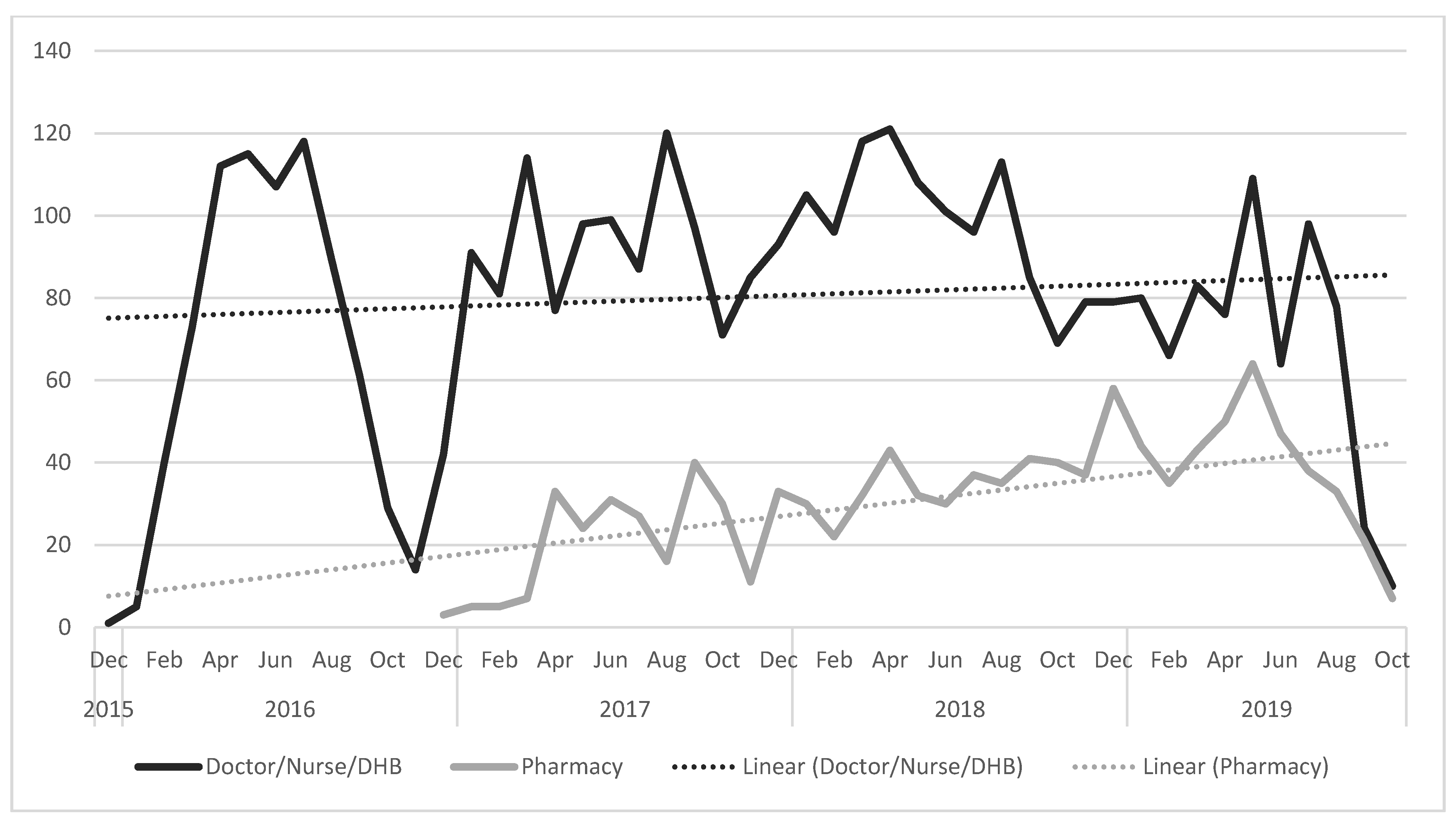
3.2. Pertussis Vaccine Uptake
4. Discussion
4.1. Comparison with Other Literature
4.2. Implications
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somerville, R.L.; Grant, C.C.; Grimwood, K.; Murdoch, D.; Graham, D.; Jackson, P.; Meates-Dennis, M.; Nicholson, R.; Purvis, D. Infants hospitalised with pertussis: Estimating the true disease burden. J. Paediatr. Child Health 2007, 43, 617–622. [Google Scholar] [CrossRef]
- Gopal, D.P.; Barber, J.; Toeg, D. Pertussis (whooping cough). BMJ 2019, 364, l401. [Google Scholar] [CrossRef]
- Kolos, V.; Menzies, R.; McIntyre, P. Higher pertussis hospitalization rates in indigenous Australian infants, and delayed vaccination. Vaccine 2007, 25, 588–590. [Google Scholar] [CrossRef]
- Gil-Prieto, R.; Walter, S.; San-Román-Montero, J.; Marín-García, P.; González-Escalada, A.; Gil-De-Miguel, A. Paediatric hospitalizations due to whooping cough in Spain (1997–2017). Vaccine 2019, 37, 6342–6347. [Google Scholar] [CrossRef]
- Vizzotti, C.; Neyro, S.; Katz, N.; Juárez, M.; Carrega, M.P.; Aquino, A.; Fullone, F.K. Maternal immunization in Argentina: A storyline from the prospective of a middle income country. Vaccine 2015, 33, 6413–6419. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Edwards, K.M. Optimizing the Timing of Vaccine Administration During Pregnancy. J. Am. Med. Assoc. 2019, 321, 935–936. [Google Scholar] [CrossRef]
- World Health Organisation. Pertussis vaccines: WHO position paper—September 2015. Wkly. Epidemiol. Rec. 2015, 90, 433–458. [Google Scholar]
- Public Health England. Pertussis vaccination programme for pregnant women update: Vaccine coverage in England, April to September 2019. Health Prot. Rep. 2019, 13. [Google Scholar]
- Psarris, A.; Sindos, M.; Theodora, M.; Antsaklis, P.; Pergialiotis, V.; Loutradis, D.; Daskalakis, G. Routine immunizations during pregnancy, doctors’ compliance and patient hesitancy: A two stage study on vaccination uptake. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 243, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.S.; Pointon, L.; Gauld, N.; Paynter, J.; Willing, E.; Turner, N. Pertussis and influenza immunisation coverage of pregnant women in New Zealand. Vaccine 2020, 38, 6766–6776. [Google Scholar] [CrossRef] [PubMed]
- Byrne, L.; Ward, C.; White, J.M.; Amirthalingam, G.; Edelstein, M. Predictors of coverage of the national maternal pertussis and infant rotavirus vaccination programmes in England. Epidemiol. Infect. 2018, 146, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kriss, J.L.; Albert, A.P.; Carter, V.M.; Jiles, A.J.; Liang, J.L.; Mullen, J.; Rodriguez, L.; Howards, P.P.; Orenstein, W.A.; Omer, S.B.; et al. Disparities in Tdap Vaccination and Vaccine Information Needs Among Pregnant Women in the United States. Matern. Child Health J. 2019, 23, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.Y.S.; Tarrant, M. Determinants of uptake of influenza vaccination among pregnant women—A systematic review. Vaccine 2014, 32, 4602–4613. [Google Scholar] [CrossRef]
- Gauld, N.J.; Braganza, C.S.; Babalola, O.O.; Huynh, T.T.; Hook, S.M. Reasons for use and non-use of the pertussis vaccine in pregnancy: An interview study in New Zealand women. J. Prim. Health Care 2016, 8, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Descamps, A.; Launay, O.; Bonnet, C.; Blondel, B. Seasonal influenza vaccine uptake and vaccine refusal among pregnant women in France: Results from a national survey. Hum. Vaccines Immunother. 2019, 16, 1093–1100. [Google Scholar] [CrossRef]
- Meharry, P.M.; Colson, E.R.; Grizas, A.P.; Stiller, R.; Vázquez, M. Reasons why women accept or reject the trivalent inactivated influenza vaccine (TIV) during pregnancy. Matern. Child Health J. 2013, 17, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; McMillan, M.; Roberts, C.; Marshall, H.S. A systematic review of interventions to improve uptake of pertussis vaccination in pregnancy. PLoS ONE 2019, 14, e0214538. [Google Scholar] [CrossRef]
- Mohammed, H.; Clarke, M.; Koehler, A.; Watson, M.; Marshall, H. Factors associated with uptake of influenza and pertussis vaccines among pregnant women in South Australia. PLoS ONE 2018, 13, e0197867. [Google Scholar] [CrossRef]
- Krishnaswamy, S.; Wallace, E.M.; Buttery, J.; Giles, M. Strategies to implement maternal vaccination: A comparison between standing orders for midwife delivery, a hospital based maternal immunisation service and primary care. Vaccine 2018, 36, 1796–1800. [Google Scholar] [CrossRef]
- Vishram, B.; Letley, L.; Van Hoek, A.J.; Silverton, L.; Donovan, H.; Adams, C.; Green, D.; Edwards, A.; Yarwood, J.; Bedford, H.; et al. Vaccination in pregnancy: Attitudes of nurses, midwives and health visitors in England. Hum. Vaccines Immunother. 2018, 14, 179–188. [Google Scholar] [CrossRef]
- Gauld, N.; Martin, S.; Sinclair, O.; Petousis-Harris, H.; Dumble, F.; Grant, C.C. Influences on Pregnant Women’s and Health Care Professionals’ Behaviour Regarding Maternal Vaccinations: A Qualitative Interview Study. Vaccines 2022, 10, 76. [Google Scholar] [CrossRef]
- Isenor, J.E.; Edwards, N.T.; Alia, T.A.; Slayter, K.L.; MacDougall, D.M.; McNeil, S.A.; Bowles, S.K. Impact of pharmacists as immunizers on vaccination rates: A systematic review and meta-analysis. Vaccine 2016, 34, 5708–5723. [Google Scholar] [CrossRef] [PubMed]
- Blin, A. Pregnancy and vaccination. Actual. Pharm. 2019, 58, 46–48. [Google Scholar] [CrossRef]
- Mills, B.; Fensterheim, L.; Taitel, M.; Cannon, A. Pharmacist-led Tdap vaccination of close contacts of neonates in a women’s hospital. Vaccine 2014, 32, 521–525. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hughes, C. Pharmacists and vaccination in pregnancy. Can. Pharm. J. (Ott.) 2019, 152, 424–426. [Google Scholar] [CrossRef]
- Waikato District Health Board. Waikato Health System Plan, Te Korowai Wairoa; Waikato District Health Board: Hamilton, New Zealand, 2019. [Google Scholar]
- Ministry of Health. Population of Northland DHB. Available online: https://www.health.govt.nz/new-zealand-health-system/my-dhb/northland-dhb/population-northland-dhb (accessed on 5 March 2020).
- Gauld, N.; Martin, S.; Sinclair, O.; Petousis-Harris, H.; Dumble, F.; Grant, C.C. A qualitative study of views and experiences of women and health care professionals about free maternal vaccinations administered at community pharmacies. Vaccines 2020, 8, 152. [Google Scholar] [CrossRef]
- Gauld, N.; Johnstone, E.; McMichael, I.; Braund, R. Pharmacists’ views and desires regarding pharmacist administration of vaccines in New Zealand. Int. J. Pharm. Pract. 2020, 29, 126–133. [Google Scholar] [CrossRef]
- Gauld, N.J.; Kelly, F.S.; Kurosawa, N.; Bryant, L.J.M.; Emmerton, L.M.; Buetow, S.A. Widening consumer access to medicines through switching medicines to non-prescription: A six country comparison. PLoS ONE 2014, 9, e107726. [Google Scholar] [CrossRef]
- National Health Board Business Unit. National Maternity Collection Data Mart Data Dictionary; Ministry of Health: Wellington, New Zealand, 2011.
- Pharmaceutical Management Agency. Decision to Widen Access to Pertussis (Whooping Cough) Vaccine. Available online: https://pharmac.govt.nz/news-and-resources/consultations-and-decisions/decision-to-widen-access-to-pertussis-whooping-cough-vaccine/ (accessed on 25 June 2021).
- Statistics New Zealand. 2018 Census—NZ Stat Tables. Available online: https://www.stats.govt.nz/tools/2018-census-place-summaries/new-zealand (accessed on 5 April 2021).
- Atkinson, J.; Salmond, C.; Crampton, P. NZDep2013 Index of Deprivation; Department of Public Health, University of Otago: Wellington, New Zealand, 2014. [Google Scholar]
- Mouzoon, M.E.; Munoz, F.M.; Greisinger, A.J.; Brehm, B.J.; Wehmanen, O.A.; Smith, F.A.; Markee, J.A.; Glezen, W.P. Improving influenza immunization in pregnant women and healthcare workers. Am. J. Manag. Care 2010, 16, 209–216. [Google Scholar]
- Chamberlain, A.; Seib, K.; Ault, K.; Rosenberg, E.; Frew, P.; Cortés, M.; Whitney, E.; Berkelman, R.; Orenstein, W.; Omer, S. Improving influenza and Tdap vaccination during pregnancy: A cluster-randomized trial of a multi-component antenatal vaccine promotion package in late influenza season. Vaccine 2015, 33, 3571–3579. [Google Scholar] [CrossRef]
- Koehler, A. How will the aging nurse workforce impact on rural healthcare and what can be done to address this issue? Sch. Nurs. Online J. 2019, 6. [Google Scholar]
- Petousis-Harris, H.; Jiang, Y.; Yu, L.; Watson, D.; Walls, T.; Turner, N.; Howe, A.S.; Griffin, J.B. A retrospective cohort study of safety outcomes in New Zealand infants exposed to tdap vaccine in utero. Vaccines 2019, 7, 147. [Google Scholar] [CrossRef] [PubMed]
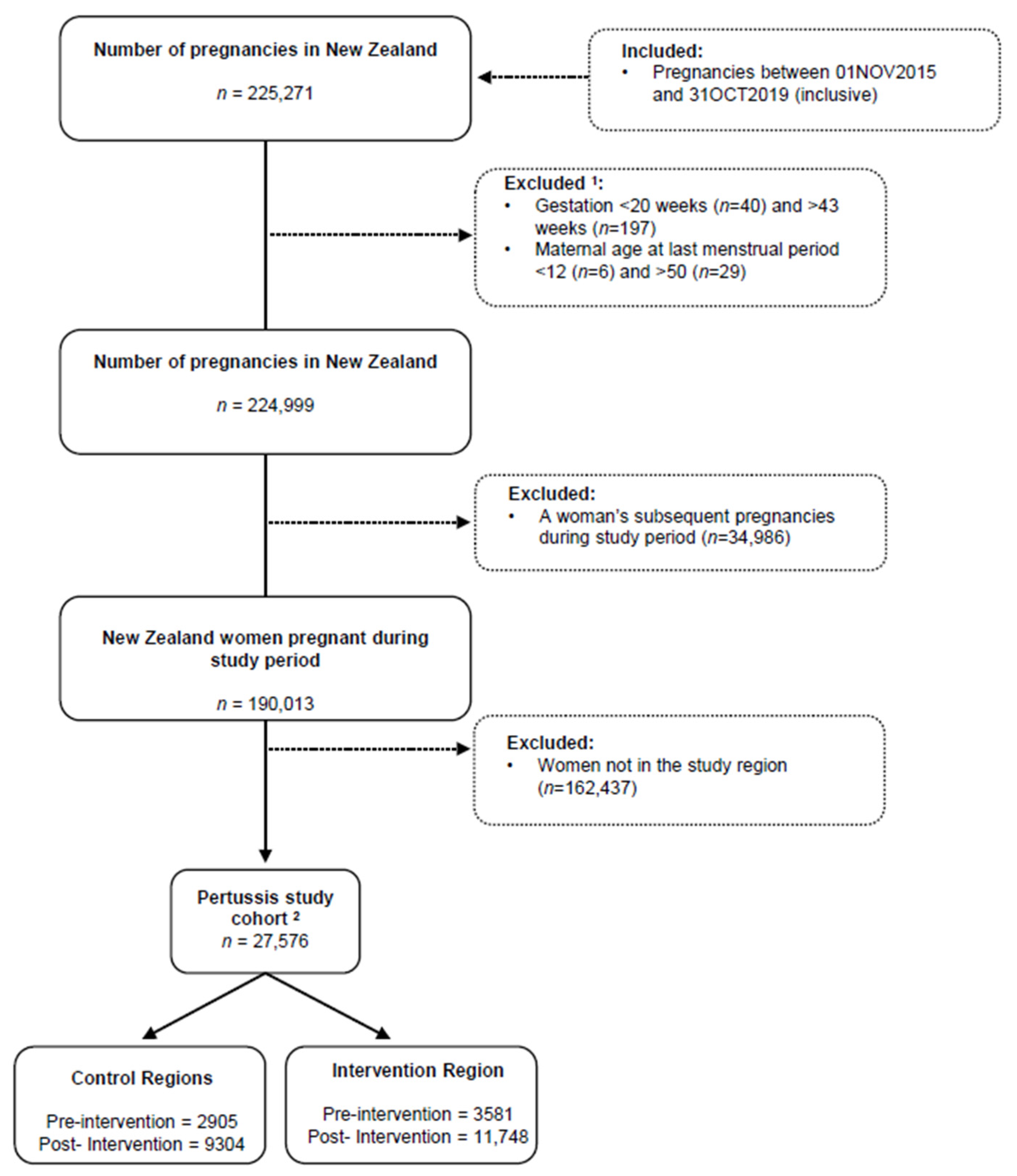
| Total Pertussis Cohort | Pertussis Cohort | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Intervention Period | Post-Intervention Period | |||||||||
| Control Areas | Intervention Area | Control Areas | Intervention Area | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Total | 27,576 | (100.0) | 2905 | (44.8) | 3581 | (55.2) | 9342 | (44.3) | 11,748 | (55.7) |
| Maternal age at LMP (years) 2,3 | ||||||||||
| 12–19 | 1948 | (7.1) | 234 | (8.1) | 239 | (6.7) | 734 | (7.9) | 741 | (6.3) |
| 20–24 | 5458 | (19.8) | 674 | (23.2) | 760 | (21.2) | 1885 | (20.2) | 2139 | (18.2) |
| 25–29 | 8268 | (30.0) | 845 | (29.1) | 1079 | (30.1) | 2733 | (29.3) | 3611 | (30.7) |
| 30–34 | 7498 | (27.2) | 704 | (24.2) | 993 | (27.7) | 2458 | (26.3) | 3343 | (28.5) |
| 35–39 | 3508 | (12.7) | 359 | (12.4) | 424 | (11.8) | 1208 | (12.9) | 1517 | (12.9) |
| 40–45 | 869 | (3.2) | 86 | (3.0) | 82 | (2.3) | 315 | (3.4) | 386 | (3.3) |
| 46+ | 27 | (0.1) | 3 | (0.1) | 4 | (0.1) | 9 | (0.1) | 11 | (0.1) |
| Prioritised Ethnicity 2,3 | ||||||||||
| Māori | 11,302 | (41.0) | 1534 | (52.8) | 1289 | (36.0) | 4429 | (47.4) | 4050 | (34.5) |
| Pacific | 1137 | (4.1) | 114 | (3.9) | 146 | (4.1) | 380 | (4.1) | 497 | (4.2) |
| Asian | 2889 | (10.5) | 167 | (5.7) | 430 | (12.0) | 642 | (6.9) | 1650 | (14.0) |
| Other | 457 | (1.7) | 31 | (1.1) | 59 | (1.6) | 84 | (0.9) | 283 | (2.4) |
| European | 11,791 | (42.8) | 1059 | (36.5) | 1657 | (46.3) | 3807 | (40.8) | 5268 | (44.8) |
| Area Level Deprivation 2,3 | ||||||||||
| Missing | 1 | (0.0) | 1 | (0.0) | ||||||
| 1 (least deprived) | 2201 | (8.0) | 117 | (4.0) | 392 | (10.9) | 323 | (3.5) | 1369 | (11.7) |
| 2 | 2610 | (9.5) | 340 | (11.7) | 283 | (7.9) | 1135 | (12.1) | 852 | (7.3) |
| 3 | 4478 | (16.2) | 309 | (10.6) | 709 | (19.8) | 1097 | (11.7) | 2363 | (20.1) |
| 4 | 7180 | (26.0) | 670 | (23.1) | 976 | (27.3) | 2207 | (23.6) | 3327 | (28.3) |
| 5 (most deprived) | 11,106 | (40.3) | 1468 | (50.5) | 1221 | (34.1) | 4580 | (49.0) | 3837 | (32.7) |
| District Health Board 2,3 | ||||||||||
| Northland | 6359 | (23.1) | 1539 | (53.0) | - | - | 4820 | (51.6) | - | - |
| Waikato | 15,329 | (55.6) | - | - | 3581 | (100.0) | - | - | 11,748 | (100.0) |
| Hawke’s Bay | 5888 | (21.4) | 1366 | (47.0) | - | - | 4522 | (48.4) | - | - |
| Model of maternity care at registration 2,3 | ||||||||||
| DHB | 322 | (1.2) | 120 | (4.1) | 0 | (0.0) | 201 | (2.2) | 1 | (0.0) |
| General Practice | 13 | (0.0) | 1 | (0.0) | 0 | (0.0) | 4 | (0.0) | 8 | (0.1) |
| Midwife | 26,367 | (95.6) | 2710 | (93.3) | 3486 | (97.3) | 8782 | (94.0) | 11,389 | (96.9) |
| No LMC | 817 | (3.0) | 71 | (2.4) | 88 | (2.5) | 341 | (3.7) | 317 | (2.7) |
| Obstetrician | 57 | (0.2) | 3 | (0.1) | 7 | (0.2) | 14 | (0.1) | 33 | (0.3) |
| Number of antenatal care visits 2,3 | ||||||||||
| Missing | 1385 | (5.0) | 218 | (7.5) | 110 | (3.1) | 618 | (6.6) | 439 | (3.7) |
| 0–5 | 4813 | (17.5) | 351 | (12.1) | 441 | (12.3) | 1208 | (12.9) | 2813 | (23.9) |
| 6–10 | 11,422 | (41.4) | 1191 | (41.0) | 1734 | (48.4) | 3557 | (38.1) | 4940 | (42.0) |
| 11–15 | 8896 | (32.3) | 1002 | (34.5) | 1188 | (33.2) | 3460 | (37.0) | 3246 | (27.6) |
| 16–20 | 941 | (3.4) | 126 | (4.3) | 99 | (2.8) | 434 | (4.6) | 282 | (2.4) |
| 21+ | 119 | (0.4) | 17 | (0.6) | 9 | (0.3) | 65 | (0.7) | 28 | (0.2) |
| Parity of eligible pregnancy 1,3 | ||||||||||
| Missing | 863 | (3.1) | 95 | (3.3) | 88 | (2.5) | 361 | (3.9) | 319 | (2.7) |
| 0 | 11,556 | (41.9) | 964 | (33.2) | 1277 | (35.7) | 3952 | (42.3) | 5363 | (45.7) |
| 1 | 7597 | (27.5) | 914 | (31.5) | 1158 | (32.3) | 2351 | (25.2) | 3174 | (27.0) |
| 2 | 4122 | (14.9) | 478 | (16.5) | 565 | (15.8) | 1420 | (15.2) | 1659 | (14.1) |
| 3 | 1844 | (6.7) | 219 | (7.5) | 274 | (7.7) | 663 | (7.1) | 688 | (5.9) |
| 4+ | 1594 | (5.8) | 235 | (8.1) | 219 | (6.1) | 595 | (6.4) | 545 | (4.6) |
| Gestational age at delivery (weeks) 2,3 | ||||||||||
| 28–38 | 7974 | (28.9) | 842 | (29.0) | 921 | (25.7) | 2736 | (29.3) | 3475 | (29.6) |
| 39–41 | 18,702 | (67.8) | 1998 | (68.8) | 2503 | (69.9) | 6410 | (68.6) | 7791 | (66.3) |
| 42–43 | 900 | (3.3) | 65 | (2.2) | 157 | (4.4) | 196 | (2.1) | 482 | (4.1) |
| Total | Pre-Intervention | Post-Intervention | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Unvaccinated | Vaccinated | Unvaccinated | Vaccinated | |||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Control areas 1 | 12,247 | (100) | 2138 | (73.6) | 767 | (26.4) | 5797 | (62.1) | 3545 | (38.0) |
| Intervention area 2 | 15,329 | (100) | 2832 | (79.1) | 749 | (20.9) | 7636 | (65.0) | 4112 | (35.0) |
| Pre-Intervention | Post-Intervention | Adjusted Model 1,2 | ||||
|---|---|---|---|---|---|---|
| n | Proportion Vaccinated (95% CI) | n | Proportion Vaccinated (95% CI) | OR | (95% CI) | |
| Region 2 | ||||||
| Control areas | 2904 | 26.4 (24.5, 28.3) | 9342 | 38.0 (36.7, 39.3) | 1.67 | (1.52, 1.84) |
| Intervention area | 3581 | 20.9 (19.4, 22.4) | 11,748 | 35.0 (33.9, 36.1) | 2.07 | (1.89, 2.27) |
| Non-Māori 3 | ||||||
| Control areas | 1370 | 36.4 (33.2, 39.6) | 4913 | 50.4 (48.4, 52.4) | 1.79 | (1.58, 2.03) |
| Intervention area | 2292 | 27.4 (25.3, 29.5) | 7698 | 42.7 (41.2, 44.2) | 1.98 | (1.79, 2.20) |
| Māori 3 | ||||||
| Control areas | 1534 | 17.5 (15.4, 19.6) | 4429 | 24.2 (22.8, 25.6) | 1.50 | (1.29, 1.74) |
| Intervention area | 1289 | 9.4 (7.7, 11.1) | 4050 | 20.4 (19.0, 21.8) | 2.42 | (1.99, 2.97) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Howe, A.S.; Gauld, N.J.; Cavadino, A.Y.; Petousis-Harris, H.; Dumble, F.; Sinclair, O.; Grant, C.C. Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies. Vaccines 2022, 10, 150. https://doi.org/10.3390/vaccines10020150
Howe AS, Gauld NJ, Cavadino AY, Petousis-Harris H, Dumble F, Sinclair O, Grant CC. Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies. Vaccines. 2022; 10(2):150. https://doi.org/10.3390/vaccines10020150
Chicago/Turabian StyleHowe, Anna S., Natalie J. Gauld, Alana Y. Cavadino, Helen Petousis-Harris, Felicity Dumble, Owen Sinclair, and Cameron C. Grant. 2022. "Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies" Vaccines 10, no. 2: 150. https://doi.org/10.3390/vaccines10020150
APA StyleHowe, A. S., Gauld, N. J., Cavadino, A. Y., Petousis-Harris, H., Dumble, F., Sinclair, O., & Grant, C. C. (2022). Increasing Uptake of Maternal Pertussis Vaccinations through Funded Administration in Community Pharmacies. Vaccines, 10(2), 150. https://doi.org/10.3390/vaccines10020150






