Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Omicron Variant (B.1.1.529): A Systematic Review with Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy and Selection of Studies
2.3. Data Extraction
2.4. Quality Assessment
2.5. Outcomes Measure
2.6. Statistical Analysis
3. Results
3.1. Study Selection and Quality Assessment
3.2. Study Characteristics
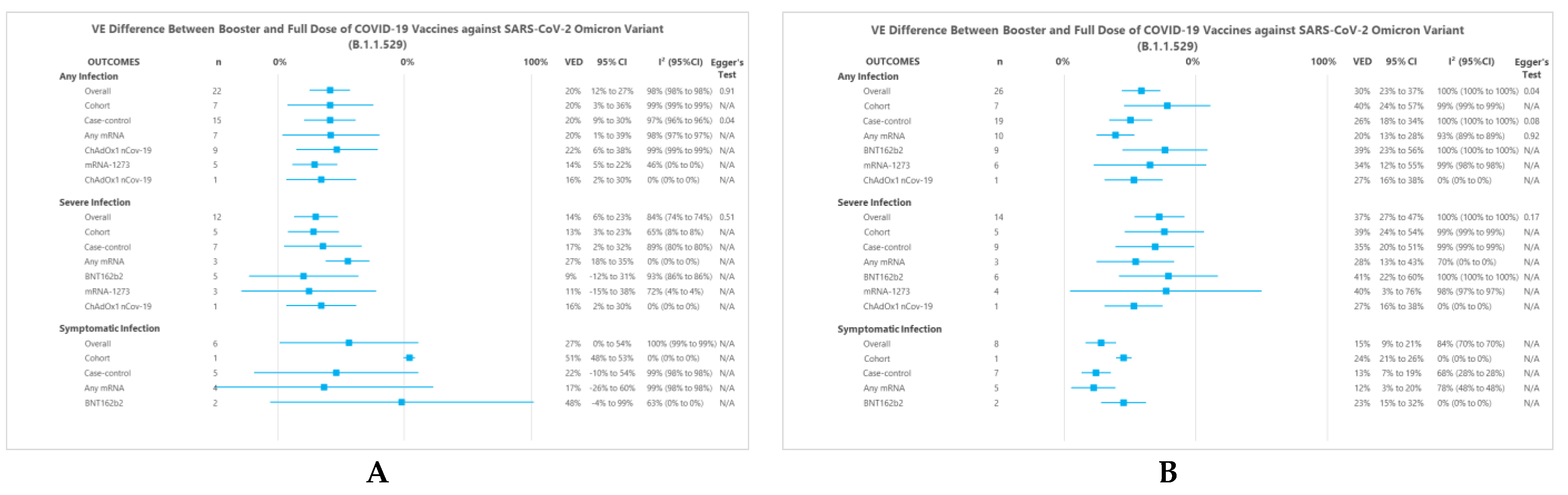
3.3. VED Estimates between Booster and Full Dose
3.3.1. Overall Analysis
3.3.2. Subgroup Analysis of ‘Within 3 Months’ Model
3.3.3. Subgroup Analysis of ‘Within 3 Months or More’ Model
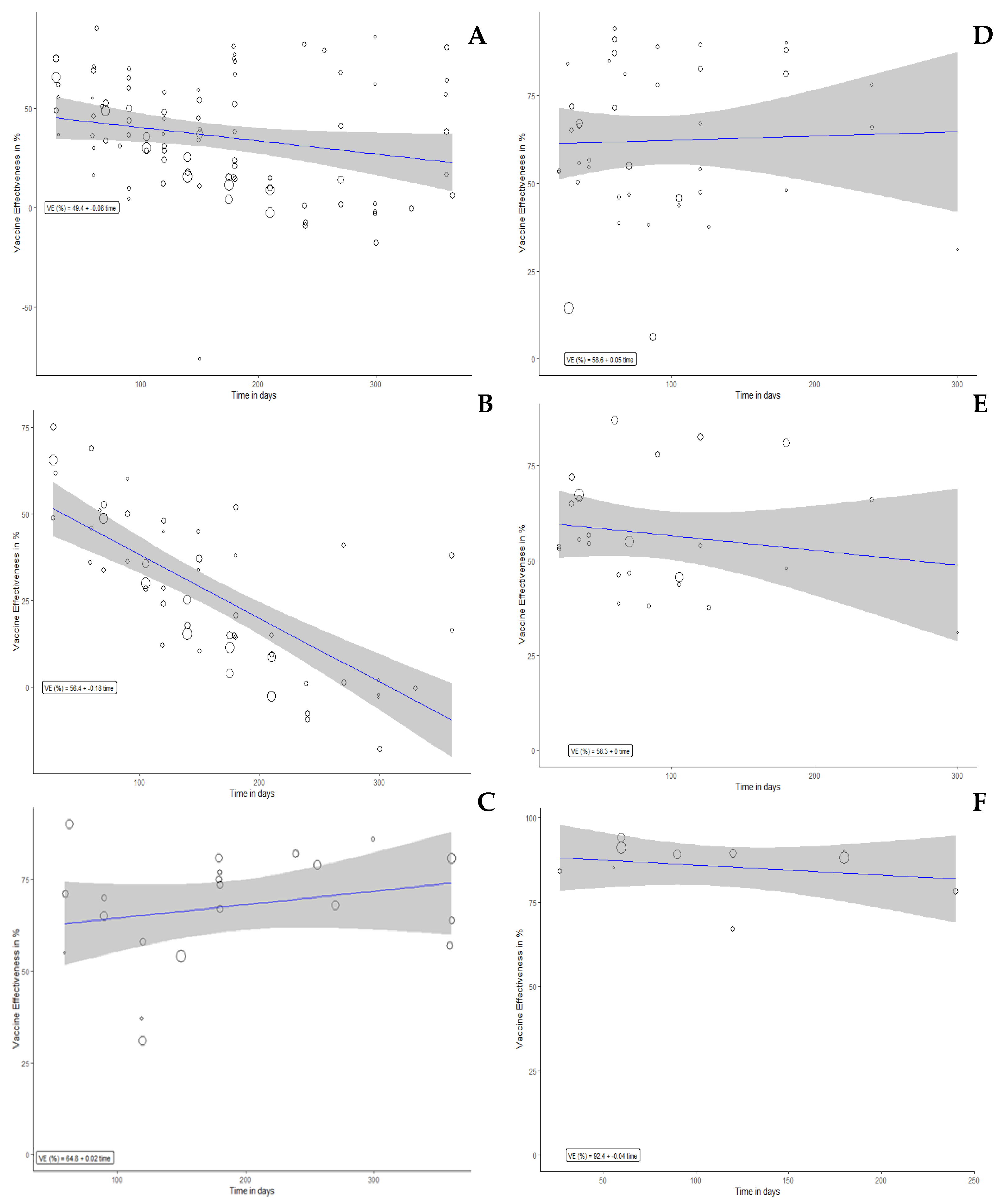
3.4. VE Estimates and VE Reduction for Booster Dose and Full Dose
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 17 April 2022).
- Science Brief: Omicron (B.1.1.529) Variant|CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/scientific-brief-omicron-variant.html (accessed on 17 April 2022).
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after MRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron Variant of SARS-CoV-2: Genomics, Transmissibility, and Responses to Current COVID-19 Vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Principles of Epidemiology|Lesson 3—Section 6. Available online: https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html (accessed on 27 April 2022).
- Lauring, A.S.; Hodcroft, E.B. Genetic Variants of SARS-CoV-2—What Do They Mean? JAMA-J. Am. Med. Assoc. 2021, 325, 529–531. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in Creating Herd Immunity to SARS-CoV-2 Infection by Mass Vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef] [PubMed]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; el Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine, BBIBP-CorV: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Trial. Lancet Infect Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; st. Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. MRNA-Based COVID-19 Vaccine Boosters Induce Neutralizing Immunity against SARS-CoV-2 Omicron Variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Peng, L.; Filler, R.; Suzuki, K.; McNamara, A.; Lin, Q.; Renauer, P.A.; Yang, L.; Menasche, B.; Sanchez, A.; et al. Omicron-Specific MRNA Vaccination Alone and as a Heterologous Booster against SARS-CoV-2. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lou, F.; Fan, H. SARS-CoV-2 Variant Omicron: Currently the Most Complete “Escapee” from Neutralization by Antibodies and Vaccines. Signal Transduct. Target. Ther. 2022, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against COVID-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Zeng, G.; Wu, Q.; Pan, H.; Li, M.; Yang, J.; Wang, L.; Wu, Z.; Jiang, D.; Deng, X.; Chu, K.; et al. Immunogenicity and Safety of a Third Dose of CoronaVac, and Immune Persistence of a Two-Dose Schedule, in Healthy Adults: Interim Results from Two Single-Centre, Double-Blind, Randomised, Placebo-Controlled Phase 2 Clinical Trials. Lancet Infect. Dis. 2022, 22, 483–495. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Zarandi, P.K.; Zinatizadeh, M.; Yousefi, M.H.; Amani, J.; Rezaei, N. Efficacy of MRNA, Adenoviral Vector, and Perfusion Protein COVID-19 Vaccines. Biomed. Pharmacother. 2022, 146, 112527. [Google Scholar] [CrossRef]
- CDC COVID Data Tracker: Global COVID-19 Vaccinations. Available online: https://covid.cdc.gov/covid-data-tracker/#global-vaccinations (accessed on 25 April 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Ga, S.W.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. PLoS Negl. Trop. Dis. 2014, 7, e2195. [Google Scholar] [CrossRef]
- Buchan, S.A.; Chung, H.; Brown, K.A.; Austin, P.C.; Fell, D.B.; Gubbay, J.B.; Nasreen, S.; Schwartz, K.L.; Sundaram, M.E.; Tadrous, M.; et al. Effectiveness of COVID-19 Vaccines against Omicron or Delta Symptomatic Infection and Severe Outcomes. medRxiv 2022. [Google Scholar] [CrossRef]
- Gray, G.E.; Collie, S.; Garrett, N.; Goga, A.; Champion, J.; Zylstra, M.; Reddy, T.; Yende, N.; Seocharan, I.; Takalani, A.; et al. Vaccine Effectiveness against Hospital Admission in South African Health Care Workers Who Received a Homologous Booster of Ad26.COV2 during an Omicron COVID19 Wave: Preliminary Results of the Sisonke 2 Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Accorsi, E.K.; Britton, A.; Fleming-Dutra, K.E.; Smith, Z.R.; Shang, N.; Derado, G.; Miller, J.; Schrag, S.J.; Verani, J.R. Association between 3 Doses of MRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA-J. Am. Med. Assoc. 2022, 327, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. Effectiveness of COVID-19 Vaccines against the Omicron (B.1.1.529) Variant of Concern. medRxiv 2021. [Google Scholar] [CrossRef]
- Chemaitelly, H.; Ayoub, H.H.; Almukdad, S.; Tang, P.; Hasan, M.R.; Yassine, H.M.; al Khatib, H.A.; Smatti, M.K.; Coyle, P.; al Kanaani, Z.; et al. Duration of Protection of BNT162b2 and MRNA-1273 COVID-19 Vaccines against Symptomatic SARS-CoV-2 Omicron Infection in Qatar. medRxiv 2022. [Google Scholar] [CrossRef]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.-G.; Gray, G. Effectiveness of BNT162b2 Vaccine against Omicron Variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Ferdinands, J.M.; Rao, S.; Dixon, B.E.; Mitchell, P.K.; DeSilva, M.B.; Irving, S.A.; Lewis, N.; Natarajan, K.; Stenehjem, E.; Grannis, S.J.; et al. Waning 2-Dose and 3-Dose Effectiveness of MRNA Vaccines Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, Aug. MMWR Recomm. Rep. 2022, 71, 255–263. [Google Scholar] [CrossRef]
- Klein, N.P.; Stockwell, M.S.; Demarco, M.; Gaglani, M.; Kharbanda, A.B.; Irving, S.A.; Rao, S.; Grannis, S.J.; Dascomb, K.; Murthy, K.; et al. Effectiveness of COVID-19 Pfizer-BioNTech BNT162b2 MRNA Vaccination in Preventing COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Nonimmunocompromised Children and Adolescents Aged 5–17 Years—VISION Network. MMWR Recomm. Rep. 2022, 71, 352–358. [Google Scholar] [CrossRef]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical Severity and MRNA Vaccine Effectiveness for Omicron, Delta, and Alpha SARS-CoV-2 Variants in the United States: A Prospective Observational Study. medRxiv 2022. [Google Scholar] [CrossRef]
- Natarajan, K.; Prasad, N.; Dascomb, K.; Irving, S.A.; Yang, D.-H.; Gaglani, M.; Klein, N.P.; DeSilva, M.B.; Ong, T.C.; Grannis, S.J.; et al. Effectiveness of Homologous and Heterologous COVID-19 Booster Doses Following 1 Ad.26.COV2.S (Janssen [Johnson & Johnson]) Vaccine Dose Against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations Among Adults—VISION. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 495–502. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Puzniak, L.; Hong, V.; Xie, F.; Ackerson, B.K.; Valluri, S.R.; Jodar, L.; McLaughlin, J.M. BNT162b2 (Pfizer–Biontech) MRNA COVID-19 Vaccine against Omicron-Related Hospital and Emergency Department Admission in a Large US Health System: A Test-Negative Design. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Talbot, H.K. Effectiveness of MRNA Vaccination in Preventing COVID-19—Associated Invasive Mechanical Ventilation and Death—United States, March 2021–January 2022. Morb. Mortal. Wkly. Rep. 2022, 71, 459–465. [Google Scholar] [CrossRef]
- Thompson, M.G.; Natarajan, K.; Irving, S.A.; Rowley, E.A.; Griggs, E.P.; Gaglani, M.; Klein, N.P.; Grannis, S.J.; DeSilva, M.B.; Stenehjem, E.; et al. Effectiveness of a Third Dose of MRNA Vaccines against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicron Variant Predominance—VISION Network, 10 States, August 2021–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Fu Tseng, H.; Ackerson, B.K.; Luo, Y.; Sy, L.S.; Talarico, C.A.; Tian, Y.; Bruxvoort, K.J.; Tubert, J.E.; Florea, A.; Ku, J.H.; et al. Effectiveness of MRNA-1273 against SARS-CoV-2 Omicron and Delta Variants. medRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Young-Xu, Y.; Zwain, G.M.; Izurieta, H.S.; Korves, C.; Powell, E.I.; Smith, J.; Balajee MPH, A.S.; Holodniy, M.; Beenhouwer, D.O.; Rodriguez-Barradas, M.C.; et al. Effectiveness of MRNA COVID-19 Booster Vaccines against Omicron and Delta Variants among US Veterans. medRxiv 2022. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M. Effectiveness of BNT162b2 (Pfizer-BioNTech) MRNA Vaccination against Multisystem Inflammatory Syndrome in Children among Persons Aged 12–18 Years—United States, July–December 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raddad, L.J.; Chemaitelly, H.; Ayoub, H.H.; Almukdad, S.; Tang, P.; Hasan, M.R.; Coyle, P.; Yassine, H.M.; Al-Khatib, H.A.; Smatti, M.K.; et al. Effectiveness of BNT162b2 and MRNA-1273 COVID-19 Boosters against SARS-CoV-2 Omicron (B.1.1.529) Infection in Qatar. medRxiv 2022. [Google Scholar] [CrossRef]
- Holm, C.; Phd, H.; Blicher, A.; Phd, S.; Rask, I.; Phd, M.-H.; Emborg, H.-D.; Krause, T.G.; Mølbak Dmsc, K.; Valentiner-Branth, P. TITLE PAGE Title: Vaccine Effectiveness against SARS-CoV-2 Infection with the Omicron or Delta Variants Following a Two-Dose or Booster BNT162b2 or MRNA-1273 Vaccination Series: A Danish Cohort Study. medRxiv 2021. [Google Scholar] [CrossRef]
- Monge, S.; Rojas-Benedicto, A.; Olmedo, C.; Mazagatos, C.; Sierra, M.J.; Limia, A.; Martín-Merino, E.; Larrauri, A.; Hernán, M.A. The Effectiveness of MRNA Vaccine Boosters for Laboratory-Confirmed COVID-19 during a Period of Predominance of the Omicron Variant of SARS-CoV-2. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) MRNA Vaccine in Preventing SARS-CoV-2 Infection among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef]
- Khoury, D.S.; Steain, M.; Triccas, J.A.; Sigal, A.; Davenport, M.P.; Cromer, D. A Meta-Analysis of Early Results to Predict Vaccine Efficacy against Omicron. medRxiv 2021. [Google Scholar] [CrossRef]
- Ratajczak, W.; Niedźwiedzka-Rystwej, P.; Tokarz-Deptuła, B.; DeptuŁa, W. Immunological Memory Cells. Cent. Eur. J. Immunol. 2018, 43, 194–203. [Google Scholar] [CrossRef]
- Stevenson, F.K.; di Genova, G.; Ottensmeier, C.; Savelyeva, N. Cancer Vaccines. In Cancer Immunotherapy; Elsevier Inc.: Amsterdam, The Netherlands, 2007; pp. 183–204. ISBN 9780123725516. [Google Scholar]
- Kyei-Barffour, I.; Addo, S.A.; Aninagyei, E.; Ghartey-Kwansah, G.; Acheampong, D.O. Sterilizing Immunity against COVID-19: Developing Helper T Cells I and II Activating Vaccines Is Imperative. Biomed. Pharmacother. 2021, 144, 112282. [Google Scholar] [CrossRef] [PubMed]
- Palm, A.K.E.; Henry, C. Remembrance of Things Past: Long-Term B Cell Memory After Infection and Vaccination. Front. Immunol. 2019, 10, 1787. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G.C.; Light, A.; Nossal, G.J.V.; Tarlinton, D.M. The Extent of Affinity Maturation Differs between the Memory and Antibody-Forming Cell Compartments in the Primary Immune Response. EMBO J. 1997, 16, 2996–3006. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Hahn, M.; Coyle, E.M.; King, L.R.; Lin, T.L.; Treanor, J.; Sant, A.; Golding, H. Repeat Vaccination Reduces Antibody Affinity Maturation across Different Influenza Vaccine Platforms in Humans. Nat. Commun. 2019, 10, 3338. [Google Scholar] [CrossRef]
- Munro, A.P.S.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and Immunogenicity of Seven COVID-19 Vaccines as a Third Dose (Booster) Following Two Doses of ChAdOx1 NCov-19 or BNT162b2 in the UK (COV-BOOST): A Blinded, Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Choi, A.; Koch, M.; Wu, K.; Chu, L.; Ma, L.Z.; Hill, A.; Nunna, N.; Huang, W.; Oestreicher, J.; Colpitts, T.; et al. Safety and Immunogenicity of SARS-CoV-2 Variant MRNA Vaccine Boosters in Healthy Adults: An Interim Analysis. Nat. Med. 2021, 27, 2025–2031. [Google Scholar] [CrossRef]
- Gray, K.J.; Bordt, E.A.; Atyeo, C.; Deriso, E.; Akinwunmi, B.; Young, N.; Baez, A.M.; Shook, L.L.; Cvrk, D.; James, K.; et al. Coronavirus Disease 2019 Vaccine Response in Pregnant and Lactating Women: A Cohort Study. Am. J. Obstet. Gynecol. 2021, 225, 303.e1–303.e17. [Google Scholar] [CrossRef]
- Muik, A.; Lui, B.G.; Wallisch, A.K.; Bacher, M.; Mühl, J.; Reinholz, J.; Ozhelvaci, O.; Beckmann, N.; de la Caridad Güimil Garcia, R.; Poran, A.; et al. Neutralization of SARS-CoV-2 Omicron by BNT162b2 MRNA Vaccine-Elicited Human Sera. Science 2022, 375, 678–680. [Google Scholar] [CrossRef]
- Earle, K.A.; Ambrosino, D.M.; Fiore-Gartland, A.; Goldblatt, D.; Gilbert, P.B.; Siber, G.R.; Dull, P.; Plotkin, S.A. Evidence for Antibody as a Protective Correlate for COVID-19 Vaccines. Vaccine 2021, 39, 4423–4428. [Google Scholar] [CrossRef]
- Koch, T.; Mellinghoff, S.C.; Shamsrizi, P.; Addo, M.M.; Dahlke, C.; Sobrino, F. Correlates of Vaccine-Induced Protection against SARS-CoV-2 Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD). Vaccines 2021, 9, 238. [Google Scholar] [CrossRef]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising Antibody Titres as Predictors of Protection against SARS-CoV-2 Variants and the Impact of Boosting: A Meta-Analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 COVID-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Peacock, S.J.; et al. SARS-CoV-2 Variants, Spike Mutations and Immune Escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Dingens, A.S.; Bloom, J.D. Complete Map of SARS-CoV-2 RBD Mutations That Escape the Monoclonal Antibody LY-CoV555 and Its Cocktail with LY-CoV016. Cell Rep. Med. 2021, 2, 100255. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta Variant Replication and Immune Evasion. Nature 2021, 599, 41. [Google Scholar] [CrossRef]
- Sun, C.; Kang, Y.F.; Liu, Y.T.; Kong, X.W.; Xu, H.Q.; Xiong, D.; Xie, C.; Liu, Y.H.; Peng, S.; Feng, G.K.; et al. Parallel Profiling of Antigenicity Alteration and Immune Escape of SARS-CoV-2 Omicron and Other Variants. Signal Transduct. Target. Ther. 2022, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Altmann, D.M.; Boyton, R.J. SARS-CoV-2 T Cell Immunity: Specificity, Function, Durability, and Role in Protection. Sci. Immunol. 2020, 5, eabd6160. [Google Scholar] [CrossRef]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183. [Google Scholar] [CrossRef]
- Grifoni, A.; Weiskopf, D.; Ramirez, S.I.; Mateus, J.; Dan, J.M.; Moderbacher, C.R.; Rawlings, S.A.; Sutherland, A.; Premkumar, L.; Jadi, R.S.; et al. Targets of T Cell Responses to SARS-CoV-2 Coronavirus in Humans with COVID-19 Disease and Unexposed Individuals. Cell 2020, 181, 1489–1501. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. Publisher Correction: A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, D.; Schmitz, K.S.; Raadsen, M.P.; Grifoni, A.; Okba, N.M.A.; Endeman, H.; van den Akker, J.P.C.; Molenkamp, R.; Koopmans, M.P.G.; van Gorp, E.C.M.; et al. Phenotype and Kinetics of SARS-CoV-2-Specific T Cells in COVID-19 Patients with Acute Respiratory Distress Syndrome. Sci. Immunol. 2020, 5, eabd2071. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.-Y.; Wang, W.-B.; Gao, R.-D.; Zhou, A.-M. Omicron Variant (B.1.1.529) of SARS-CoV-2: Mutation, Infectivity, Transmission, and Vaccine Resistance. World J. Clin. Cases 2022, 10, 1–11. [Google Scholar] [CrossRef]
- Iuliano, A.D.; Brunkard, J.M.; Boehmer, T.K.; Peterson, E.; Adjei, S.; Binder, A.M.; Cobb, S.; Graff, P.; Hidalgo, P.; Panaggio, M.J.; et al. Trends in Disease Severity and Health Care Utilization During the Early Omicron Variant Period Compared with Previous SARS-CoV-2 High Transmission Periods—United States, December 2020–January 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Nakayama, J.Y.; O’Hegarty, M.; McGowan, A.; Teran, R.A.; Bart, S.M.; Mosack, K.; Roberts, N.; Campos, B.; Paegle, A.; et al. SARS-CoV-2 B.1.1.529 (Omicron) Variant Transmission Within Households—Four U.S. Jurisdictions, November 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 341–346. [Google Scholar] [CrossRef]
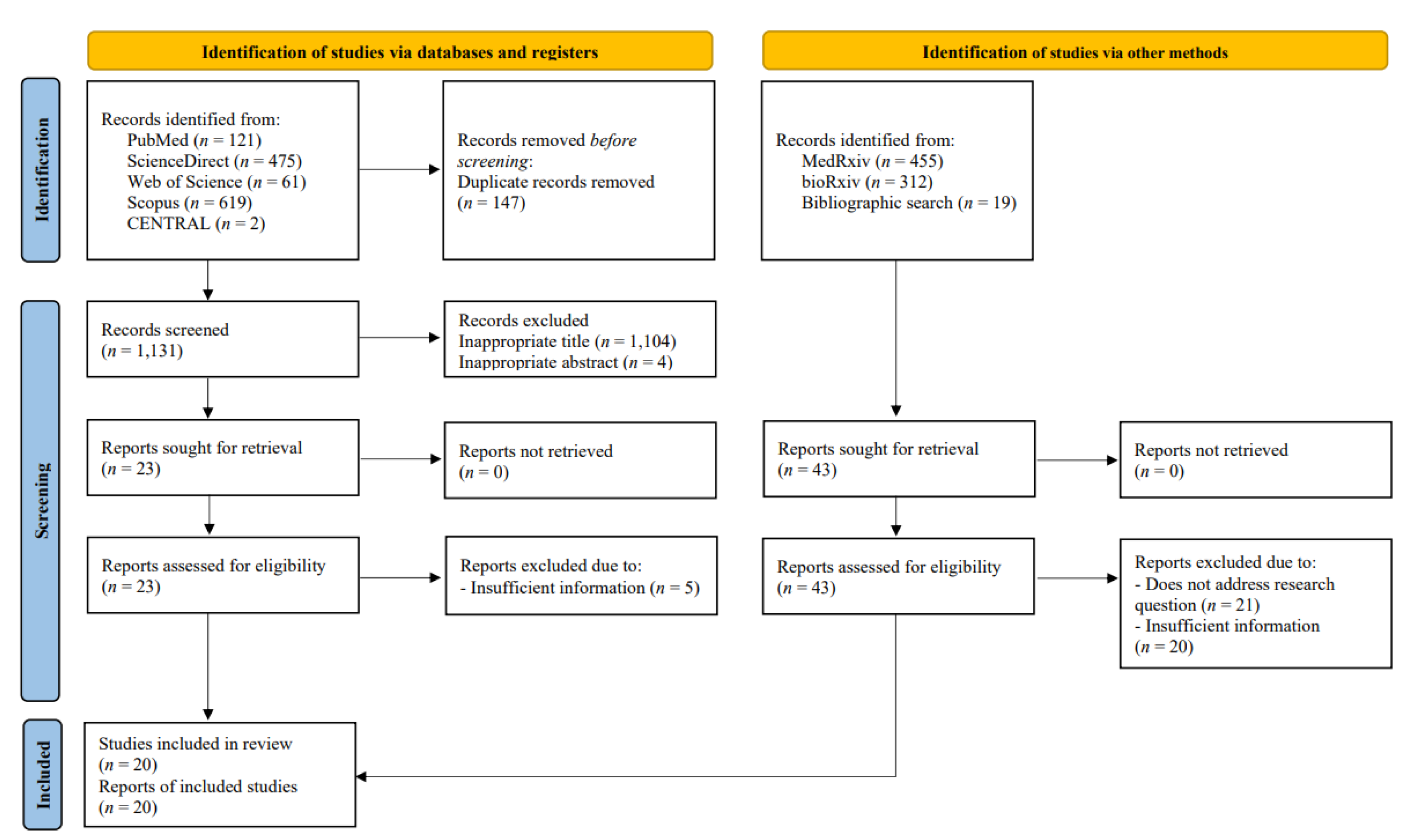
| Reference | Study Design | County of Origin | Sample Sizes | Age /Years | Omicron Strain Confirmation Method | Follow-up Duration & (Median (IQR)) or Range) +/Days | Type of Vaccines | Endpoints | Vaccine Effectiveness § /100% | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dose 2 | Dose 3 | |||||||||
| Buchan et al. [23] | Case- control ^ | Canada | 134,435 | ≥18 | Viral whole genome or S-gene sequencing | NR | NR | BNT162b2, mRNA-1273 | Symptomatic infection and severe infection | 1-OR |
| Gray et al. [24] | Case- control ^ | South Africa | 52,468 | ≥18 | Omicron period | N/A | 0–13 days group 8 (5–11) days 14–27 days group 20 (17–24) days 1–2 months group 32 (29–34) days | Ad26. COV2 | Positive COVID-19 and severe infection | 1-OR |
| Accorsi et al. [25] | Case- control ^ | United States | 70,155 | ≥18 | Viral ORFlab, S, and N gene sequencing | 8.0 (1.0) months | 1.0 (1.0) month | BNT162b2, mRNA-1273 | Symptomatic infection | 1-OR |
| Andrews et al. [26] | Case-control ^ | United Kingdom | 2,663,549 | ≥18 | Viral whole genome and S-gene sequencing | NR | 39 (range, 14–118) | ChAdOx1, BNT162b2, mRNA-1273 | Symptomatic infection | 1-OR |
| Chemaitelly et al. [27] | Case- control ^ | Qatar | 133,327 | No ≥ restriction | Viral whole genome sequencing | NR | NR | BNT162b2, mRNA-1273 | Symptomatic infection | 1-OR |
| Collie et al. [28] | Case- control ^ | South Africa | 211,610 | ≥18 | Viral S-gene sequencing | NR | NR | BNT162b2 | Symptomatic infection and severe infection | 1-OR |
| Ferdinand et al. [29] | Case- control ^ | United States | 93,408 | ≥18 | Omicron- predominance period | 214 (164–259) | 49 (30–73) | any mRNA vaccines *** | Symptomatic infection | 1-OR |
| Klein et al. [30] | Case- control ^ | United States | 39,217 | ≥18 | Omicron- predominance period | 5 to 11 y.: 14–67 12–15 y.: NR 16–17 y.: NR | NR | BNT162b2 | Symptomatic infection | 1-OR |
| Lauring et al. [31] | Case- control ^ | United States | 11,690 | ≥18 | Viral whole genome sequencing | NR | 69.5 (41.5–97) | any mRNA vaccines *** | Severe infection | 1-OR |
| Natarajan et al. [32] | Case- control ^ | United States | 80,287 | ≥18 | Omicron- predominance period | Ad26. COV2.S: 52 (33–71) any mRNA: 48 (32–71) | Median (IQR) 59 (38–79) | any mRNA vaccines *** | Symptomatic infection and Hospitalization | 1-OR |
| Tartof et al. [33] | Case- control ^ | United States | 14,137 | ≥18 | Viral whole genome and S-gene sequencing | NR | NR | BNT162b2 | Symptomatic infection and severe infection | 1-OR |
| Tenforde et al. [34] | Case- control ^ | United States | 7544 | ≥18 | Omicron- predominance period | 256 | 60 | any mRNA vaccines *** | Severe infection # | 1-OR |
| Thompson et al. [35] | Case- control ^ | United States | 222,772 | ≥18 | Omicron- predominant period | <180 days group: 137 ≥180 days group: 223 | Median interval: 41–44 | any mRNA vaccines *** | Positive COVID-19 and severe infection | 1-OR |
| Tseng et al. [36] | Case- control ^ | United States | 136,345 | ≥18 | Viral whole genome and S-gene sequencing | 14–365 days | NR | mRNA-1273 | Positive COVID-19 and severe infection | 1-OR |
| Young-Xu et al. [37] | Case- control ^ | United States | 69,215 | ≥18 | Omicron- predominant period | NR | NR | any mRNA vaccines *** | Positive COVID-19 | 1-OR |
| Zambrano et al. [38] | Case- control ^ | United States | 283 | 12 to 18 | Viral genome sequencing | MIS-C: 63 (48–89) | N/A | BNT162b2 | Severe infection & | 1-OR |
| Abu-Raddad et al. [39] | Retrospective Cohort | Qatar | 2,239,193 | No restriction | Viral genome sequencing | 21 (11–38) | 22 (12–38) | BNT162b2, mRNA-1273 | Symptomatic infection and severe infection | 1-HR |
| Hansen et al. [40] | Retrospective Cohort | Denmark | 5767 | 12 to 60 | Sequencing of viral whole genome or a novel variant specific targeting the 452L mutation | 1–150 | 1–30 | BNT162b2, mRNA-1273 | Positive COVID-19 | 1-HR |
| Monge et al. [41] | Retrospective Cohort | Spain | 6,222,318 | ≥40 | Omicron- predominant period | 0–34 | 0–34 | ChAdOx1-S, Ad26. COV2.S, mRNA-1273, BNT162b2 | Positive COVID-19 | 1-RR |
| Fowlkes et al. [42] | Prospective cohort | United States | 1364 | 5 to 18 | Viral genome sequencing | 5 to 11 y.: 14–82 12 to 15 y.: NR | N/A | BNT162b2 | Any infection | 1-HR |
| Vaccine | Full Vaccination Dose | Booster Vaccination Dose | ||||
|---|---|---|---|---|---|---|
| n | VE Estimate (%) (95%CI) | VE Reduction § (% per Month) (95%CI) | n | VE Estimate (%) (95%CI) | VE Reduction § (% per Month) (95%CI) | |
| Any infection | ||||||
| Overall Results | 89 | 49.45 (38.01 to 60.88) | −2.45 (−4.26 to −0.63) | 52 | 56.78 (47.11 to 66.45) | 1.79 (−1.25 to 4.82) |
| Any mRNA | 23 | 54.96 (30.63 to 79.29) | −0.93 (−4.64 to 2.78) | 15 | 76.81 (59.99 to 93.63) | 0.05 (−3.79 to 3.89) |
| Ad26.COV2.S | 2 | 26.38 (−15.77 to 68.54) | N/A | 8 | 48.66 (0.31 to 97) | 0.58 (−19.47 to 20.62) |
| BNT162b2 | 39 | 52.15 (33.84 to 70.47) | −3.36 (−6.19 to −0.53) | 17 | 53.04 (34.27 to 71.81) | 1.82 (−4.61 to 8.24) |
| mRNA-1273 | 19 | 47.8 (25.48 to 70.12) | −2.98 (−6.44 to 0.47) | 9 | 57.12 (30.57 to 83.68) | −0.88 (−15.27 to 13.5) |
| ChAdOx1 nCov-19 | 6 | 56.03 (47.81 to 64.25) | −8.53 (−9.98 to −7.09) | 3 | 61.12 (25.33 to 96.91) | −5.87 (−27.76 to 16.02) |
| Symptomatic infection | ||||||
| Overall Results | 53 | 56.36 (47.21 to 65.51) | −5.5 (−7.01 to −3.99) | 32 | 53.58 (44.18 to 62.98) | 1.14 (−1.99 to 4.26) |
| Any mRNA | 11 | 51.69 (22.71 to 80.68) | −3.3 (−7.86 to 1.25) | 7 | 63.93 (30.38 to 97.47) | 0.89 (−5.97 to 7.74) |
| Ad26.COV2.S | 1 | 24 (18.5 to 29.5) | N/A | 1 | 54 (44 to 64) | N/A |
| BNT162b2 | 26 | 58.24 (45.84 to 70.64) | −5.8 (−7.69 to −3.91) | 14 | 55.65 (39.04 to 72.25) | −1.68 (−8 to 4.65) |
| mRNA-1273 | 9 | 79.61 (68.83 to 90.39) | −10.76 (−12.91 to −8.6) | 7 | 52.88 (8.61 to 97.15) | 0.91 (−37.41 to 39.23) |
| ChAdOx1 nCov-19 | 6 | 56.03 (47.81 to 64.25) | −8.53 (−9.98 to −7.09) | 3 | 61.12 (25.33 to 96.91) | −5.87 (−27.76 to 16.02) |
| Severe infection | ||||||
| Overall Results | 21 | 64.81 (49.9 to 79.73) | 0.59 (−1.49 to 2.67) | 13 | 92.53 (85.54 to 99.52) | −1.27 (−3.07 to 0.53) |
| Any mRNA | 12 | 64.3 (46.19 to 82.42) | 0.72 (−2 to 3.43) | 7 | 94.54 (90.94 to 98.14) | −1.24 (−2.11 to −0.38) |
| Ad26.COV2.S | 1 | 31 (21.5 to 40.5) | N/A | 4 | 81.74 (38.01 to 125.46) | −3.02 (−20.41 to 14.37) |
| BNT162b2 | 6 | 80.17 (55.61 to 104.73) | −0.58 (−3.72 to 2.56) | 2 | 89.05 (86.39 to 91.71) | N/A |
| mRNA-1273 | 2 | 66.86 (−1.23 to 134.94) | N/A | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratama, N.R.; Wafa, I.A.; Budi, D.S.; Sutanto, H.; Asmarawati, T.P.; Barlian Effendi, G.; Wungu, C.D.K. Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Omicron Variant (B.1.1.529): A Systematic Review with Meta-Analysis and Meta-Regression. Vaccines 2022, 10, 2180. https://doi.org/10.3390/vaccines10122180
Pratama NR, Wafa IA, Budi DS, Sutanto H, Asmarawati TP, Barlian Effendi G, Wungu CDK. Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Omicron Variant (B.1.1.529): A Systematic Review with Meta-Analysis and Meta-Regression. Vaccines. 2022; 10(12):2180. https://doi.org/10.3390/vaccines10122180
Chicago/Turabian StylePratama, Nando Reza, Ifan Ali Wafa, David Setyo Budi, Henry Sutanto, Tri Pudy Asmarawati, Gema Barlian Effendi, and Citrawati Dyah Kencono Wungu. 2022. "Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Omicron Variant (B.1.1.529): A Systematic Review with Meta-Analysis and Meta-Regression" Vaccines 10, no. 12: 2180. https://doi.org/10.3390/vaccines10122180
APA StylePratama, N. R., Wafa, I. A., Budi, D. S., Sutanto, H., Asmarawati, T. P., Barlian Effendi, G., & Wungu, C. D. K. (2022). Effectiveness of COVID-19 Vaccines against SARS-CoV-2 Omicron Variant (B.1.1.529): A Systematic Review with Meta-Analysis and Meta-Regression. Vaccines, 10(12), 2180. https://doi.org/10.3390/vaccines10122180







