Ag85a-S2 Activates cGAS-STING Signaling Pathway in Intestinal Mucosal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ag85a-S2, Ag85a, and S2
2.2. Cell Culture
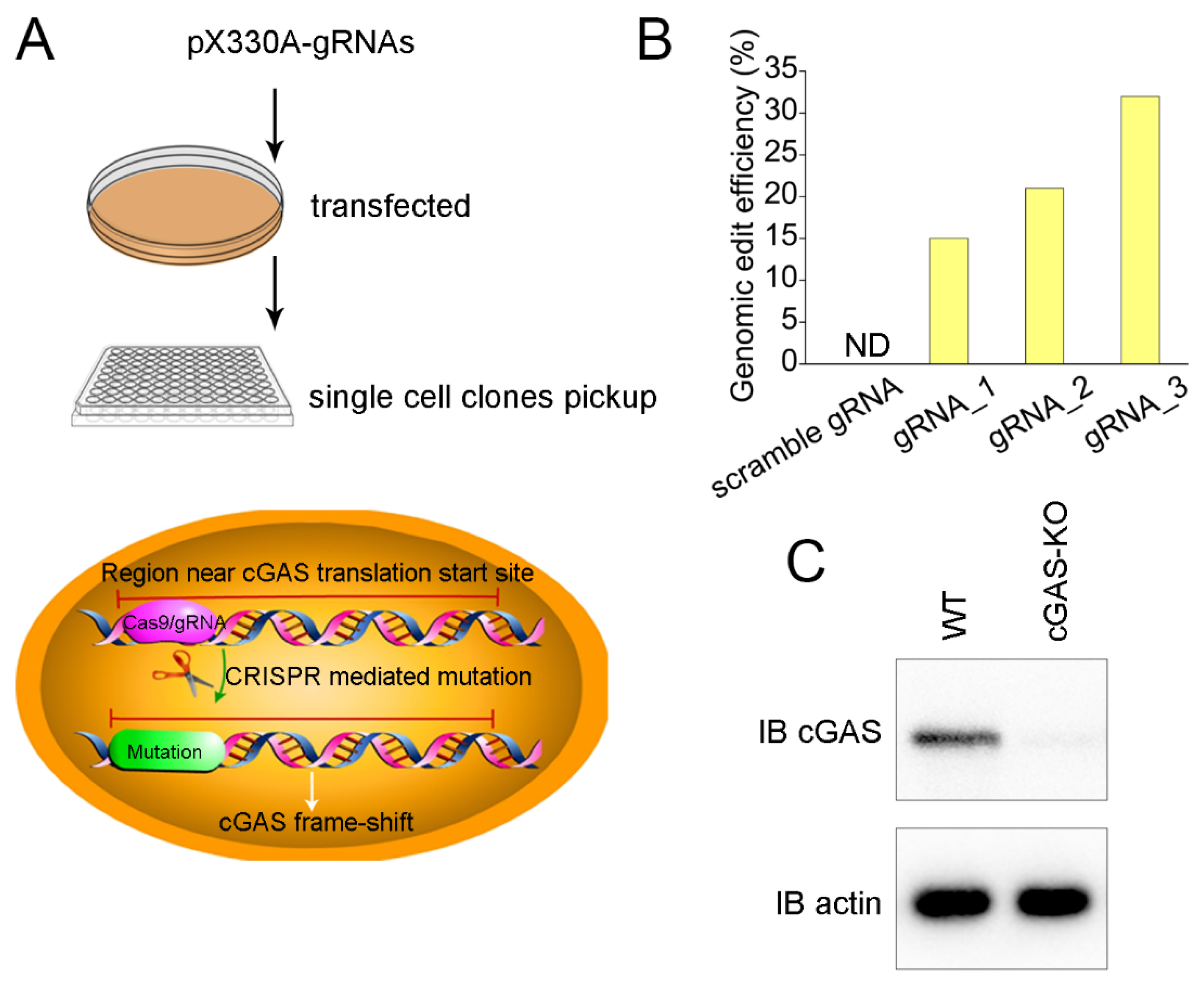
2.3. cGAS Knockout Cell Line Construction
2.4. In Vitro Stimulation
2.5. Animal Immunization
2.6. siRNA Transfection
2.7. qPCR Assay
2.8. Western Blotting Assay
2.9. ELISA Assay
2.10. RNA Sequencing and Bioinformatics Analysis
2.11. Statistical Analysis
3. Results
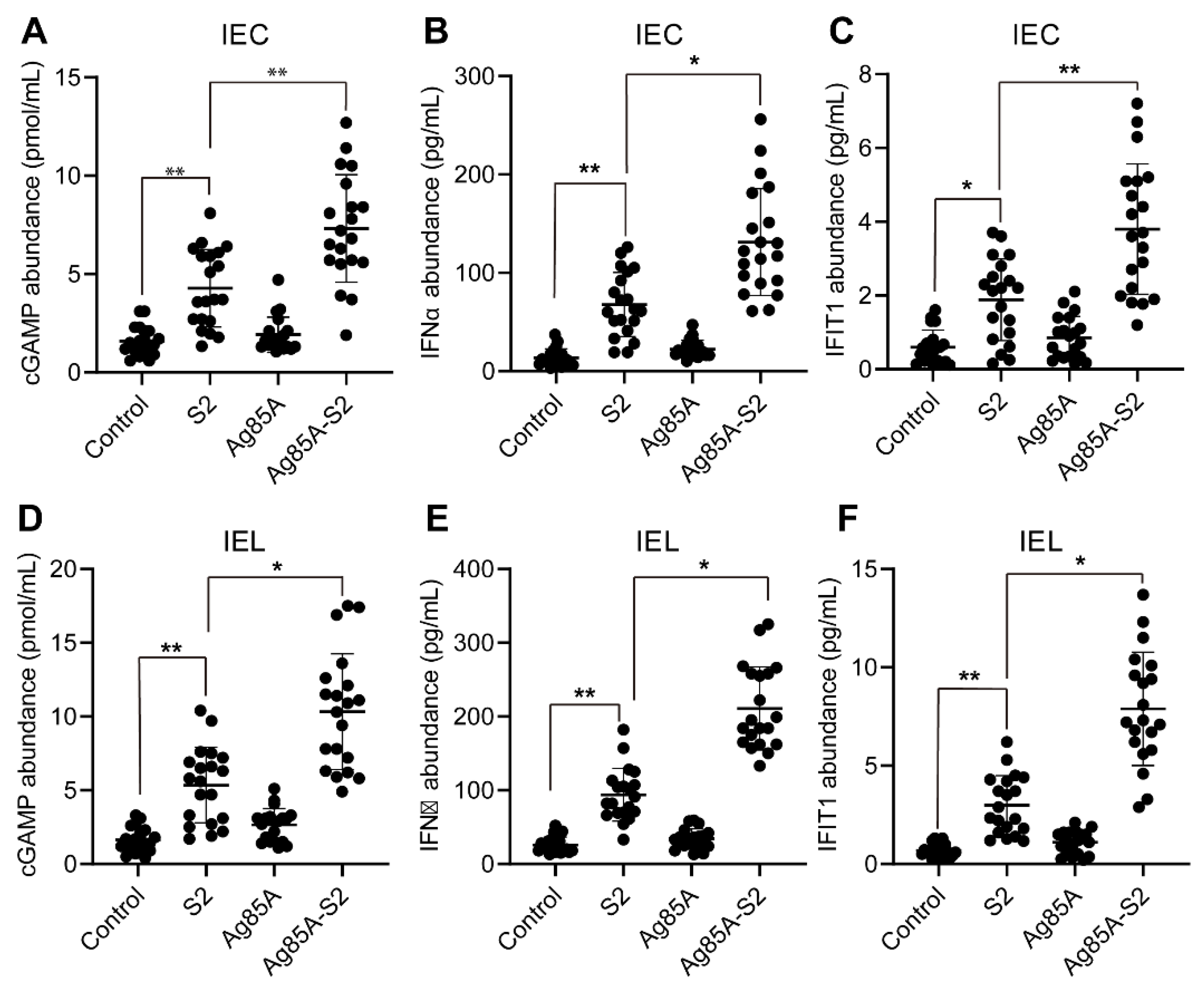
3.1. Ag85a-S2 Upregulates the Key Components in the cGAS-STING Cascade
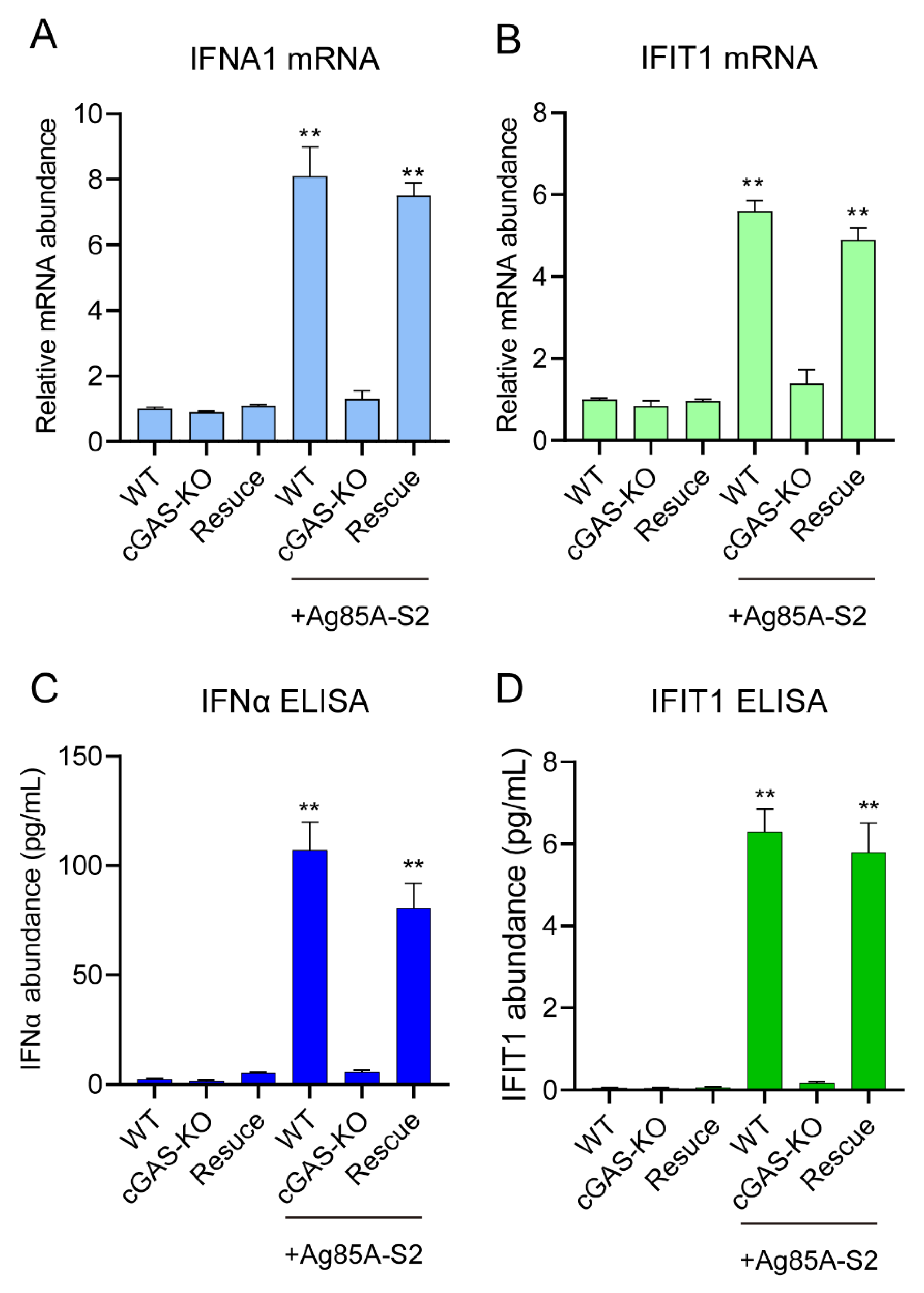
3.2. Generation of cGAS Knockout Cell Line
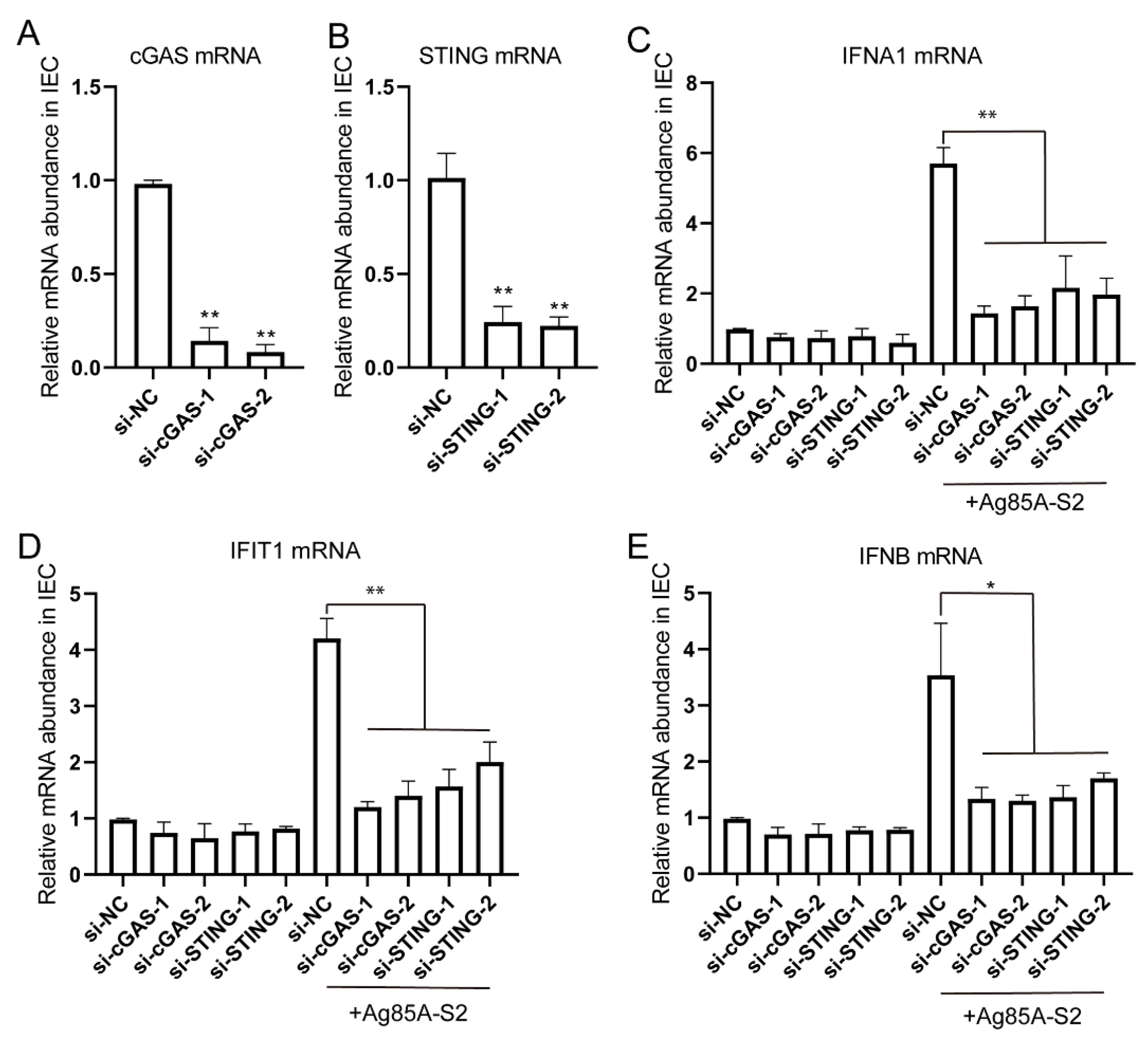
3.3. Depletion of cGAS-STING Signaling Impairs Immune Cytokines Secretion
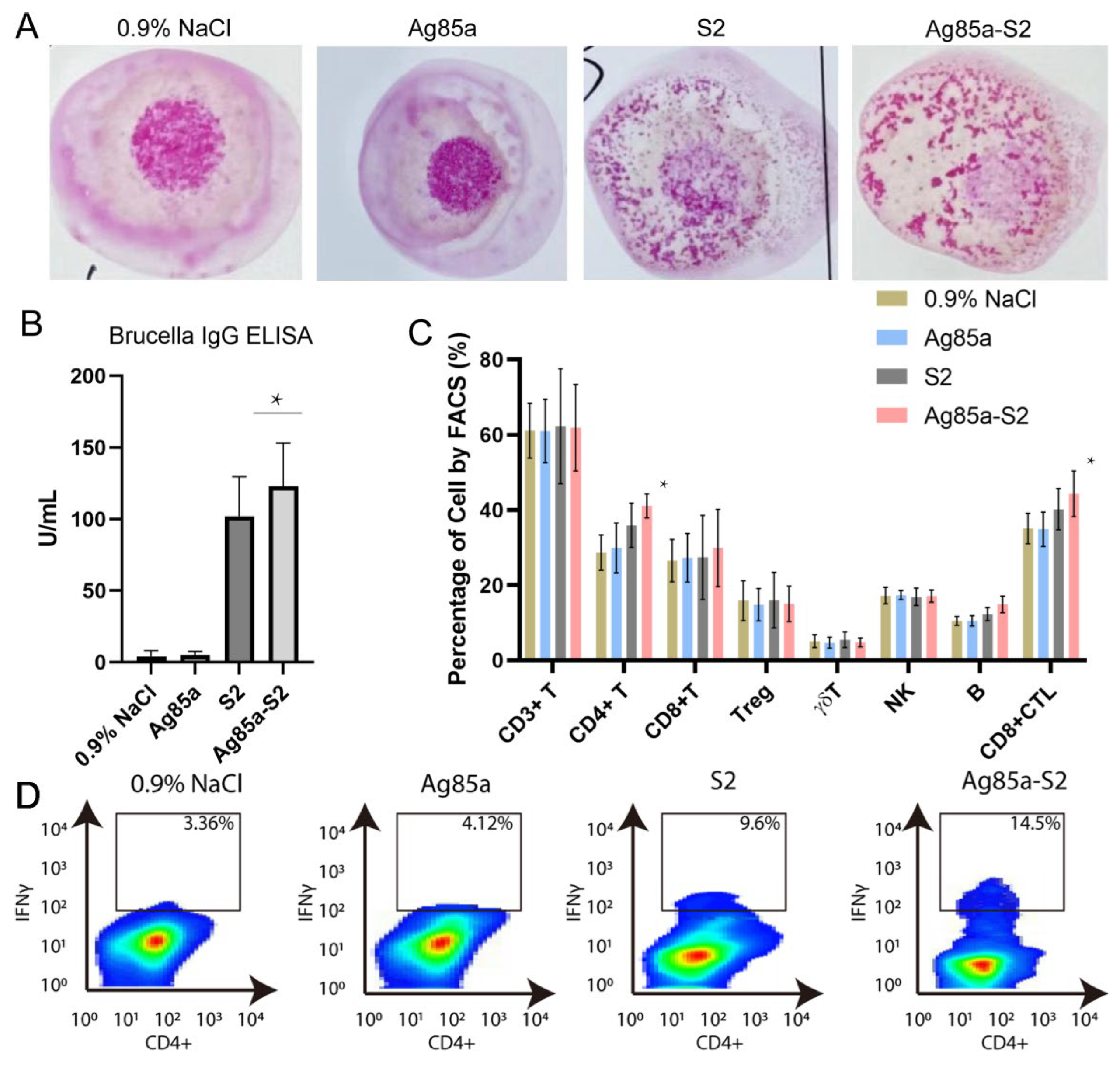
3.4. Ag85a-S2 Triggers Cellular Immune Response
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figueiredo, P.D.; Ficht, T.A.; Ficht, A.R.; Rossetti, C.A.; Adams, L.G. Pathogenesis and Immunobiology of Brucellosis Review of Brucella-Host Interactions. Am. J. Pathol. 2015, 185, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Gong, Q.L.; Feng, H.F.; Wang, Q.; Shi, J.F.; Song, Y.H.; Liu, F.; Shi, K.; Zong, Y.; Du, R. Brucellosis prevalence in yaks in China in 1980–2019: A systematic review and meta-analysis. Prev. Vet. Med. 2022, 198, 105532. [Google Scholar] [CrossRef] [PubMed]
- Dek, R.P.; Magnusson, U.; Grace, D. Bovine brucellosis: Prevalence, risk factors, economic cost and control options with particular reference to India—A review. Infect. Ecol. Epidemiol. 2018, 8, 1556548. [Google Scholar] [CrossRef]
- Liang, D.Y.; Liu, D.; Yang, M.; Wang, X.M.; Li, Y.P.; Guo, W.D.; Du, M.L.; Wang, W.R.; Xue, M.M.; Wu, J.; et al. Spatiotemporal distribution of human brucellosis in Inner Mongolia, China, in 2010–2015, and influencing factors. Sci. Rep. 2021, 11, 24213. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.; De Oliveira, F.S.; Macedo, G.C.; De Almeida, L.A.; Carvalho, N.B. The role of innate immune receptors in the control of Brucella abortus infection: Toll-like receptors and beyond. Microbes Infect. 2008, 10, 1005–1009. [Google Scholar] [CrossRef]
- Campos, P.C.; Gomes, M.T.R.; Guimaraes, E.S.; Guimaraes, G.; Oliveira, S.C. TLR7 and TLR3 Sense Brucella abortus RNA to Induce Proinflammatory Cytokine Production but They Are Dispensable for Host Control of Infection. Front. Immunol. 2017, 8, 28. [Google Scholar] [CrossRef]
- Cannella, A.P.; Tsolis, R.M.; Liang, L.; Felgner, P.L.; Saito, M.; Sette, A.; Gotuzzo, E.; Vinetz, J.M. Antigen-specific acquired immunity in human brucellosis: Implications for diagnosis, prognosis, and vaccine development. Front. Cell. Infect. Microbiol. 2012, 2, 1. [Google Scholar] [CrossRef]
- Corner, L.A.; Alton, G.G. Persistence of Brucella-Abortus Strain-19 Infection in Adult Cattle Vaccinated with Reduced Doses. Res. Vet. Sci. 1981, 31, 342–344. [Google Scholar] [CrossRef]
- Vemullpalli, R.; He, Y.Q.; Boyle, S.M.; Sriranganathan, N.; Schurig, G.G. Brucella abortus strain RB51 as a vector for heterologous protein expression and induction of specific Th1 type immune responses. Infect. Immun. 2000, 68, 3290–3296. [Google Scholar] [CrossRef]
- Bardenstein, S.; Mandelboim, M.; Ficht, T.A.; Baum, M.; Banai, M. Identification of the Brucella melitensis vaccine strain Rev.1 in animals and humans in Israel by PCR analysis of the PstI site polymorphism of its omp2 gene. J. Clin. Microbiol. 2002, 40, 1475–1480. [Google Scholar] [CrossRef]
- Cloeckaert, A.; Grayon, M.; Grepinet, O. Identification of Brucella melitensis vaccine strain Rev.1 by PCR-RFLP based on a mutation in the rpsL gene. Vaccine 2002, 20, 2546–2550. [Google Scholar] [CrossRef]
- Samartino, L.E.; Enright, F.M. Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 1993, 2, 95–101. [Google Scholar] [CrossRef]
- Xin, X. Orally administrable brucellosis vaccine: Brucella suis strain 2 vaccine. Vaccine 1986, 4, 212–216. [Google Scholar] [CrossRef]
- Zhu, L.Q.; Feng, Y.; Zhang, G.; Jiang, H.; Zhang, Z.; Wang, N.; Ding, J.B.; Suo, X. Brucella suis strain 2 vaccine is safe and protective against heterologous Brucella spp. infections. Vaccine 2016, 34, 395–400. [Google Scholar] [CrossRef]
- Bosseray, N.; Plommet, M. Brucella suis S2, Brucella melitensis Rev1 and Brucella abortus S19 living vaccines: Residual virulence and immunity induced against three Brucella species challenge strains in mice. Vaccine 1990, 8, 462–468. [Google Scholar] [CrossRef]
- Elamin, A.A.; Stehr, M.; Spallek, R.; Rohde, M.; Singh, M. The Mycobacterium tuberculosis Ag85a is a novel diacylglycerol acyltransferase involved in lipid body formation. Mol. Microbiol. 2011, 81, 1577–1592. [Google Scholar] [CrossRef]
- D’souza, S.; Rosseels, V.; Romano, M.; Tanghe, A.; Denis, O.; Jurion, F.; Castiglione, N.; Vanonckelen, A.; Palfliet, K.; Huygen, K. Mapping of murine Th1 helper T-Cell epitopes of mycolyl transferases Ag85a, Ag85B, and Ag85C from Mycobacterium tuberculosis. Infect. Immun. 2003, 71, 483–493. [Google Scholar] [CrossRef]
- Wang, D.; Xu, J.; Feng, Y.; Liu, Y.; Mchenga, S.S.; Shan, F.; Sasaki, J.; Lu, C. Liposomal oral DNA vaccine (mycobacterium DNA) elicits immune response. Vaccine 2010, 28, 3134–3142. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Chen, X.; Hu, T.; Meng, C.; Wang, X.B.; Rao, Y.; Zhang, X.M.; Yin, Y.L.; Pan, Z.M.; Jiao, X.A. Evaluation of Immunogenicity and Protective Efficacy Elicited by Mycobacterium bovis BCG Overexpressing Ag85a Protein against Mycobacterium tuberculosis Aerosol Infection. Front. Cell Infect. Microbiol. 2016, 6, 3. [Google Scholar] [CrossRef]
- Zhai, J.B.; Gao, W.; Zhao, L.H.; Gao, Z.P.; Jiang, X.F.; Lu, C.L. Dendritic cell vaccine with Ag85a enhances anti-colorectal carcinoma immunity. Exp. Ther. Med. 2018, 16, 5123–5129. [Google Scholar] [CrossRef]
- Zhai, J.B.; Gao, W.; Zhao, L.H.; Lu, C.L. Integrated transcriptomic and quantitative proteomic analysis identifies potential RNA sensors that respond to the Ag85a DNA vaccine. Microb. Pathog. 2020, 149, 104487. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.B.; Wang, Q.B.; Gao, Y.F.; Zhang, R.; Li, S.J.; Wei, B.; You, Y.; Sun, X.; Lu, C.L. The mechanisms of Ag85a DNA vaccine activates RNA sensors through new signal transduction. Int. Immunopharmacol. 2018, 59, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, J.; Du, F.; Chen, X.; Chen, Z.J. Cyclic GMP-AMP Synthase Is a Cytosolic DNA Sensor That Activates the Type I Interferon Pathway. Science 2012, 339, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Ablasser, A.; Schmid-Burgk, J.L.; Hemmerling, I.; Horvath, G.L.; Schmidt, T.; Latz, E.; Hornung, V. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature 2013, 503, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, L.; Chen, X.; Du, F.; Shi, H.; Chen, C.; Chen, Z.J. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science 2013, 339, 826–830. [Google Scholar] [CrossRef]
- Zhang, C.; Shang, G.; Gui, X.; Zhang, X.; Bai, X.C.; Chen, Z.J. Structural basis of STING binding with and phosphorylation by TBK1. Nature 2019, 567, 394–398. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Hornung, V. Molecular mechanisms and cellular functions of cGAS-STING signalling. Nat. Rev. Mol. Cell Biol. 2020, 21, 501–521. [Google Scholar] [CrossRef]
- Tan, X.; Sun, L.; Chen, J.; Chen, Z.J. Detection of Microbial Infections through Innate Immune Sensing of Nucleic Acids. Annu. Rev. Microbiol. 2018, 72, 447–478. [Google Scholar] [CrossRef]
- Franco, M.M.C.; Marim, F.; Guimarães, E.S.; Assis, N.R.G.; Cerqueira, D.M.; Alves-Silva, J.; Harms, J.; Splitter, G.; Smith, J.; Kanneganti, T.-D.; et al. S.C. Brucella abortus Triggers a cGAS-Independent STING Pathway to Induce Host Protection That Involves Guanylate-Binding Proteins and Inflammasome Activation. J. Immunol. 2018, 200, 607–622. [Google Scholar] [CrossRef]
- Li, R.; Liu, W.; Yin, X.; Zheng, F.; Wang, Z.; Zhang, X.; Du, Q.; Huang, Y.; Tong, D. Brucella spp. Omp25 Promotes Proteasome-Mediated cGAS Degradation to Attenuate IFN-β Production. Front. Microbiol. 2021, 12, 702881. [Google Scholar] [CrossRef]
- Comerci, D.J.; Pollevick, G.D.; Vigliocco, A.M.; Frasch, A.C.C.; Ugalde, R.A. Vector development for the expression of foreign proteins in the vaccine strain Brucella abortus S19. Infect. Immun. 1998, 66, 3862–3866. [Google Scholar] [CrossRef]
- Sheng, D.; Xin, S.; Dongxing, W.; Shubo, W.; Yang, S.; Zeliang, C.; Jingbo, Z. Oral S2-Ag85 DNA Vaccine Activated Intestinal Cell dsDNA and RNA Sensors to Promote the Presentation of Intestinal Antigen. J. Immunol. Res. 2022, 13, 7200379. [Google Scholar] [CrossRef]
- Toda, G.; Yamauchi, T.; Kadowaki, T.; Ueki, K. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protoc. 2021, 2, 100246. [Google Scholar] [CrossRef]
- Bonam, S.R.; Camille, C.; Mathew, M.J.; Bayry, J. IFN-Induces PD-L1 Expression in Primed Human Basophils. Cells 2022, 11, 801. [Google Scholar] [CrossRef]
- Barquero-Calvo, E.; Chaves-Olarte, E.; Weiss, D.S.; Guzman-Verri, C.; Chacon-Diaz, C.; Rucavado, A.; Moriyon, I.; Moreno, E. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2007, 2, e631. [Google Scholar] [CrossRef]
- Kohler, S.; Foulongne, V.; Ouahrani-Bettache, S.; Bourg, G.; Teyssier, J.; Ramuz, M.; Liautard, J.P. The analysis of the intramacrophagic virulome of Brucella suis deciphers the environment encountered by the pathogen inside the macrophage host cell. Proc Natl. Acad. Sci. USA 2002, 99, 15711–15716. [Google Scholar] [CrossRef]
- Li, W.; Ke, Y.; Wang, Y.; Yang, M.; Gao, J.; Zhan, S.; Xinying, D.; Huang, L.; Li, W.; Chen, Z. Brucella TIR-like protein TcpB/Btp1 specifically targets the host adaptor protein MAL/TIRAP to promote infection. Biochem. Biophys. Res. Commun. 2016, 477, 509–514. [Google Scholar] [CrossRef]
- Carroll, E.C.; Jin, L.; Mori, A.; Munoz-Wolf, N.; Oleszycka, E.; Moran, H.B.T.; Mansouri, S.; Mcentee, C.P.; Lambe, E.; Agger, E.M.; et al. The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons. Immunity 2016, 44, 597–608. [Google Scholar] [CrossRef]
- Herck, S.V.; Feng, B.; Tang, L. Delivery of STING agonists for adjuvanting subunit vaccines. Adv. Drug Deliv. Rev. 2021, 179, 114020. [Google Scholar] [CrossRef]
- Canesso, M.C.C.; Lemos, L.; Neves, T.C.; Marim, F.M.; Castro, T.B.R.; Veloso, E.S.; Queiroz, C.P.; Ahn, J.; Santiago, H.C.; Martins, F.S.; et al. The cytosolic sensor STING is required for intestinal homeostasis and control of inflammation. Mucosal. Immunology 2018, 11, 820–834. [Google Scholar]
- Hu, Q.; Ren, H.; Li, G.; Wang, D.; Zhou, Q.; Wu, J.; Zheng, J.; Huang, J.; Slade, D.A.; Wu, X.; et al. STING-mediated intestinal barrier dysfunction contributes to lethal sepsis. EBioMedicine 2019, 41, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Wottawa, F.; Bordoni, D.; Baran, N.; Rosenstiel, P.; Aden, K. The role of cGAS/STING in intestinal immunity. Eur. J. Immunol. 2021, 51, 785–797. [Google Scholar] [CrossRef] [PubMed]
- Babaki, M.K.Z.; Soleimanpour, S.; Rezaee, S.A. Antigen 85 complex as a powerful Mycobacterium tuberculosis immunogene: Biology, immune-pathogenicity, applications in diagnosis, and vaccine design. Microb. Pathog. 2017, 112, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, M.; Khairani-Bejo, S.; Hamzah, H.; Nasruddin, N.S.; Zamri-Saad, A.S.M. Pathological changes, distribution and detection of Brucella melitensis in foetuses of experimentally-infected does. Vet. Q. 2021, 41, 36–49. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, S.; Li, W.; Wen, S.; Song, Y.; Bai, M.; Li, S.; Chen, Z.; Zhai, J. Ag85a-S2 Activates cGAS-STING Signaling Pathway in Intestinal Mucosal Cells. Vaccines 2022, 10, 2170. https://doi.org/10.3390/vaccines10122170
Dang S, Li W, Wen S, Song Y, Bai M, Li S, Chen Z, Zhai J. Ag85a-S2 Activates cGAS-STING Signaling Pathway in Intestinal Mucosal Cells. Vaccines. 2022; 10(12):2170. https://doi.org/10.3390/vaccines10122170
Chicago/Turabian StyleDang, Sheng, Wanyang Li, Shubo Wen, Yang Song, Meirong Bai, Shuyan Li, Zeliang Chen, and Jingbo Zhai. 2022. "Ag85a-S2 Activates cGAS-STING Signaling Pathway in Intestinal Mucosal Cells" Vaccines 10, no. 12: 2170. https://doi.org/10.3390/vaccines10122170
APA StyleDang, S., Li, W., Wen, S., Song, Y., Bai, M., Li, S., Chen, Z., & Zhai, J. (2022). Ag85a-S2 Activates cGAS-STING Signaling Pathway in Intestinal Mucosal Cells. Vaccines, 10(12), 2170. https://doi.org/10.3390/vaccines10122170







