Correlation between Baseline 25(OH) Vitamin D Levels and Both Humoral Immunity and Breakthrough Infection Post-COVID-19 Vaccination
Abstract
1. Introduction
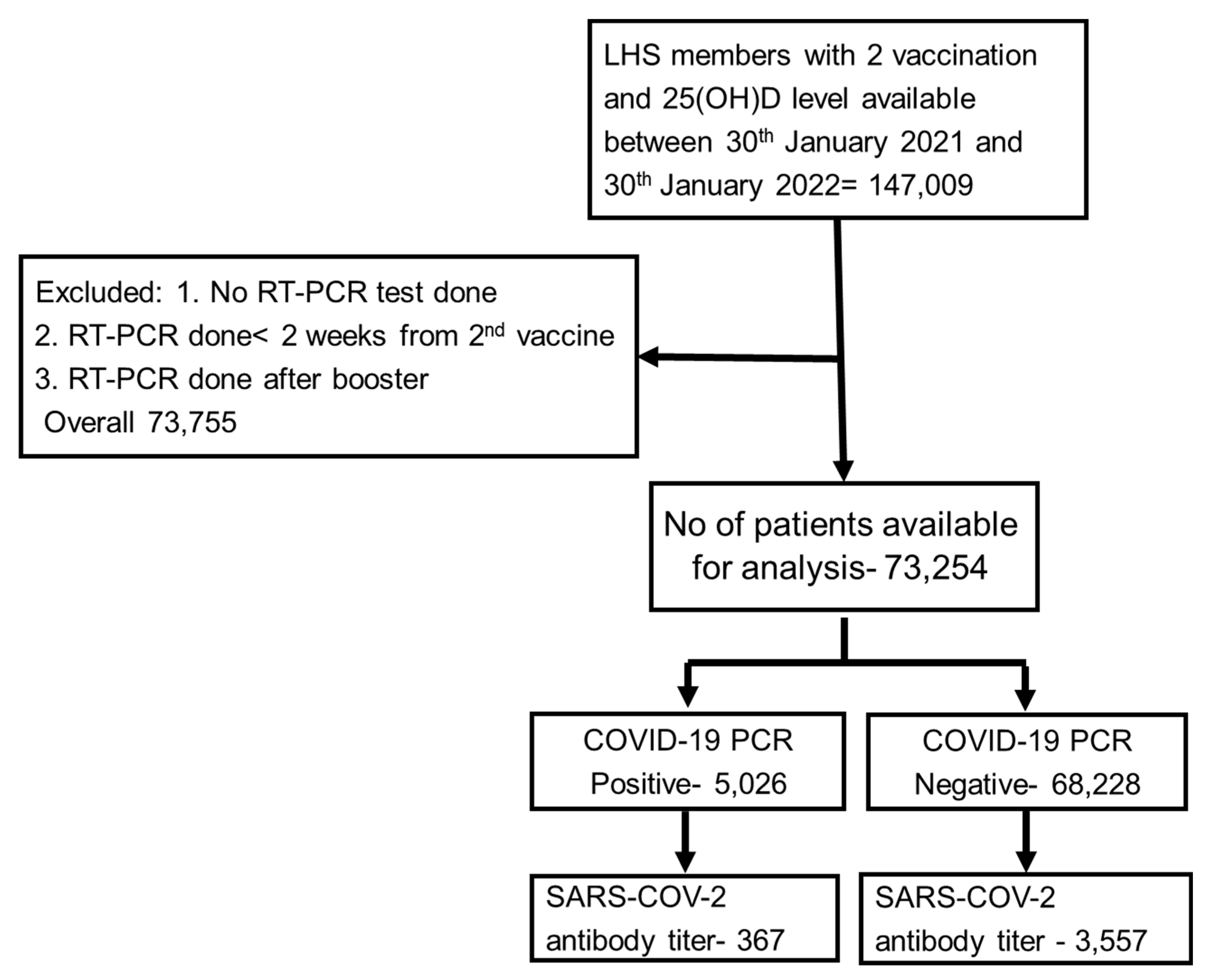
2. Methods and Patients
3. Statistical Analysis
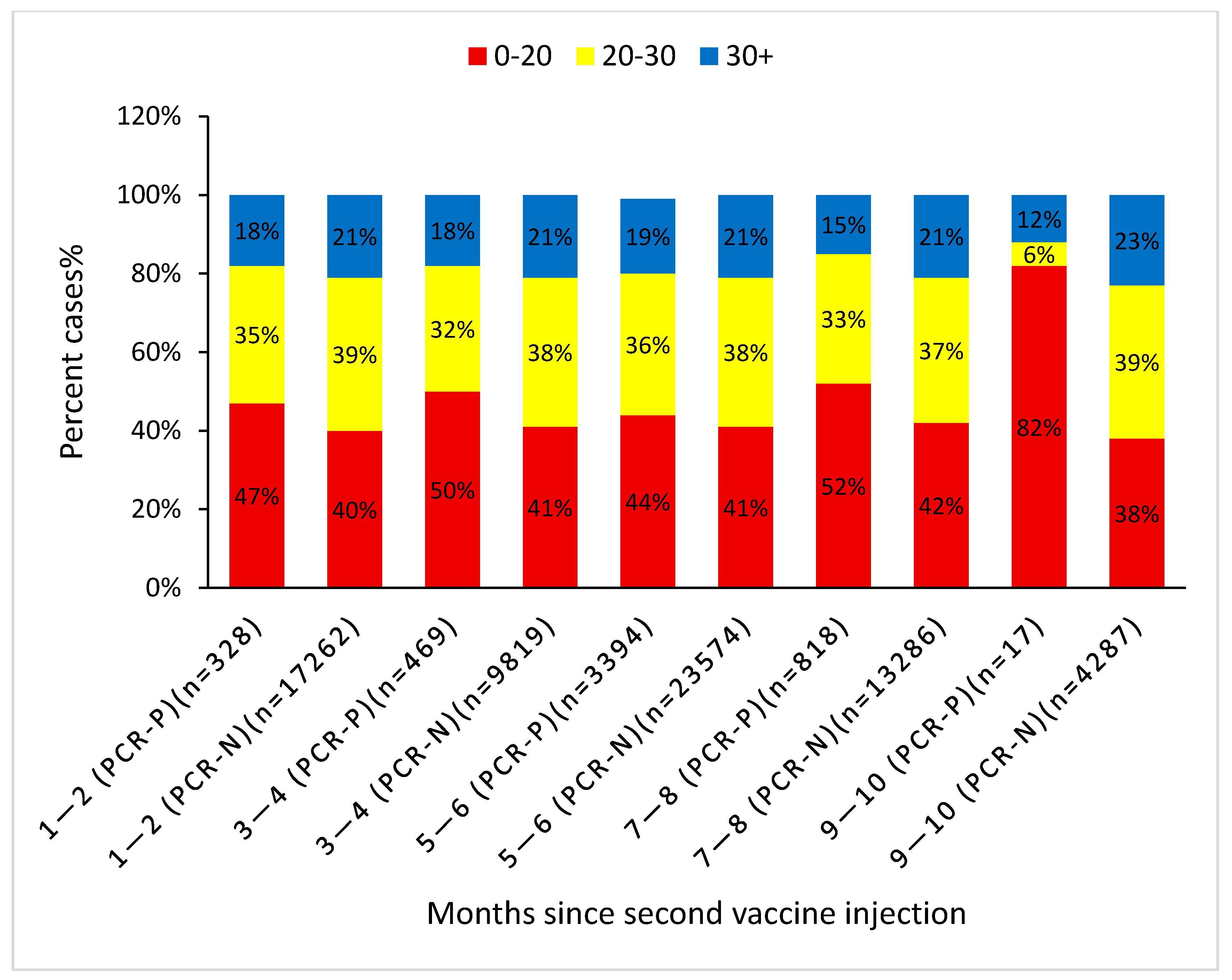
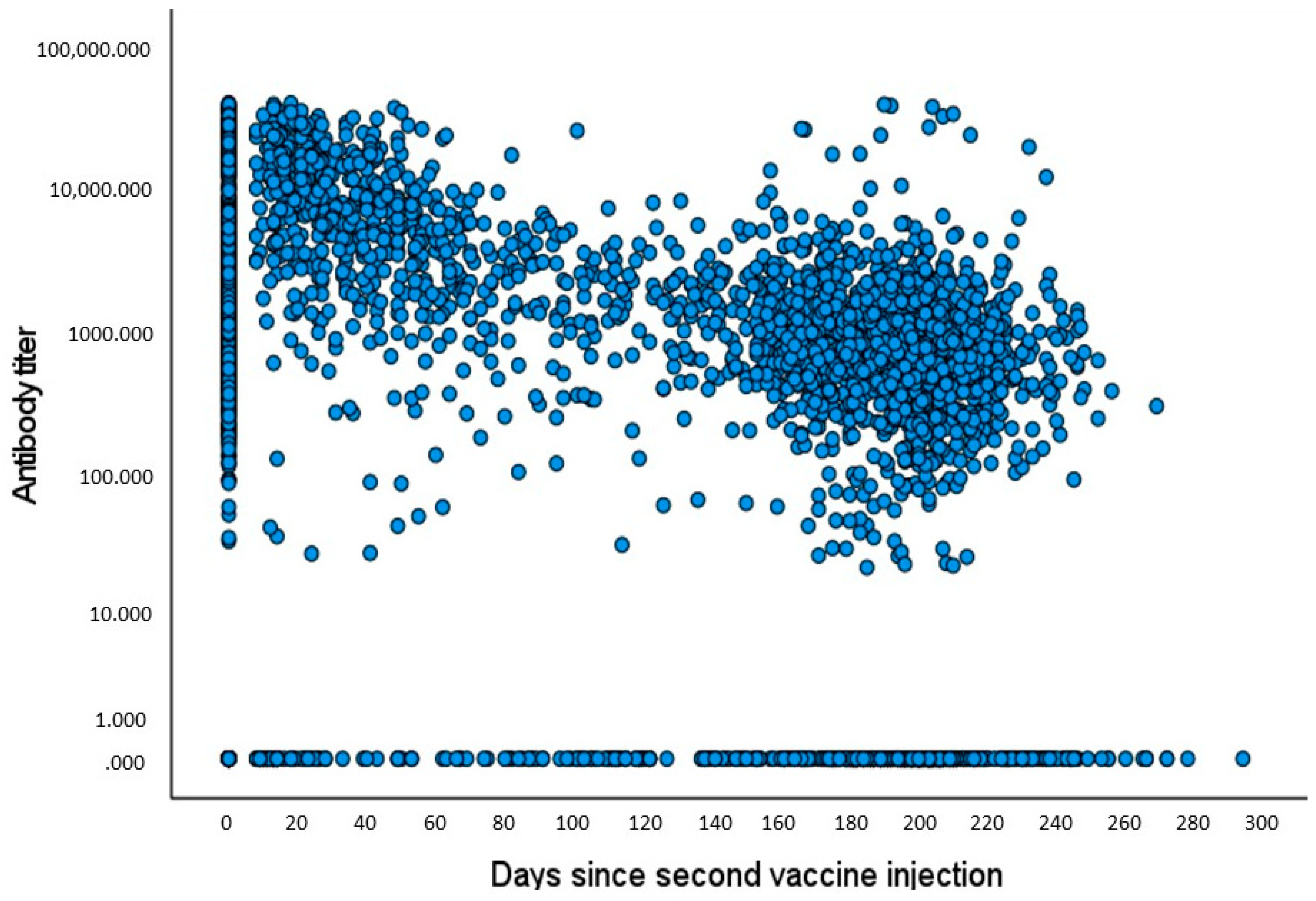
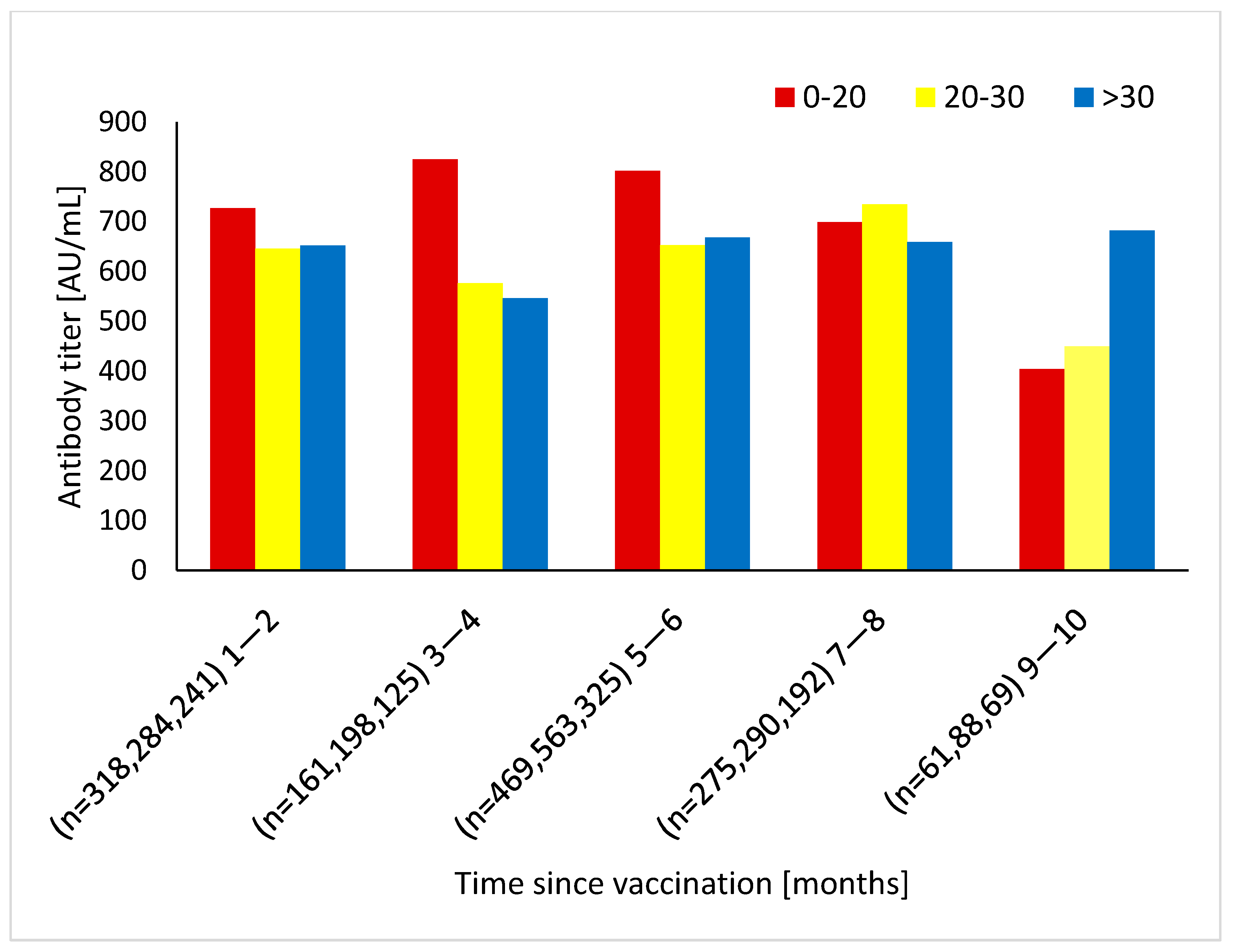
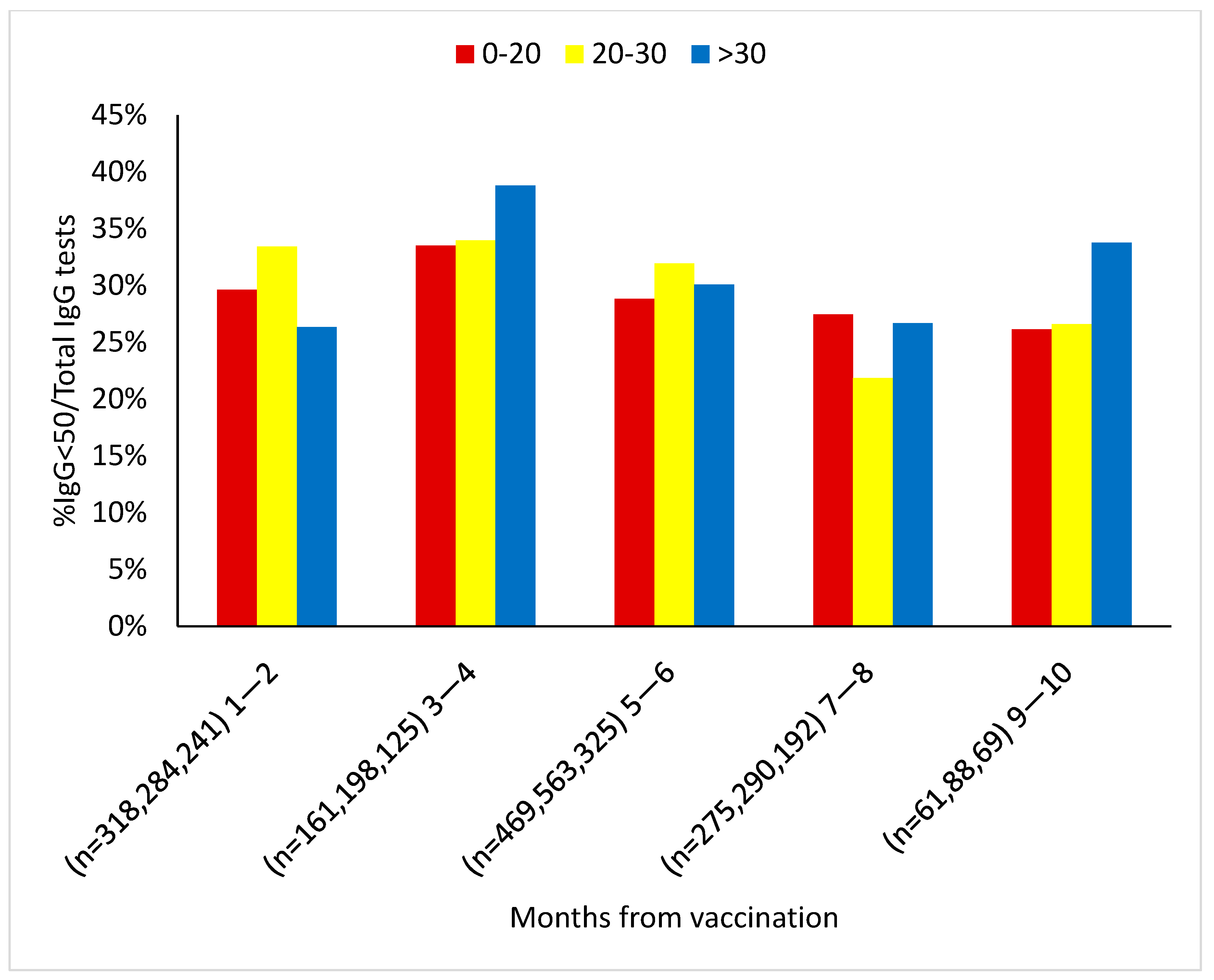
4. Results
Timing of COVID-19 Infection and the Effect of Baseline Vitamin D Level
5. Discussion
6. Study Limitation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, 6529. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-Scale Study of Antibody Titer Decay following BNT162b2 mRNA Vaccine or SARS-CoV-2 Infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Liu, M.; Wang, A.; Lu, L.; Wang, Q.; Gu, C.; Chen, J.; Wu, Y.; Xia, S.; Ling, Y.; et al. Evaluating the Association of Clinical Characteristics with Neutralizing Antibody Levels in Patients Who Have Recovered from Mild COVID-19 in Shanghai, China. JAMA Intern. Med. 2020, 180, 1356–1362. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.J.A.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Long, Q.X.; Jia, Y.J.; Wang, X.; Deng, H.J.; Cao, X.X.; Yuan, J.; Fang, L.; Cheng, X.R.; Luo, C. Immune memory in convalescent patients with asymptomatic or mild COVID-19. Cell Discov. 2021, 7, 18. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, K.; Liu, X.; Zhuang, C.; Huang, X.; Huang, Y.; Yao, X.; Quan, J.; Lin, H.; Huang, S.; et al. The Protection of Naturally Acquired Antibodies Against Subsequent SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Emerg. Microbes Infect. 2022, 11, 793–803. [Google Scholar] [CrossRef]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef]
- Jude, E.B.; Ling, S.F.; Allcock, R.; Yeap, B.X.Y.; Pappachan, J.M. Vitamin D Deficiency Is Associated with Higher Hospitalization Risk From COVID-19: A Retrospective Case-control Study. J. Clin. Endocrinol. Metab. 2021, 106, e4708–e4715. [Google Scholar] [CrossRef] [PubMed]
- Bordelon, P.; Ghetu, M.V.; Langan, R.C. Recognition and management of vitamin D deficiency. Am. Fam. Physician 2009, 80, 841–846. [Google Scholar] [PubMed]
- Campi, I.; Gennari, L.; Merlotti, D.; Mingiano, C.; Frosali, A.; Giovanelli, L.; Torlasco, C.; Pengo, M.F.; Heilbron, F.; Soranna, D.; et al. Vitamin D and COVID-19 severity and related mortality: A prospective study in Italy. BMC Infect. Dis. 2021, 21, 566. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using Open SAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Grupel, D.; Gazit, S.; Schreiber, L.; Nadler, V.; Wolf, T.; Lazar, R.; Supino-Rosin, L.; Perez, G.; Peretz, A.; Ben Tov, A.; et al. Kinetics of SARS-CoV-2 anti-S IgG after BNT162b2 vaccination. Vaccine 2021, 39, 5337–5340. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Vieth, R. What is the optimal vitamin D status for health? Prog. Biophys. Mol. Biol. 2006, 92, 26–32. [Google Scholar] [CrossRef]
- Jaun, F.; Boesing, M.; Lüthi-Corridori, G.; Abig, K.; Makhdoomi, A.; Bloch, N.; Lins, C.; Raess, A.; Grillmayr, V.; Haas, P.; et al. High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebo-controlled, multi-center study-VitCov Trial. Trials 2022, 23, 114. [Google Scholar] [CrossRef]
- Gönen, M.S.; Alaylıoğlu, M.; Durcan, E.; Özdemir, Y.; Şahin, S.; Konukoğlu, D.; Nohut, O.K.; Ürkme, S.; Küçükece, B.; Balkan, İ.İ.; et al. Rapid and Effective Vitamin D Supplementation May Present Better Clinical Outcomes in COVID-19 (SARS-CoV-2) Patients by Altering Serum INOS1, IL1B, IFNg, Cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef]
- Murai, I.H.; Fernandes, A.L.; Sales, L.P.; Pinto, A.J.; Goessler, K.F.; Duran, C.S.C.; Silva, C.B.R.; Franco, A.S.; Macedo, M.B.; Dalmolin, H.H.H.; et al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients with Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Wang, W.M.; Kao, T.W.; Chiang, C.P.; Hung, C.T.; Chen, W.L. Inverse relationship between serum vitamin D level and measles antibody titer: A cross-sectional analysis of NHANES, 2001–2004. PLoS ONE 2018, 13, e0207798. [Google Scholar] [CrossRef] [PubMed]
- Chillon, T.S.; Demircan, K.; Heller, R.A.; Hirschbil-Bremer, I.M.; Diegmann, J.; Bachmann, M.; Moghaddam, A.; Schomburg, L. Relationship between Vitamin D Status and Antibody Response to COVID-19 mRNA Vaccination in Healthy Adults. Biomedicines 2021, 9, 1714. [Google Scholar] [CrossRef] [PubMed]
- Parthymou, A.; Habeos, E.E.; Habeos, G.I.; Deligakis, A.; Livieratos, E.; Marangos, M.; Chartoumpekis, D.V. Factors associated with anti-SARS-CoV-2 antibody titres 3 months post-vaccination with the second dose of BNT162b2 vaccine: A longitudinal observational cohort study in western Greece. BMJ Open 2022, 12, e057084. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Golan Cohen, A.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef]
- Apanga, P.A.; Kumbeni, M.T. Adherence to COVID-19 preventive measures and associated factors among pregnant women in Ghana. Trop. Med. Int. Health 2021, 26, 656–663. [Google Scholar] [CrossRef]
- Arcavi, L.; Benowitz, N.L. Cigarette smoking and infection. Arch. Intern. Med. 2004, 164, 2206–2216. [Google Scholar] [CrossRef]
- Alraddadi, B.M.; Watson, J.T.; Almarashi, A.; Abedi, G.R.; Turkistani, A.; Sadran, M.; Housa, A.; Almazroa, M.A.; Alraihan, N.; Banjar, A.; et al. Risk Factors for Primary Middle East Respiratory Syndrome Coronavirus Illness in Humans, Saudi Arabia, 2014. Emerg. Infect. Dis. 2016, 22, 49–55. [Google Scholar] [CrossRef]
- Simons, D.; Shahab, L.; Brown, J.; Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: A living rapid evidence review with Bayesian meta-analyses (version 7). Addiction 2021, 116, 1319–1368. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Vallarta-Robledo, J.R.; Sandoval, J.L.; Baggio, S.; Salamun, J.; Jacquérioz, F.; Spechbach, H.; Guessous, I. Negative Association Between Smoking and Positive SARS-CoV-2 Testing: Results from a Swiss Outpatient Sample Population. Front. Public Health 2021, 9, 731981. [Google Scholar] [CrossRef] [PubMed]
- Paleiron, N.; Mayet, A.; Marbac, V.; Perisse, A.; Barazzutti, H.; Brocq, F.X.; Janvier, F.; Dautzenberg, B.; Bylicki, O. Impact of Tobacco Smoking on the Risk of COVID-19: A Large Scale Retrospective Cohort Study. Nicotine Tob. Res. 2021, 23, 1398–1404. [Google Scholar] [CrossRef] [PubMed]
- Fan, V.S.; Dominitz, J.A.; Eastment, M.C.; Locke, E.R.; Green, P.; Berry, K.; O’Hare, A.M.; Shah, J.A.; Crothers, K.; Ioannou, G.N. Risk Factors for Testing Positive for Severe Acute Respiratory Syndrome Coronavirus 2 in a National United States Healthcare System. Clin. Infect. Dis. 2021, 73, e3085–e3094. [Google Scholar] [CrossRef] [PubMed]
- De Lusignan, S.; Dorward, J.; Correa, A.; Jones, N.; Akinyemi, O.; Amirthalingam, G.; Andrews, N.; Byford, R.; Dabrera, G.; Elliot, A.; et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect. Dis. 2020, 20, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Oakes, J.M.; Fuchs, R.M.; Gardner, J.D.; Lazartigues, E.; Yue, X. Nicotine and the renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R895–R906. [Google Scholar] [CrossRef]
- Thomas, W. Smoking and COVID-19—A Review of Studies Suggesting a Protective Effect of Smoking against COVID-19; Publications Office of the European Union: Luxemburg, 2020.
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef]
- Patel, J.A.; Nielsen, F.B.H.; Badiani, A.A.; Assi, S.; Unadkat, V.A.; Patel, B.; Ravindrane, R.; Wardle, H. Poverty, inequality and COVID-19: The forgotten vulnerable. Public Health 2020, 183, 110–111. [Google Scholar] [CrossRef]
- Cardoso, M.R.; Cousens, S.N.; de Góes Siqueira, L.F.; Alves, F.M.; D’Angelo, L.A. Crowding: Risk factor or protective factor for lower respiratory disease in young children? BMC Public Health 2004, 4, 19. [Google Scholar] [CrossRef]
- Algren, M.H.; Ekholm, O.; Nielsen, L.; Ersbøll, A.K.; Bak, C.K.; Andersen, P.T. Associations between perceived stress, socioeconomic status, and health-risk behaviour in deprived neighbourhoods in Denmark: A cross-sectional study. BMC Public Health 2018, 18, 250. [Google Scholar] [CrossRef]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef]
- Howard, L.M.; Ehrlich, A.M.; Gamlen, F.; Oram, S. Gender-neutral mental health research is sex and gender biased. Lancet Psychiatry 2017, 4, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Kalman, D.; Morissette, S.B.; George, T.P. Co-morbidity of smoking in patients with psychiatric and substance use disorders. Am. J. Addict. 2005, 14, 106–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.T.C.; Cheng, G.W.H.; Lin, C.; Wong, B.H.C.; Lam, L.C.W. Do people with mental health problems have lower adherence to precautionary measures in COVID-19 pandemic? A cross-sectional observational study in Hong Kong. BMJ Open 2021, 11, e046658. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Ahn, J.; Park, J.; Kyung Kang, C.; Won, S.H.; Wook Kim, D.; Park, J.H.; Chung, K.H.; Joh, J.S.; Bang, J.H.; et al. Beneficial Effect of Statins in COVID-19-Related Outcomes-Brief Report: A National Population-Based Cohort Study. Arterioscler. Thromb. Vasc. Biol. 2021, 41, e175–e182. [Google Scholar] [CrossRef]
- Zapatero-Belinchón, F.J.; Moeller, R.; Lasswitz, L.; van Ham, M.; Becker, M.; Brogden, G.; Rosendal, E.; Bi, W.; Carriquí-Madroñal, B.; Islam, K.; et al. Fluvastatin mitigates SARS-CoV-2 infection in human lung cells. iScience 2021, 24, 103469. [Google Scholar] [CrossRef]
- Linder, N.; Abudi, Y.; Abdalla, W.; Badir, M.; Amitai, Y.; Samuels, J.; Mendelson, E.; Levy, I. Effect of season of inoculation on immune response to rubella vaccine in children. J. Trop. Pediatr. 2011, 57, 299–302. [Google Scholar] [CrossRef]
- Zimmerman, R.K.; Lin, C.J.; Raviotta, J.M.; Nowalk, M.P. Do vitamin D levels affect antibody titers produced in response to HPV vaccine? Hum. Vaccin. Immunother. 2015, 11, 2345–2349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hewison, M.; Freeman, L.; Hughes, S.V.; Evans, K.N.; Bland, R.; Eliopoulos, A.G.; Kilby, M.D.; Moss, P.A.; Chakraverty, R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J. Immunol. 2003, 170, 5382–5390. [Google Scholar] [CrossRef]
- Alroy, I.; Towers, T.L.; Freedman, L.P. Transcriptional repression of the interleukin-2 gene by vitamin D3: Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell. Biol. 1995, 15, 5789–5799. [Google Scholar] [CrossRef]
- Mahon, B.D.; Wittke, A.; Weaver, V.; Cantorna, M.T. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J. Cell. Biochem. 2003, 89, 922–932. [Google Scholar] [CrossRef]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Holick, M.F. Immunologic Effects of Vitamin D on Human Health and Disease. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Xu, P.; Li, G.; Qiao, Y.; Han, W.; Geng, C.; Liao, D.; Yang, M.; Chen, D.; Jiang, P. Vitamin D receptor activation regulates microglia polarization and oxidative stress in spontaneously hypertensive rats and angiotensin II-exposed microglial cells: Role of renin-angiotensin system. Redox Biol. 2019, 26, 101295. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J. Steroid Biochem. Mol. Biol. 2010, 121, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef]
- Gunville, C.F.; Mourani, P.M.; Ginde, A.A. The role of vitamin D in prevention and treatment of infection. Inflamm. Allergy Drug Targets 2013, 12, 239–245. [Google Scholar] [CrossRef]
- Tan, L.; Wang, Q.; Zhang, D.; Ding, J.; Huang, Q.; Tang, Y.Q.; Wang, Q.; Miao, H. Lymphopenia predicts disease severity of COVID-19: A descriptive and predictive study. Signal Transduct. Target Ther. 2020, 5, 33. [Google Scholar] [CrossRef]
- Tarke, A.; Sidney, J.; Kidd, C.K.; Dan, J.M.; Ramirez, S.I.; Yu, E.D.; Mateus, J.; da Silva Antunes, R.; Moore, E.; Rubiro, P.; et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021, 2, 100204. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Gaffney-Stomberg, E.; Lutz, L.J.; Rood, J.C.; Cable, S.J.; Pasiakos, S.M.; Young, A.J.; McClung, J.P. Calcium and vitamin D supplementation maintains parathyroid hormone and improves bone density during initial military training: A randomized, double-blind, placebo controlled trial. Bone 2014, 68, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Oren, Y.; Shapira, Y.; Agmon-Levin, N.; Kivity, S.; Zafrir, Y.; Altman, A.; Lerner, A.; Shoenfeld, Y. Vitamin D insufficiency in a sunny environment: A demographic and seasonal analysis. Isr. Med. Assoc. J. 2010, 12, 751–756. [Google Scholar] [PubMed]
| PCR Status | |||
|---|---|---|---|
| Positive (n = 5026) | Negative (n = 68,228) | p-Value | |
| Age | 45.4 ± 17.8 | 50.8 ± 18.9 | p < 0.001 |
| Gender Male Female | (33%) (67%) | (37%) (63%) | p < 0.001 |
| Ethnicity Arab Orthodox Jewish Other | (20%) (19%) (61%) | (13%) (16%) (71%) | p < 0.001 |
| 25(OH)D level 0–20 ng/mL 20–30 ng/mL >30 ng/ml | (46.5%) (35%) (18.5%) | (41%) (38%) (21%) | p < 0.001 |
| Anxiety | (36%) | (36%) | p = 0.96 |
| Schizophrenia | (2.0%) | (2.2%) | p = 0.52 |
| Depression | (23.0%) | (26.1%) | p < 0.001 |
| Dementia | (4.0%) | (4.4%) | p = 0.31 |
| Nephrotic syndrome | (0.6%) | (0.4%) | p = 0.084 |
| Chronic Renal failure | (6.5%) | (7.4%) | p = 0.072 |
| CVA | (4.4%) | (6.0%) | p < 0.001 |
| CHF | (4.8%) | (5.1%) | p = 0.46 |
| PVD | (3.9%) | (5.3%) | p < 0.001 |
| IHD | (9.0%) | (10.9%) | p < 0.001 |
| Hyperlipidemia | (53.6%) | (63.4%) | p < 0.001 |
| HTN | (2.0%) | (2.1%) | p = 0.88 |
| DM | (11.7%) | (13.1%) | p = 0.032 |
| COPD | (7.8%) | (10.5%) | p < 0.001 |
| Asthma | (16.6%) | (14.9%) | p = 0.016 |
| SES 1–10 10–20 | (55%) (45%) | (43%) (57%) | p < 0.001 |
| Smoking status Active smokers Non-smokers Former smokers | (14%) (84%) (2%) | (19%) (79%) (2%) | p < 0.0001 |
| BMI 16.5–18.5 18.5–24.9 25–29.9 30+ | (3%) (34%) (34%) (29%) | (3%) (34%) (35%) (28%) | p = 0.40 |
| [25(OH)D] < 20 | [25(OH)D] 20–30 | [25(OH)D] > 30 | |
|---|---|---|---|
| 1–2 Months | 2.18% (n = 7067) | 1.7% (n = 6826) | 1.57% (n = 3694) |
| 3–4 Months | 5.6% (n = 4214) | 3.87 (n = 3898) | 3.77% (n = 2174) |
| 5–6 Months | 13.56% (n = 11,094) | 11.94% (n = 10,291) | 11.85% (n = 5579) |
| 7–8 Months | 7.13% (n = 5992) | 5.1% (n = 5236) | 4.35% (n = 2874) |
| 9–10 Months | 0.86% (n = 1637) | 0.06% (n = 1672) | 0.20% (n = 994) |
| 1–10 Months | 7.78% (n = 30,004) * | 6.31% (n = 27,923) # | 6.06% (n = 15,315) |
| Antibody Titer < 50 (n = 1014) | Antibody Titer >= 50 (n = 2645) | Total; n = 3659 | p | |
|---|---|---|---|---|
| Age | 51.8 ± 16.9 | 51.2 ± 16.5 | 51.4 ± 16.6 | p = 0.32 |
| Gender Code1 = male Code2 = female | (36%) (64%) | (31%) (69%) | (32%) (68%) | p = 0.005 |
| PCR positive | 13.2% | 7.7% | 9.3% | p < 0.05 |
| Ethnicity Arab Orthodox Jewish Other | (13.5%) (7%) (79%) | (19%) (12%) (69%) | (17.5%) (10.5%) (72%) | p < 0.001 |
| 25(OH)D levels 0–20 ng/mL 20–30 ng/mL >30 ng/ml | (34%) (40%) (26%) | (34%) (41%) (25%) | (34%) (41%) (25%) | p = 0.87 |
| Anxiety | (36%) | (40%) | (39%) | p = 0.11 |
| Schizophrenia | (1.5%) | (1.3%) | (1.3%) | p = 0.71 |
| Depression | (26.4%) | (26.5%) | (26.5%) | p = 0.96 |
| Dementia | (1.9%) | (1.4%) | (1.6%) | p = 0.38 |
| Nephrotic syndrome | (1.2%) | (0.3%) | (0.6%) | p = 0.016 |
| Chronic Renal failure | (10.3%) | (5.8%) | (7.1%) | p < 0.001 |
| CVA | (5.0%) | (3.7%) | (4.1%) | p = 0.13 |
| CHF | (5.3%) | (4.6%) | (4.8%) | p = 0.48 |
| PVD | (4.7%) | (4.2%) | (4.3%) | p = 0.52 |
| IHD | (11.2%) | (9.8%) | (10.2%) | p = 0.28 |
| Hyperlipidemia | (65.0%) | (63.1%) | (63.6%) | p = 0.37 |
| HTN | (2.7%) | (2.0%) | (2.2%) | p = 0.30 |
| DM | (11.0%) | (10.8%) | (10.8%) | p = 0.89 |
| COPD | (10.7%) | (10.5%) | (10.5%) | p = 0.88 |
| asthma | (14.9%) | (15.8%) | (15.5%) | p = 0.59 |
| SES 1–10 10–20 | (30.4%) (69.6%) | (40.8%) (59.2%) | (37.9%) (62.1%) | p < 0.001 |
| Smoking status Active smoker Non-smoker Former smoker | (14.6%) (82.9%) (2.5%) | (14.0%) (84.3%) (1.7%) | (14.1%) (83.9%) (1.9%) | p = 0.27 |
| BMI 16.5–18.5 18.5–24.9 25–29.9 30+ | (2.6%) (36.7%) (36.4%) (24.3%) | (2.4%) (35.9%) (35.7%) (26.0%) | (2.5%) (36.1%) (35.9%) (25.5%) | p = 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abu Fanne, R.; Lidawi, G.; Maraga, E.; Moed, M.; Roguin, A.; Meisel, S.-R. Correlation between Baseline 25(OH) Vitamin D Levels and Both Humoral Immunity and Breakthrough Infection Post-COVID-19 Vaccination. Vaccines 2022, 10, 2116. https://doi.org/10.3390/vaccines10122116
Abu Fanne R, Lidawi G, Maraga E, Moed M, Roguin A, Meisel S-R. Correlation between Baseline 25(OH) Vitamin D Levels and Both Humoral Immunity and Breakthrough Infection Post-COVID-19 Vaccination. Vaccines. 2022; 10(12):2116. https://doi.org/10.3390/vaccines10122116
Chicago/Turabian StyleAbu Fanne, Rami, Ghalib Lidawi, Emad Maraga, Mahmud Moed, Ariel Roguin, and Simcha-Ron Meisel. 2022. "Correlation between Baseline 25(OH) Vitamin D Levels and Both Humoral Immunity and Breakthrough Infection Post-COVID-19 Vaccination" Vaccines 10, no. 12: 2116. https://doi.org/10.3390/vaccines10122116
APA StyleAbu Fanne, R., Lidawi, G., Maraga, E., Moed, M., Roguin, A., & Meisel, S.-R. (2022). Correlation between Baseline 25(OH) Vitamin D Levels and Both Humoral Immunity and Breakthrough Infection Post-COVID-19 Vaccination. Vaccines, 10(12), 2116. https://doi.org/10.3390/vaccines10122116




