Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018)
Abstract
1. Introduction
2. Materials and Methods
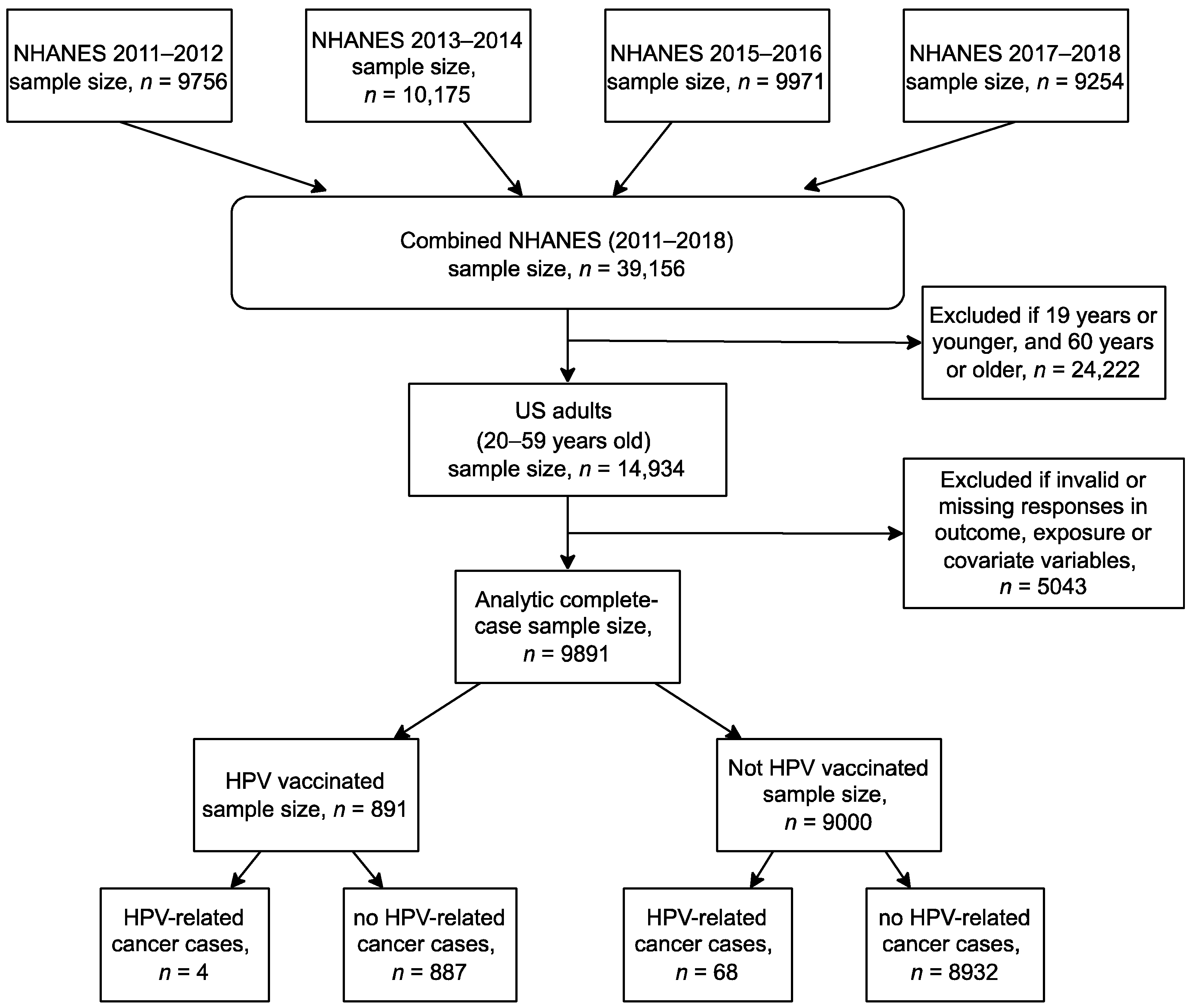
2.1. Study Design and Data Source
2.2. Study Population
2.3. Study Variables
2.4. Statistical Analysis
2.4.1. Primary Analysis
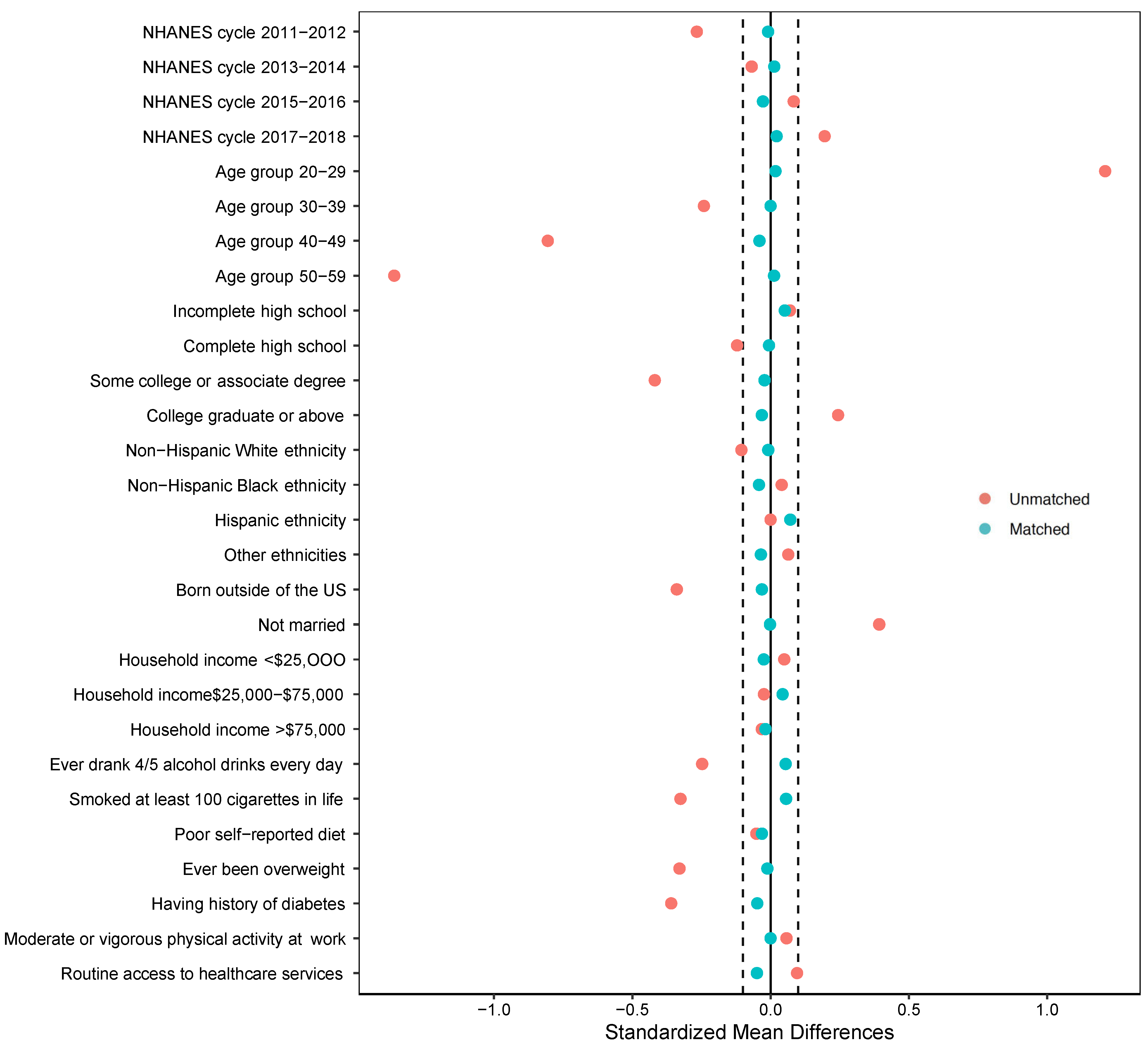
2.4.2. Secondary Analysis (Propensity Score Matching)
2.4.3. Sensitivity Analysis
3. Results
3.1. Sample Characteristics and Univariate Analysis
3.2. Primary Analysis
3.3. Secondary Analysis
3.4. Sensitivity Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. Ca Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Wong, I.C.K.; Ng, Y.-K.; Lui, V.W.Y. Cancers of the lung, head and neck on the rise: Perspectives on the genotoxicity of air pollution. Chin. J. Cancer 2014, 33, 476. [Google Scholar] [CrossRef] [PubMed]
- Maino Vieytes, C.A.; Mondul, A.M.; Li, Z.; Zarins, K.R.; Wolf, G.T.; Rozek, L.S.; Arthur, A.E. Dietary fiber, whole grains, and head and neck cancer prognosis: Findings from a prospective cohort study. Nutrients 2019, 11, 2304. [Google Scholar] [CrossRef]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C. US assessment of HPV types in cancers: Implications for current and 9-valent HPV vaccines. JNCI J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef]
- Morka, N.; Norris, J.M.; Emberton, M.; Kelly, D. Prostate cancer and the human papilloma virus: Causative association, role of vaccines, and the impact of the COVID-19 pandemic. Prostate Cancer Prostatic Dis. 2022, 25, 55–57. [Google Scholar] [CrossRef]
- Garolla, A.; Vitagliano, A.; Muscianisi, F.; Valente, U.; Ghezzi, M.; Andrisani, A.; Ambrosini, G.; Foresta, C. Role of viral infections in testicular cancer etiology: Evidence from a systematic review and meta-analysis. Front. Endocrinol. 2019, 10, 355. [Google Scholar] [CrossRef]
- Athanasiou, A.; Bowden, S.; Paraskevaidi, M.; Fotopoulou, C.; Martin-Hirsch, P.; Paraskevaidis, E.; Kyrgiou, M. HPV vaccination and cancer prevention. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 65, 109–124. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices; Wiley Online Library: Hoboken, NJ, USA, 2019; Volume 19, pp. 3202–3206. [Google Scholar]
- Canadian Cancer Society. Human Papillomavirus. Available online: https://cancer.ca/en/cancer-information/reduce-your-risk/get-vaccinated/human-papillomavirus-hpv (accessed on 15 November 2022).
- Lehtinen, M.; Paavonen, J.; Wheeler, C.M.; Jaisamrarn, U.; Garland, S.M.; Castellsagué, X.; Skinner, S.R.; Apter, D.; Naud, P.; Salmerón, J. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012, 13, 89–99. [Google Scholar] [CrossRef]
- Muñoz, N.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.-E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J. Natl. Cancer Inst. 2010, 102, 325–339. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L.; Simoens, C.; Martin-Hirsch, P.P. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018. [Google Scholar] [CrossRef]
- Han, J.J.; Beltran, T.H.; Song, J.W.; Klaric, J.; Choi, Y.S. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013–2014. JAMA Oncol. 2017, 3, 810–816. [Google Scholar] [CrossRef]
- Van Dyne, E.A.; Henley, S.J.; Saraiya, M.; Thomas, C.C.; Markowitz, L.E.; Benard, V.B. Trends in human papillomavirus–associated cancers—United States, 1999–2015. Morb. Mortal. Wkly. Rep. 2018, 67, 918. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Liu, G.; Hariri, S.; Steinau, M.; Dunne, E.F.; Unger, E.R. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics 2016, 137, e20151968. [Google Scholar] [CrossRef]
- Luostarinen, T.; Apter, D.; Dillner, J.; Eriksson, T.; Harjula, K.; Natunen, K.; Paavonen, J.; Pukkala, E.; Lehtinen, M. Vaccination protects against invasive HPV-associated cancers. Int. J. Cancer 2018, 142, 2186–2187. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Elfström, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundström, K.; Dillner, J.; Sparén, P. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Holman, D.M.; Benard, V.; Roland, K.B.; Watson, M.; Liddon, N.; Stokley, S. Barriers to human papillomavirus vaccination among US adolescents: A systematic review of the literature. JAMA Pediatr. 2014, 168, 76–82. [Google Scholar] [CrossRef]
- Szilagyi, P.G.; Albertin, C.S.; Gurfinkel, D.; Saville, A.W.; Vangala, S.; Rice, J.D.; Helmkamp, L.; Zimet, G.D.; Valderrama, R.; Breck, A. Prevalence and characteristics of HPV vaccine hesitancy among parents of adolescents across the US. Vaccine 2020, 38, 6027–6037. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). About the National Health and Nutrition Examination Survey. Available online: https://www.cdc.gov/nchs/nhanes/about_nhanes.htm (accessed on 15 November 2022).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design (accessed on 15 November 2022).
- Johnson, C.L.; Dohrmann, S.M.; Burt, V.L.; Mohadjer, L.K. National Health and Nutrition Examination Survey: Sample Design, 2011–2014; US Department of Health and Human Services: Washington, DC, USA; Centers for Disease Control and Prevention: Washington, DC, USA, 2014.
- Board of Governors. Policy # 89: Research Involving Human Participants. Available online: https://bog2.sites.olt.ubc.ca/files/2012/01/4.2_2012.02.Policy-89.pdf (accessed on 15 November 2022).
- Government of Canada. TCPS 2 (2018)—Chapter 2: Scope and Approach. Available online: https://ethics.gc.ca/eng/tcps2-eptc2_2018_chapter2-chapitre2.html (accessed on 15 November 2022).
- Centers for Disease Control and Prevention (CDC); National Center for Health Statistics (NCHS). NHANES Questionnaires, Datasets, and Related Documentation. Available online: https://wwwn.cdc.gov/nchs/nhanes/ (accessed on 15 November 2022).
- Hirth, J. Disparities in HPV vaccination rates and HPV prevalence in the United States: A review of the literature. Hum. Vaccines Immunother. 2019, 15, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Stenson, K.M.; Brockstein, B.E.; Ross, M.E. Epidemiology and Risk Factors for Head and Neck Cancer. UpToDate. 2014. Available online: https://www.uptodate.com/contents/epidemiology-and-risk-factors-for-head-and-neck-cancer (accessed on 15 November 2022).
- Skinner, C. Analysis of categorical data for complex surveys. Int. Stat. Rev. 2019, 87, S64–S78. [Google Scholar] [CrossRef]
- Pearl, J. Causal diagrams for empirical research. Biometrika 1995, 82, 669–688. [Google Scholar] [CrossRef]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef]
- Archer, K.J.; Lemeshow, S. Goodness-of-fit test for a logistic regression model fitted using survey sample data. Stata J. 2006, 6, 97–105. [Google Scholar] [CrossRef]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef]
- Cepeda, M.S.; Boston, R.; Farrar, J.T.; Strom, B.L. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am. J. Epidemiol. 2003, 158, 280–287. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- DuGoff, E.H.; Schuler, M.; Stuart, E.A. Generalizing observational study results: Applying propensity score methods to complex surveys. Health Serv. Res. 2014, 49, 284–303. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: Abingdon, UK, 2013. [Google Scholar]
- Austin, P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009, 28, 3083–3107. [Google Scholar] [CrossRef]
- Bhaskaran, K.; Smeeth, L. What is the difference between missing completely at random and missing at random? Int. J. Epidemiol. 2014, 43, 1336–1339. [Google Scholar] [CrossRef]
- Von Hippel, P.T. 4. Regression with missing Ys: An improved strategy for analyzing multiply imputed data. Sociol. Methodol. 2007, 37, 83–117. [Google Scholar] [CrossRef]
- Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 2016, 4, 30. [Google Scholar]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 81. [Google Scholar]
- Wood, A.M.; White, I.R.; Royston, P. How should variable selection be performed with multiply imputed data? Stat. Med. 2008, 27, 3227–3246. [Google Scholar] [CrossRef]
- Tierney, L. The R statistical computing environment. In Statistical Challenges in Modern Astronomy V; Springer: Berlin/Heidelberg, Germany, 2012; pp. 435–447. [Google Scholar]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Dehlendorff, C.; Baandrup, L.; Kjaer, S.K. Real-world effectiveness of Human Papillomavirus vaccination against vulvovaginal high-grade precancerous lesions and cancers. JNCI J. Natl. Cancer Inst. 2021, 113, 869–874. [Google Scholar] [CrossRef]
- Canfell, K.; Hall, M.; Simms, K.; Smith, M.; Saville, M. Australia on-track to be the first country to achieve cervical cancer elimination. Mortality 2018, 1, 80. [Google Scholar]
- Patel, C.; Brotherton, J.M.; Pillsbury, A.; Jayasinghe, S.; Donovan, B.; Macartney, K.; Marshall, H. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: What additional disease burden will a nonavalent vaccine prevent? Eurosurveillance 2018, 23, 1700737. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Gee, J.; Chesson, H.; Stokley, S. Ten years of human papillomavirus vaccination in the United States. Acad. Pediatr. 2018, 18, S3–S10. [Google Scholar] [CrossRef]
- Stokley, S.; Jeyarajah, J.; Yankey, D.; Cano, M.; Gee, J.; Roark, J.; Curtis, C.R.; Markowitz, L. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. Morb. Mortal. Wkly. Rep. 2014, 63, 620. [Google Scholar]
- Lu, P.-J.; Hung, M.-C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of vaccination coverage among adult populations—United States, 2018. MMWR Surveill. Summ. 2021, 70, 1. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.E.; Unger, E.R.; Lewis, R.; McDaniel, D.; Gargano, J.W.; Steinau, M.; Markowitz, L.E. Prevalence of human papillomavirus among females after vaccine introduction—National Health and Nutrition Examination Survey, United States, 2003–2014. J. Infect. Dis. 2017, 216, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E.R. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Flagg, E.W.; Torrone, E.A. Declines in anogenital warts among age groups most likely to be impacted by human papillomavirus vaccination, United States, 2006–2014. Am. J. Public Health 2018, 108, 112–119. [Google Scholar] [CrossRef]
- Mann, L.M.; Llata, E.; Flagg, E.W.; Hong, J.; Asbel, L.; Carlos-Henderson, J.; Kerani, R.P.; Kohn, R.; Pathela, P.; Schumacher, C. Trends in the prevalence of anogenital warts among patients at sexually transmitted disease clinics—Sexually Transmitted Disease Surveillance Network, United States, 2010–2016. J. Infect. Dis. 2019, 219, 1389–1397. [Google Scholar] [CrossRef]
- Norwegian Institute of Public Health. High Vaccine Uptake in the Childhood Immunisation Programme. Available online: https://www.fhi.no/en/news/2022/high-vaccine-uptake-in-the-childhood-immunisation-programme/ (accessed on 15 November 2022).
- Bjerke, R.D.; Laake, I.; Feiring, B.; Aamodt, G.; Trogstad, L. Time trends in HPV vaccination according to country background: A nationwide register-based study among girls in Norway. BMC Public Health 2021, 21, 854. [Google Scholar] [CrossRef]
- Bigaard, J.; Franceschi, S. Vaccination against HPV: Boosting coverage and tackling misinformation. Mol. Oncol. 2021, 15, 770–778. [Google Scholar] [CrossRef]
- World Health Organization. Uzbekistan Achieves High HPV Vaccination Coverage against Cervical Cancer. Available online: https://www.who.int/europe/news/item/07-09-2022-uzbekistan-achieves-high-hpv-vaccination-coverage-against-cervical-cancer (accessed on 15 November 2022).
- Beavis, A.L.; Levinson, K.L. Preventing cervical cancer in the United States: Barriers and resolutions for HPV vaccination. Front. Oncol. 2016, 6, 19. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem. Available online: https://apps.who.int/iris/bitstream/handle/10665/336583/9789240014107-eng.pdf (accessed on 15 November 2022).
- Bruni, L.; Diaz, M.; Barrionuevo-Rosas, L.; Herrero, R.; Bray, F.; Bosch, F.X.; De Sanjosé, S.; Castellsagué, X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob. Health 2016, 4, e453–e463. [Google Scholar] [CrossRef]
- Tjalma, W.A.; Fiander, A.; Reich, O.; Powell, N.; Nowakowski, A.M.; Kirschner, B.; Koiss, R.; O’Leary, J.; Joura, E.A.; Rosenlund, M. Differences in human papillomavirus type distribution in high-grade cervical intraepithelial neoplasia and invasive cervical cancer in Europe. Int. J. Cancer 2013, 132, 854–867. [Google Scholar] [CrossRef]
- Depuydt, C.E.; Criel, A.M.; Benoy, I.H.; Arbyn, M.; Vereecken, A.J.; Bogers, J.J. Changes in type-specific human papillomavirus load predict progression to cervical cancer. J. Cell. Mol. Med. 2012, 16, 3096–3104. [Google Scholar] [CrossRef]
- Burger, E.A.; De Kok, I.M.; Groene, E.; Killen, J.; Canfell, K.; Kulasingam, S.; Kuntz, K.M.; Matthijsse, S.; Regan, C.; Simms, K.T. Estimating the natural history of cervical carcinogenesis using simulation models: A CISNET comparative analysis. JNCI J. Natl. Cancer Inst. 2020, 112, 955–963. [Google Scholar] [CrossRef]
 ” is the primary exposure variable (HPV vaccination). The variable with “
” is the primary exposure variable (HPV vaccination). The variable with “ 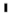 ” sign is the outcome (HPV-related cancers).
” sign is the outcome (HPV-related cancers).
 ” is the primary exposure variable (HPV vaccination). The variable with “
” is the primary exposure variable (HPV vaccination). The variable with “  ” sign is the outcome (HPV-related cancers).
” sign is the outcome (HPV-related cancers).

| Variables | No HPV-Related Cancers (n = 9819) | HPV-Related Cancers (n = 72) | p-Value b | All c (n = 9891) | Missing Data d (n = 4102) |
|---|---|---|---|---|---|
| n (%) a | n (%) a | n (%) a | n (%) a | ||
| NHANES cycle | 0.25 | ||||
| 2011–2012 | 2479 (24.9) | 17 (28.2) | 2496 (24.9) | 1098 (25.9) | |
| 2013–2014 | 2682 (25.3) | 26 (33.9) | 2708 (25.3) | 1033 (25.0) | |
| 2015–2016 | 2402 (24.5) | 14 (20.3) | 2416 (24.5) | 1118 (26.8) | |
| 2017–2018 | 2256 (25.4) | 15 (17.6) | 2271 (25.3) | 853 (22.4) | |
| HPV vaccination history | 0.01 | ||||
| Not vaccinated | 8932 (90.6) | 68 (97.4) | 9000 (90.6) | 3779 (91.6) | |
| Vaccinated | 887 (9.4) | 4 (2.6) | 891 (9.4) | 323 (8.4) | |
| Age groups | 0.001 | ||||
| 20–29 years | 2358 (24.1) | 5 (6.0) | 2363 (24.0) | 995 (25.5) | |
| 30–39 years | 2484 (23.5) | 19 (20.2) | 2503 (23.5) | 1046 (26.6) | |
| 40–49 years | 2457 (25.2) | 26 (40.2) | 2483 (25.3) | 1030 (23.2) | |
| 50–59 years | 2520 (27.2) | 22 (33.7) | 2542 (27.2) | 1031 (24.6) | |
| Sex | <0.001 | ||||
| Males | 4942 (50.3) | 3 (4.0) | 4945 (49.8) | 1626 (42.0) | |
| Females | 4877 (49.7) | 69 (96.0) | 4946 (50.2) | 2476 (58.0) | |
| Education | <0.01 | ||||
| Did not complete high school | 2712 (33.5) | 6 (12.2) | 2718 (33.3) | 1019 (28.0) | |
| Completed high school | 2155 (21.4) | 16 (21.2) | 2171 (21.4) | 870 (22.5) | |
| Some college or associate degree | 1648 (11.8) | 17 (15.5) | 1665 (11.8) | 1015 (19.1) | |
| College graduate or above | 3304 (33.4) | 33 (51.1) | 3337 (33.5) | 1192 (30.4) | |
| Ethnicity | 0.01 | ||||
| Non-Hispanic White | 2275 (15.5) | 9 (6.1) | 2284 (15.4) | 1126 (22.7) | |
| Non-Hispanic Black | 2181 (11.2) | 8 (3.5) | 2189 (11.2) | 984 (15.7) | |
| Hispanic | 3814 (65.1) | 49 (82.6) | 3863 (65.3) | 919 (47.5) | |
| Other ethnicities | 1549 (8.1) | 6 (7.8) | 1555 (8.1) | 1073 (14.0) | |
| Where born | 0.15 | ||||
| In US | 7262 (84.4) | 65 (91.3) | 7327 (84.5) | 2123 (66.4) | |
| Outside US | 2557 (15.6) | 7 (8.7) | 2564 (15.5) | 1975 (33.6) | |
| Marital status | 0.20 | ||||
| Married or living with partner | 5839 (63.2) | 33 (54.6) | 5872 (63.1) | 2507 (61.3) | |
| Not married | 3980 (36.8) | 39 (45.4) | 4019 (36.9) | 1590 (38.7) | |
| Household income | 0.001 | ||||
| <$25,000 | 4253 (39.9) | 33 (53.8) | 4286 (40.1) | 1420 (43.0) | |
| $25,000-$75,000 | 3210 (43.4) | 10 (19.9) | 3220 (43.1) | 867 (33.5) | |
| >$75,000 | 2356 (16.7) | 29 (26.3) | 2385 (16.8) | 937 (23.5) | |
| Ever drank 4/5 alcohol drinks every day | 0.58 | ||||
| Yes | 1539 (15.7) | 15 (19.1) | 1554 (15.7) | 81 (15.5) | |
| No | 8280 (84.3) | 57 (80.9) | 8337 (84.3) | 449 (84.5)) | |
| Smoked at least 100 cigarettes in life | <0.001 | ||||
| Yes | 4340 (44.5) | 55 (72.8) | 4395 (44.7) | 1055 (28.7) | |
| No | 5479 (55.5) | 17 (27.2) | 5496 (55.3) | 3041 (71.3) | |
| Self-reported diet ¶ | 0.99 | ||||
| Healthy | 6423 (68.9) | 43 (68.8) | 6466 (68.9) | 2885 (70.6) | |
| Poor | 3396 (31.1) | 29 (31.2) | 3425 (31.1) | 1215 (29.4) | |
| Ever been overweight † | 0.31 | ||||
| Yes | 5047 (52.5) | 48 (60.6) | 5095 (52.6) | 1835 (46.3) | |
| No | 4772 (47.5) | 24 (39.4) | 4796 (47.4) | 2264 (53.7)) | |
| History of diabetes | 0.27 | ||||
| Yes | 947 (8.4) | 10 (12.4) | 957 (8.4) | 390 (8.1) | |
| No | 8872 (91.6) | 62 (87.6) | 8934 (91.6) | 3702 (91.9) | |
| Moderate or vigorous physical activity at work | 0.60 | ||||
| Yes | 4621 (49.8) | 38 (54.1) | 4659 (49.8) | 1474 (39.1) | |
| No | 5198 (50.2) | 34 (45.9) | 5232 (50.2) | 2622 (60.9) | |
| Routine access to healthcare services ‡ | <0.01 | ||||
| Yes | 7695 (80.1) | 66 (93.2) | 7761 (80.2) | 3208 (78.2) | |
| No | 2124 (19.9) | 6 (6.8) | 2130 (19.8) | 893 (21.8) |
| Variables | aOR (95% CI) a Complete-Case Data b | Crude OR (95% CI) PS-Matched Data c | aOR (95% CI) d MI Data e |
|---|---|---|---|
| HPV vaccination history | |||
| Not vaccinated | Reference | Reference | Reference |
| Vaccinated | 0.58 (0.19; 1.75) | 0.40 (0.10; 1.69) | 0.47 (0.15; 1.45) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Issanov, A.; Karim, M.E.; Aimagambetova, G.; Dummer, T.J.B. Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines 2022, 10, 2113. https://doi.org/10.3390/vaccines10122113
Issanov A, Karim ME, Aimagambetova G, Dummer TJB. Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines. 2022; 10(12):2113. https://doi.org/10.3390/vaccines10122113
Chicago/Turabian StyleIssanov, Alpamys, Mohammad Ehsanul Karim, Gulzhanat Aimagambetova, and Trevor J. B. Dummer. 2022. "Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018)" Vaccines 10, no. 12: 2113. https://doi.org/10.3390/vaccines10122113
APA StyleIssanov, A., Karim, M. E., Aimagambetova, G., & Dummer, T. J. B. (2022). Does Vaccination Protect against Human Papillomavirus-Related Cancers? Preliminary Findings from the United States National Health and Nutrition Examination Survey (2011–2018). Vaccines, 10(12), 2113. https://doi.org/10.3390/vaccines10122113






