Exploiting the Macrophage Production of IL-12 in Improvement of Vaccine Development against Toxoplasma gondii and Neospora caninum Infections
Abstract
1. Overview
2. Introduction
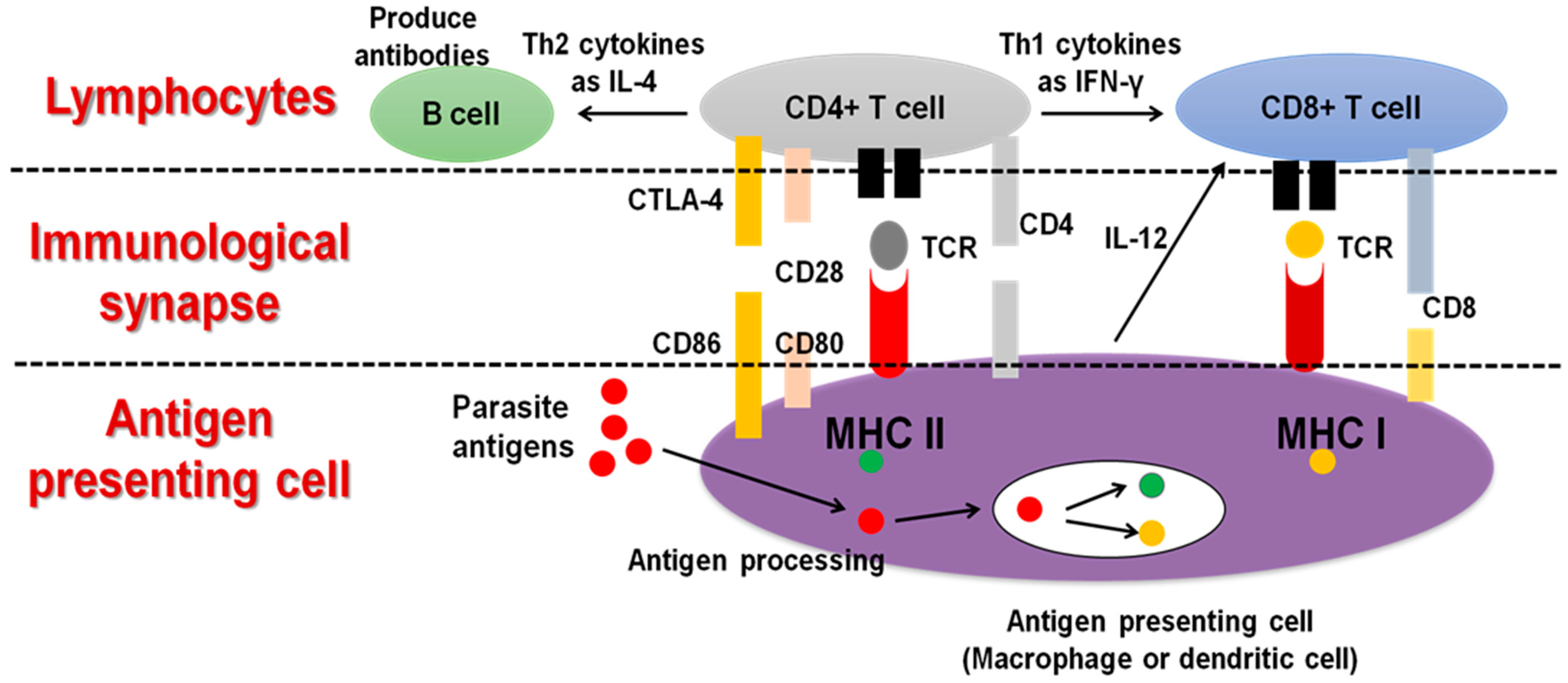
3. Protective Immunity against Toxoplasmosis and Neosporosis
4. Role of Macrophages in Host-Parasite Interaction during Toxoplasmosis and Neosporosis
5. IL-12 Production from Macrophages during T. gondii or N. caninum Infection
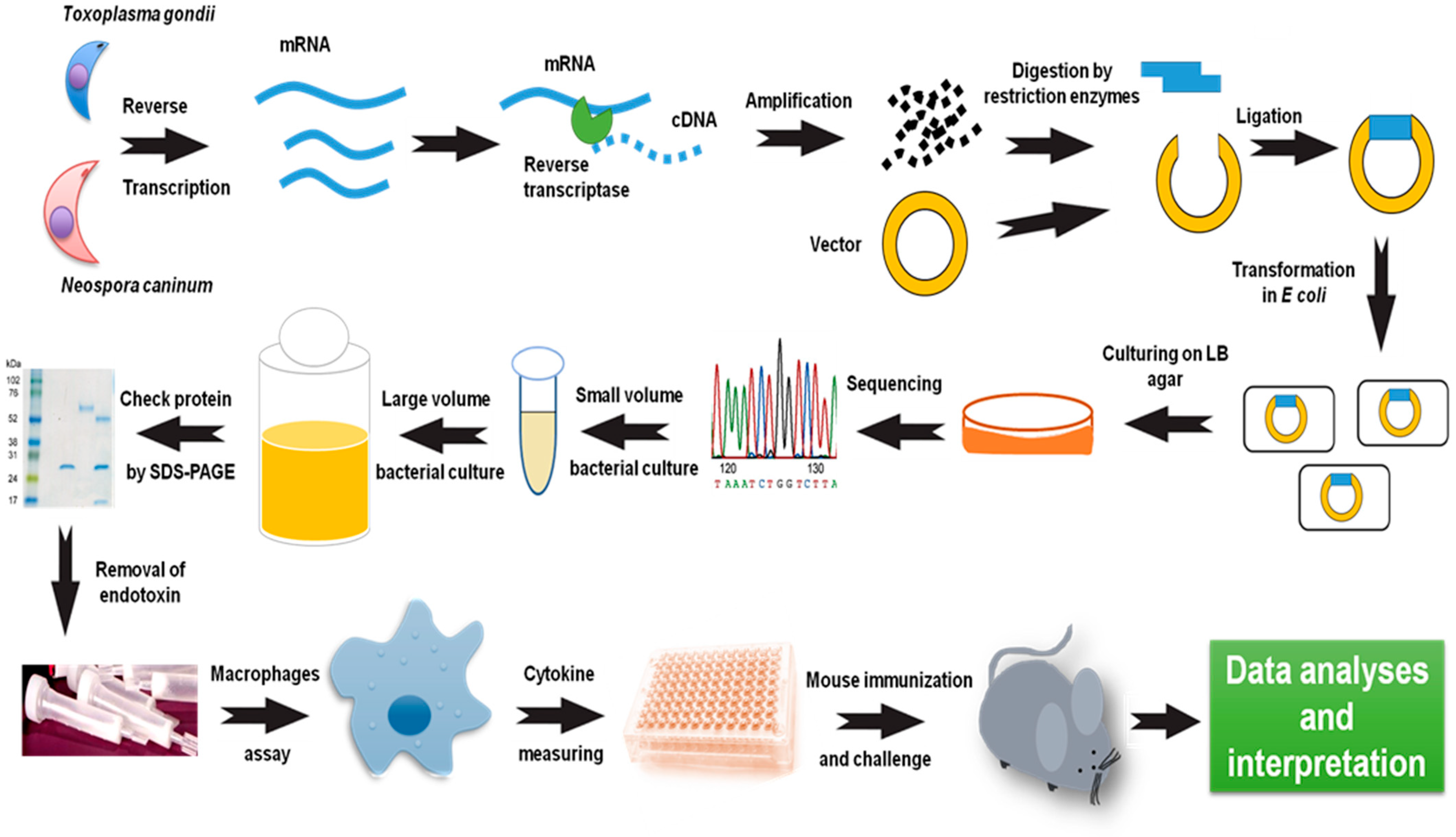
6. Macrophage Role in Vaccine Development against T. gondii and N. caninum
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, J.P.; Beattie, C.P. Toxoplasmosis of Animals and Man; CRC Press: Boca Raton, FL, USA, 1988; p. 320. [Google Scholar]
- Dubey, J.P.; Hemphil, A.; Calero-Bernal, R.; Schares, G. Neosporosis in Animals; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Hill, D.; Dubey, J.P. Toxoplasma gondii: Transmission, diagnosis and prevention. Clin. Microbiol. Infect. 1988, 8, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Grigg, M.E. Recent epidemiologic and clinical importance of Toxoplasma gondii infections in marine mammals: 2009–2020. Vet. Parasitol. 2020, 288, 109296. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P. Review of Neospora caninum and neosporosis in animals. Korean J. Parasitol. 2003, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Nishikawa, Y. From signaling pathways to distinct immune responses: Key factors for establishing or combating Neospora caninum infection in different susceptible hosts. Pathogens 2020, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D. Protozoan infections (Toxoplasma gondii, Neospora caninum and Sarcocystis spp.) in sheep and goats: Recent advances. Vet. Res. 1998, 29, 289–310. [Google Scholar]
- Buxton, D.; McAllister, M.M.; Dubey, J.P. The comparative pathogenesis of neosporosis. Trends Parasitol. 2002, 18, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Schares, G. Neosporosis in animals--the last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef]
- Porto, W.J.N.; Regidor-Cerrillo, J.; Kim, P.d.C.P.; Benavides, J.; dos Santos Silva, A.C.; Horcajo, P.; da Fonseca Oliveira, A.A.; Ferre, I.; Mota, R.A.; Ortega-Mora, L.M. Experimental caprine neosporosis: The influence of gestational stage on the outcome of infection. Vet. Res. 2016, 47, 29. [Google Scholar] [CrossRef]
- Gutiérrez-Expósito, D.; Tejerina, F.; Gutiérrez, J.; Fernández-Escobar, M.; Ortega-Mora, L.M.; Mantecón, A.R.; Dagleish, M.P.; Pérez, V.; Benavides, J. Direct economic losses of Toxoplasma gondii abortion outbreaks in two Spanish sheep flocks. Vet. Parasitol. Reg. Stud. Rep. 2021, 26, 100623. [Google Scholar] [CrossRef]
- Lindsay, D.S.; Dubey, J.P. Neosporosis, toxoplasmosis, and sarcocystosis in ruminants: An update. Vet. Clin. North Am. Food Anim. Pract. 2020, 36, 205–222. [Google Scholar] [CrossRef] [PubMed]
- González-Warleta, M.; Castro-Hermida, J.A.; Calvo, C.; Pérez, V.; Gutiérrez-Expósito, D.; Regidor-Cerrillo, J.; Ortega-Mora, L.M.; Mezo, M. Endogenous transplacental transmission of Neospora caninum during successive pregnancies across three generations of naturally infected sheep. Vet. Res. 2018, 49, 106. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Dubey, J.P. Foodborne toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Pfaff, A.W.; Liesenfeld, O.; Candolfi, E. Congenital toxoplasmosis. In Toxoplasma: Molecular and Cellular Biology; Ajioka, J.W., Soldati, D., Eds.; Horizon Bioscience: Norfolk, UK, 2007; pp. 93–110. [Google Scholar]
- Montoya, J.G.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, H.M.; Huang, P.; Salem, T.A.; Talaat, R.M.; Nasr, M.I.; Xuan, X.; Nishikawa, Y. Short report: Prevalence of Neospora caninum and Toxoplasma gondii antibodies in northern Egypt. Am. J. Trop. Med. Hyg. 2009, 80, 263–267. [Google Scholar] [CrossRef]
- Duarte, P.O.; Csordas, B.G.; Oshiro, L.M.; Higa, L.O.S.; Zimmermann, N.P.; Martins, K.R.; Barros, J.C.; Andreotti, R. Serological evaluation of Neospora caninum in pregnant women treated at referral center for prenatal screening in Mato Grosso do Sul, Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e010820. [Google Scholar] [CrossRef] [PubMed]
- Duarte, P.O.; Oshiro, L.M.; Zimmermann, N.P.; Csordas, B.G.; Dourado, D.M.; Barros, J.C.; Andreotti, R. Serological and molecular detection of Neospora caninum and Toxoplasma gondii in human umbilical cord blood and placental tissue samples. Sci. Rep. 2020, 10, 9043. [Google Scholar] [CrossRef] [PubMed]
- Black, M.W.; Boothroyd, J.C. Lytic cycle of Toxoplasma gondii. Microbiol. Mol. Biol. Rev. 2000, 64, 607–623. [Google Scholar] [CrossRef]
- Reid, A.J.; Vermont, S.J.; Cotton, J.A.; Harris, D.; Hill-Cawthorne, G.A.; Könen-Waisman, S.; Latham, S.M.; Mourier, T.; Norton, R.; Quail, M.A.; et al. Comparative genomics of the apicomplexan parasites Toxoplasma gondii and Neospora caninum: Coccidia differing in host range and transmission strategy. PLoS Pathog. 2012, 8, e1002567. [Google Scholar] [CrossRef]
- Zhao, Y.; Marple, A.H.; Ferguson, D.J.; Bzik, D.J.; Yap, G.S. Avirulent strains of Toxoplasma gondii infect macrophages by active invasion from the phagosome. Proc. Natl. Acad. Sci. USA 2014, 111, 6437–6442. [Google Scholar] [CrossRef]
- Garcia-Sanchez, M.; Jiménez-Pelayo, L.; Horcajo, P.; Regidor-Cerrillo, J.; Ólafsson, E.B.; Bhandage, A.K. Differential responses of bovine monocyte-derived macrophages to infection by Neospora caninum isolates of high and low virulence. Front. Immunol. 2019, 10, 915. [Google Scholar] [CrossRef]
- Al-Bajalan, M.M.M.; Xia, D.; Armstrong, S.; Randle, N.; Wastling, J.M. Toxoplasma gondii and Neospora caninum induce different host cell responses at proteome-wide phosphorylation events; a step forward for uncovering the biological differences between these closely related parasites. Parasitol. Res. 2017, 116, 2707–2719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lee, E.-G.; Yu, L.; Kawano, S.; Huang, P.; Liao, M.; Kawase, O.; Zhang, G.; Zhou, J.; Fujisaki, K.; et al. Identification of the cross-reactive and species-specific antigens between Neospora caninum and Toxoplasma gondii tachyzoites by a proteomics approach. Parasitol. Res. 2011, 109, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.; Zhao, Y.; Shah, S.; Fox, B.A.; Rommereim, L.M.; Bzik, D.J. Co-existence of classical and alternative activation programs in macrophages responding to Toxoplasma gondii. Int. J. Parasitol. 2014, 44, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Martínez, A.; Basto, A.P.; Leitão, A.; Hemphill, A. Neospora caninum in non-pregnant and pregnant mouse models: Cross-talk between infection and immunity. Int. J. Parasitol. 2017, 47, 723–735. [Google Scholar] [CrossRef]
- Fereig, R.M.; Abdelbaky, H.H.; Mohamed, A.E.A.; Nishikawa, Y. Recombinant subunit vaccines against Toxoplasma gondii: Successful experimental trials using recombinant DNA and proteins in mice in a period from 2006 to 2018. J. Vet. Med. Animal Sci. 2018, 1, 1005. [Google Scholar]
- Nishikawa, Y. Towards a preventive strategy for neosporosis: Challenges and future perspectives for vaccine development against infection with Neospora caninum. J. Vet. Med. Sci. 2017, 79, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Zhang, N.Z.; Li, T.T.; He, J.J.; Elsheikha, H.M.; Zhu, X.Q. Advances in the Development of anti-Toxoplasma gondii vaccines: Challenges, opportunities, and perspectives. Trends Parasitol. 2019, 35, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Haldar, A.K.; Saka, H.A.; Piro, A.S.; Dunn, J.D.; Henry, S.C.; Taylor, G.A.; Frickel, E.M.; Valdivia, R.H.; Coers, J. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013, 9, e1003414. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.W.; Feng, G.S. Relationship between interferon-gamma, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991, 5, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Schwartzman, J.D.; Matsuura, T.; Kasper, L.H. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 1997, 94, 13955–13960. [Google Scholar] [CrossRef]
- Lees, M.P.; Fuller, S.J.; McLeod, R.; Boulter, N.R.; Miller, C.M.; Zakrzewski, A.M.; Mui, E.J.; Witola, W.H.; Coyne, J.J.; Hargrave, A.C.; et al. P2X7 receptor-mediated killing of an intracellular parasite, Toxoplasma gondii, by human and murine macrophages. J. Immunol. 2010, 184, 7040–7046. [Google Scholar] [CrossRef] [PubMed]
- Aline, F.; Bout, D.; Dimier-Poisson, I. Dendritic cells as effector cells: Gamma interferon activation of murine dendritic cells triggers oxygen-dependent inhibition of Toxoplasma gondii replication. Infect. Immun. 2002, 70, 2368–2374. [Google Scholar] [CrossRef] [PubMed]
- Collazo, C.M.; Yap, G.S.; Sempowski, G.D.; Lusby, K.C.; Tessarollo, L.; Woude, G.F.; Sher, A.; Taylor, G.A. Inactivation of LRG-47 and IRG-47 reveals a family of interferon gamma-inducible genes with essential, pathogen-specific roles in resistance to infection. J. Exp. Med. 2001, 194, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Collazo, C.M.; Yap, G.S.; Hieny, S.; Caspar, P.; Feng, C.G.; Taylor, G.A.; Sher, A. The function of gamma interferon-inducible GTP-binding protein IGTP in host resistance to Toxoplasma gondii is Stat1 dependent and requires expression in both hematopoietic and nonhematopoietic cellular compartments. Infect. Immun. 2002, 70, 6933–6939. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.O.; Khaminets, A.; Hunn, J.P.; Howard, J.C. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFN-gamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009, 5, e1000288. [Google Scholar] [CrossRef]
- Selleck, E.; Fentress, S.J.; Beatty, W.L.; Degrandi, D.; Pfeffer, K.; Iv, H.W.V.; MacMicking, J.D.; Sibley, L.D. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013, 9, e1003320. [Google Scholar] [CrossRef]
- Ceravolo, I.P.; Chaves, A.C.; Bonjardim, C.A.; Sibley, D.; Romanha, A.J.; Gazzinelli, R.T. Replication of Toxoplasma gondii, but not Trypanosoma cruzi, is regulated in human fibroblasts activated with gamma interferon: Requirement of a functional JAK/STAT pathway. Infect. Immun. 1999, 67, 2233–2340. [Google Scholar] [CrossRef]
- Hunter, C.A.; Sibley, L.D. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat. Rev. Microbiol. 2012, 10, 766–778. [Google Scholar] [CrossRef]
- Howard, J.C.; Hunn, J.P.; Steinfeldt, T. The IRG protein-based resistance mechanism in mice and its relation to virulence in Toxoplasma gondii. Curr. Opin. Microbiol. 2011, 14, 414–421. [Google Scholar] [CrossRef]
- MacMicking, J.D. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat. Rev. Immunol. 2012, 12, 367–382. [Google Scholar] [CrossRef]
- Ohshima, J.; Lee, Y.; Sasai, M.; Saitoh, T.; Su Ma, J.; Kamiyama, N.; Matsuura, Y.; Pann-Ghill, S.; Hayashi, M.; Ebisu, S. Role of mouse and human autophagy proteins in IFN-gamma-induced cell-autonomous responses against Toxoplasma gondii. J. Immunol. 2014, 192, 3328–3335. [Google Scholar] [CrossRef]
- Johnston, A.C.; Piro, A.; Clough, B.; Siew, M.; Winter, S.V.; Coers, J.; Frickel, E. Human GBP1 does not localize to pathogen vacuoles but restricts Toxoplasma gondii. Cell. Microbiol. 2016, 18, 1056–1064. [Google Scholar] [CrossRef]
- Gazzinelli, R.T.; Wysocka, M.; Hieny, S.; Scharton-Kersten, T.; Cheever, A.; Kuhn, R.; Müller, W.; Trinchieri, G.; Sher, A. In the absence of endogenous IL-10, mice acutely infected with Toxoplasma gondii succumb to a lethal immune response dependent of CD4+ T cells and accompanied by overproduction of IL-12, IFN-gamma, and TNF-alpha. J. Immunol. 1996, 157, 798–805. [Google Scholar]
- Nishikawa, Y.; Inoue, N.; Makala, L.; Nagasawa, H. A role for balance of interferon-gamma and interleukin-4 production in protective immunity against Neospora caninum infection. Vet. Parasitol. 2003, 116, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.O.; Beiting, D.P.; Tato, C.; John, B.; Oldenhove, G.; Lombana, C.G.; Pritchard, G.H.; Silver, J.S.; Bouladoux, N.; Stumhofer, J.S.; et al. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity 2012, 37, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Kuroda, Y.; Terkawi, M.A.; Mahmoud, M.E.; Nishikawa, Y. Immunization with Toxoplasma gondii peroxiredoxin 1 induces protective immunity against toxoplasmosis in mice. PLoS ONE 2017, 12, e0176324. [Google Scholar] [CrossRef] [PubMed]
- Carreño, L.J.; González, P.A.; Bueno, S.M.; Riedel, C.A.; Kalergis, A.M. Modulation of the dendritic cell-T-cell synapse to promote pathogen immunity and prevent autoimmunity. Immunotherapy 2011, 3, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Buxton, D.; Thomson, K.; Maley, S.; Wright, S.; Bos, H.J. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet. Rec. 1991, 129, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, E.M.; Ozkan, A.T.; Bowman, D.D. Protozoa: Toxoplasma gondii. Ency. Food Safety 2014, 2, 54–62. [Google Scholar]
- Yektaeian, N.; Malekpour, A.; Atapour, A.; Davoodi, T.; Hatam, G. Genetic immunization against toxoplasmosis: A review article. Microb. Pathog. 2021, 155, 104888. [Google Scholar] [CrossRef] [PubMed]
- Dubey, J.P.; Murata, F.H.A.; Cerqueira-Cézar, C.K.; Kwok, O.C.H.; Su, C. Economic and public health importance of Toxoplasma gondii infections in sheep: 2009–2020. Vet. Parasitol. 2020, 286, 109195. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.C. Advances and prospects for subunit vaccines against protozoa of veterinary importance. Vet. Parasitol. 2001, 101, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Wikman, M.; Friedman, M.; Pinitkiatisakul, S.; Andersson, C.; Hemphill, A.; Lövgren-Bengtsson, K.; Lundén, A.; Ståhl, S. General strategies for efficient adjuvant incorporation of recombinant subunit immunogens. Vaccine 2005, 23, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Nishikawa, Y. Peroxiredoxin 3 promotes IL-12 production from macrophages and partially protects mice against infection with Toxoplasma gondii. Parasitol. Int. 2016, 65, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Fereig, R.M.; Nishikawa, Y. Macrophage stimulation as a useful approach for immunoscreening of potential vaccine candidates against Toxoplasma gondii and Neospora caninum infections. Meth. Mol. Biol. 2022, 2411, 129–144. [Google Scholar] [CrossRef]
- Fereig, R.M.; Nishikawa, Y. Towards a preventive strategy for toxoplasmosis: Current trends, challenges, and future perspectives for vaccine development. Meth. Mol. Biol. 2016, 1404, 153–164. [Google Scholar]
- Khan, I.A.; Ouellette, C.; Chen, K.; Moretto, M. Toxoplasma: Immunity and pathogenesis. Curr. Clin. Microbiol. Rep. 2019, 6, 44–50. [Google Scholar] [CrossRef]
- Bonnardel, J.; Guilliams, M. Developmental control of macrophage function. Curr. Opin. Immunol. 2018, 50, 64–74. [Google Scholar] [CrossRef]
- Park, J.; Hunter, C.A. The role of macrophages in protective and pathological responses to Toxoplasma gondii. Parasite Immunol. 2020, 42, e12712. [Google Scholar] [CrossRef]
- Ryter, A. Relationship between ultrastructure and specific functions of macrophages. Comp. Immunol. Microbiol. Infect. Dis. 1985, 8, 119–133. [Google Scholar] [CrossRef]
- Ryan, D.G.; Knatko, E.V.; Casey, A.M.; Hukelmann, J.L.; Naidu, S.D.; Brenes, A.J.; Ekkunagul, T.; Baker, C.; Higgins, M.; Tronci, L.; et al. Nrf2 activation reprograms macrophage intermediary metabolism and suppresses the type I interferon response. iScience 2022, 25, 103827. [Google Scholar] [CrossRef] [PubMed]
- Robben, P.M.; Mordue, D.G.; Truscott, S.M.; Takeda, K.; Akira, S.; Sibley, L.D. Production of IL-12 by macrophages infected with Toxoplasma gondii depends on the parasite genotype. J. Immunol. 2004, 172, 3686–3694. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.; Butcher, B.A.; Lee, C.W.; Uematsu, S.; Akira, S.; Denkers, E.Y. Toxoplasma gondii genotype determines MyD88-dependent signaling in infected macrophages. J. Immunol. 2006, 177, 2584–2591. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.M.; Doherty, C.M.; Mercer, H.L.; Denkers, E.Y. Induction of IL-12p40 and type 1 immunity by Toxoplasma gondii in the absence of the TLR-MyD88 signaling cascade. PLoS Pathog. 2021, 17, e1009970. [Google Scholar] [CrossRef]
- Pyo, K.H.; Jung, B.K.; Xin, C.F.; Lee, Y.W.; Chai, J.Y.; Shin, E.H. Prominent IL-12 production and tumor reduction in athymic nude mice after Toxoplasma gondii lysate antigen treatment. Kor. J. Parasitol. 2014, 52, 605–612. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Bannai, H.; Xuan, X.; Nishikawa, Y. Toxoplasma gondii cyclophilin 18-mediated production of nitric oxide induces Bradyzoite conversion in a CCR5-dependent manner. Infect. Immun. 2009, 77, 3686–3695. [Google Scholar] [CrossRef] [PubMed]
- Pyo, K.H.; Lee, Y.W.; Lim, S.M.; Shin, E.H. Immune adjuvant effect of a Toxoplasma gondii profilin-like protein in autologous whole-tumor-cell vaccination in mice. Oncotarget 2016, 7, 74107–77419. [Google Scholar] [CrossRef]
- Debierre-Grockiego, F.; Campos, M.A.; Azzouz, N.; Schmidt, J.; Bieker, U.; Resende, M.G.; Mansur, D.S.; Weingart, R.; Schmidt, R.R.; Golenbock, D.T.; et al. Activation of TLR2 and TLR4 by glycosylphosphatidylinositols derived from Toxoplasma gondii. J. Immunol. 2007, 179, 1129–1137. [Google Scholar] [CrossRef]
- Niehus, S.; Smith, T.K.; Azzouz, N.; Campos, M.A.; Dubremetz, J.-F.; Gazzinelli, R.T.; Schwarz, R.T.; Debierre-Grockiego, F. Virulent and avirulent strains of Toxoplasma gondii which differ in their glycosylphosphatidylinositol content induce similar biological functions in macrophages. PLoS ONE 2014, 9, e85386. [Google Scholar] [CrossRef]
- Kucera, K.; Koblansky, A.A.; Saunders, L.P.; Frederick, K.B.; De La Cruz, E.M.; Ghosh, S.; Modis, Y. Structure-based analysis of Toxoplasma gondii profilin: A parasite-specific motif is required for recognition by Toll-like receptor 11. J. Mol. Biol. 2010, 403, 616–629. [Google Scholar] [CrossRef]
- Rosowski, E.E.; Lu, D.; Julien, L.; Rodda, L.; Gaiser, R.A.; Jensen, K.D.; Saeij, J.P. Strain-specific activation of the NF-kappaB pathway by GRA15, a novel Toxoplasma gondii dense granule protein. J. Exp. Med. 2011, 208, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Koblansky, A.A.; Jankovic, D.; Oh, H.; Hieny, S.; Sungnak, W.; Mathur, R.; Hayden, M.S.; Akira, S.; Sher, A.; Ghosh, S. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity 2013, 38, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.; Brenier-Pinchart, M.-P.; Yogavel, M.; Curt-Varesano, A.; Curt-Bertini, R.-L.; Hussain, T.; Kieffer-Jaquinod, S.; Couté, Y.; Pelloux, H.; Tardieux, I.; et al. A Toxoplasma dense granule protein, GRA24, modulates the early immune response to infection by promoting a direct and sustained host p38 MAPK activation. J. Exp. Med. 2013, 210, 2071–2086. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Standley, D.M.; Takashima, S.; Saiga, H.; Okuyama, M.; Kayama, H.; Kubo, E.; Ito, H.; Takaura, M.; Matsuda, T.; et al. A single polymorphic amino acid on Toxoplasma gondii kinase ROP16 determines the direct and strain-specific activation of Stat3. J. Exp. Med. 2009, 206, 2747–2760. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; An, R.; Chen, L.; Shen, Y.; Chen, Y.; Cheng, L.; Jiang, Z.; Zhang, A.; Yu, L.; Chu, D.; et al. Toxoplasma gondii virulence factor ROP18 inhibits the host NF-κB pathway by promoting p65 degradation. J. Biol. Chem. 2014, 289, 12578–12592. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.J.; Sibley, L.D. Rhoptries: An arsenal of secreted virulence factors. Curr. Opinion Microbiol. 2007, 10, 582–587. [Google Scholar] [CrossRef]
- Abe, C.; Tanaka, S.; Ihara, F.; Nishikawa, Y. Macrophage depletion prior to Neospora caninum infection results in severe neosporosis in mice. Clin. Vaccine Immunol. 2014, 21, 1185–1188. [Google Scholar] [CrossRef]
- Mota, C.M.; Oliveira, A.C.; Davoli-Ferreira, M.; Silva, M.V.; Santiago, F.M.; Nadipuram, S.M.; Vashisht, A.A.; Wohlschlegel, J.A.; Bradley, P.J.; Silva, J.S.; et al. Neospora caninum Activates p38 MAPK as an evasion mechanism against innate immunity. Front. Microbiol. 2016, 7, 1456. [Google Scholar] [CrossRef]
- He, X.; Gong, P.; Wei, Z.; Liu, W.; Wang, W.; Li, J. Peroxisome proliferator-activated receptor-γ-mediated polarization of macrophages in Neospora caninum. Exp. Parasitol. 2017, 178, 37–44. [Google Scholar] [CrossRef]
- Jin, X.; Gong, P.; Zhang, X.; Li, G.; Zhu, T.; Zhang, M.; Li, J. Activation of ERK signaling via TLR11 induces IL-12p40 production in peritoneal macrophages challenged by Neospora caninum. Front. Microbiol. 2017, 8, 1393. [Google Scholar] [CrossRef]
- Baszler, T.V.; Long, M.T.; McElwain, T.F.; Mathison, B.A. Interferon-gamma and interleukin-12 mediate protection to acute Neospora caninum infection in BALB/c mice. Int. J. Parasitol. 1999, 29, 1635–1646. [Google Scholar] [CrossRef]
- Débare, H.; Schmidt, J.; Moiré, N.; Ducournau, C.; Paguay, Y.D.A.; Schwarz, R.T.; Dimier-Poisson, I.; Debierre-Grockiego, F. In vitro cellular responses to Neospora caninum glycosylphosphatidylinositols depend on the host origin of antigen presenting cells. Cytokine 2019, 119, 119–128. [Google Scholar] [CrossRef]
- Li, S.; Gong, P.; Zhang, N.; Li, X.; Tai, L.; Wang, X.; Yang, Z.; Yang, J.; Zhu, X.; Zhang, X.; et al. 14-3-3 protein of Neospora caninum modulates host cell innate immunity through the activation of MAPK and NF-κB Pathways. Front. Microbiol. 2019, 25, 37. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shimoda, N.; Fereig, R.M.; Moritaka, T.; Umeda, K.; Nishimura, M.; Ihara, F.; Kobayashi, K.; Himori, Y.; Suzuki, Y.; et al. Neospora caninum dense granule protein 7 regulates the pathogenesis of neosporosis by modulating host immune response. Appl. Environ. Microbiol. 2018, 84, e01350-18. [Google Scholar] [CrossRef]
- Fereig, R.M.; Shimoda, N.; Abdelbaky, H.H.; Kuroda, Y.; Nishikawa, Y. Neospora GRA6 possesses immune-stimulating activity and confers efficient protection against Neospora caninum infection in mice. Vet. Parasitol. 2019, 267, 61–68. [Google Scholar] [CrossRef] [PubMed]
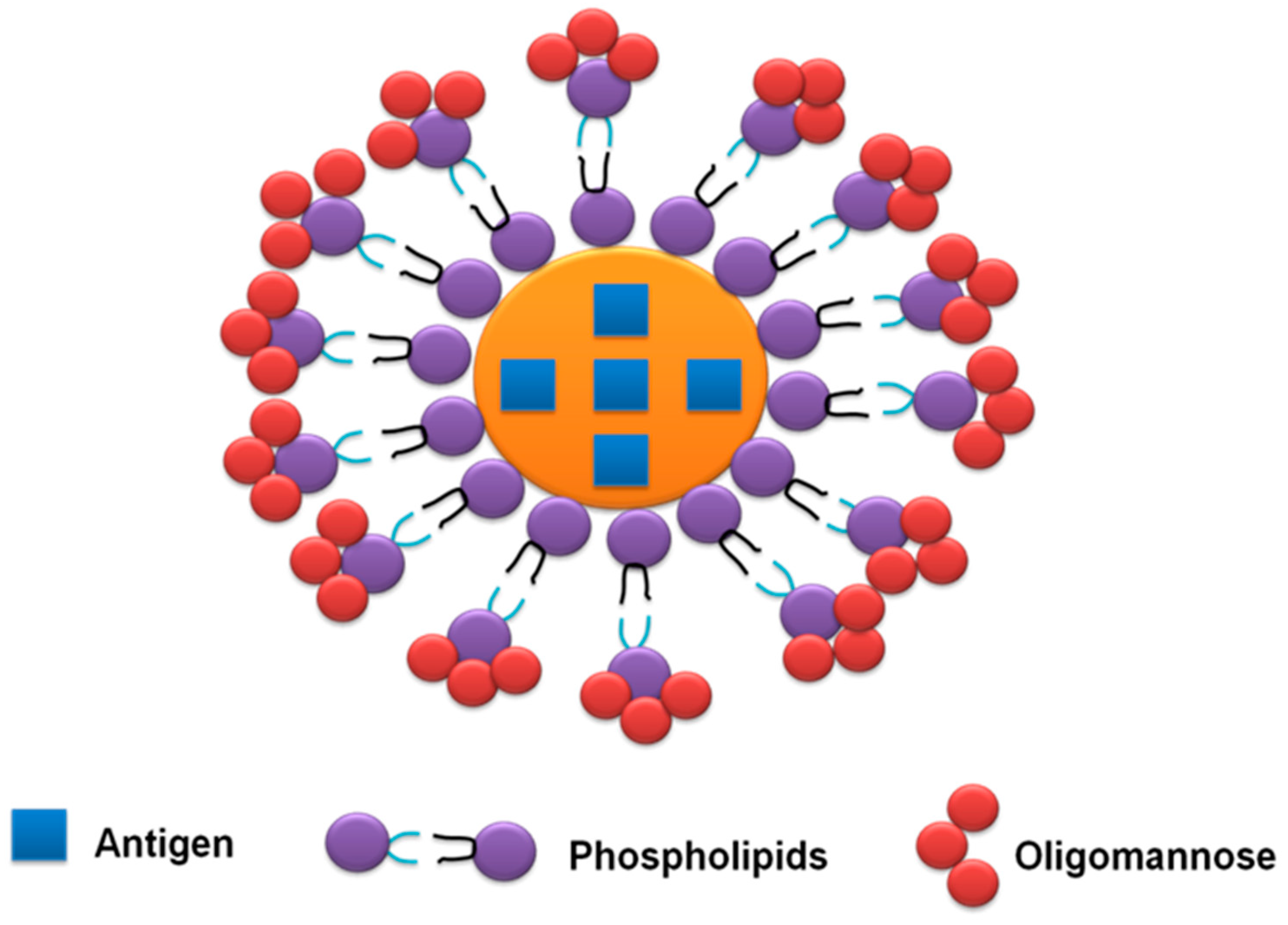
- Fereig, R.M.; Abdelbaky, H.H.; Kuroda, Y.; Nishikawa, Y. Critical role of TLR2 in triggering protective immunity with cyclophilin entrapped in oligomannose-coated liposomes against Neospora caninum infection in mice. Vaccine 2019, 37, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.K.; Geba, G.P.; Molfino, N. Investigational therapeutics targeting the IL-4/IL-13/STAT-6 pathway for the treatment of asthma. Eur. Respir. Rev. 2010, 19, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Martínez, A.; Basto, A.; Tanaka, S.; Ryser, L.T.; Nunes, T.; Ortega-Mora, L.-M.; Solís, D.A.; Leitao, A.; Hemphill, A. Immunization with a cocktail of antigens fused with OprI reduces Neospora caninum vertical transmission and postnatal mortality in mice. Vaccine 2019, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- O’Garra, A.; Murphy, K.M. From IL-10 to IL-12: How pathogens and their products stimulate APCs to induce T(H)1 development. Nat. Immunol. 2009, 10, 929–932. [Google Scholar] [CrossRef]
- Mun, H.-S.; Aosai, F.; Norose, K.; Chen, M.; Piao, L.-X.; Takeuchi, O.; Akira, S.; Ishikura, H.; Yano, A. TLR2 as an essential molecule for protective immunity against Toxoplasma gondii infection. Int. Immunol. 2003, 15, 1081–1087. [Google Scholar] [CrossRef]
- Mineo, T.W.; Oliveira, C.J.; Gutierrez, F.R.; Silva, J.S. Recognition by Toll-like receptor 2 induces antigen presenting cell activation and Th1 programming during infection by Neospora caninum. Immunol. Cell Biol. 2010, 88, 825–833. [Google Scholar] [CrossRef]
- Gibson, J.; Gow, N.; Wong, S.Y.C. Expression and functions of innate pattern recognition receptors in T and B cells. Immunol. Endocr. Metab. Agents. Med. Chem. 2010, 10, 11–20. [Google Scholar] [CrossRef]
- Rahman, M.M.; McFadden, G. Modulation of NF-kB signalling by microbial pathogens. Nat. Rev. Microbiol. 2011, 9, 291–306. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Joo, D.; Sun, S.C. NF-kB signaling in inflammation. Signal. Transduct. Target Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Marshall, E.S.; Elshekiha, H.M.; Hakimi, M.A.; Flynn, R.J. Toxoplasma gondii peroxiredoxin promotes altered macrophage function, caspase-1-dependent IL-1β secretion enhances parasite replication. Vet. Res. 2011, 42, 80. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Nishimura, M.; Punsantsogvoo, M.; Ibrahim, H.M.; Xuan, X.; Furuoka, H.; Nishikawa, Y. Immunological characterization of Neospora caninum cyclophilin. Parasitology 2012, 139, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Antibody-based vaccine strategies against intracellular pathogens. Curr. Opin. Immunol. 2018, 53, 74–80. [Google Scholar] [CrossRef]



| Toxoplasma Antigen | Macrophage Stimulation | References |
|---|---|---|
| Tachyzoites of type I virulent and type II avirulent strains | Tachyzoites of type II strain induced high macrophage IL-12 release than type I. | [65,66] |
| Cyst of ME49 strain | Oral T. gondii infection induced high recruitment of macrophages and IL-12p40 production in the lamina propria of infected mice via MyD88-independent type 1 immunity. | [67] |
| Tachyzoite lysate antigen (TLA) from RH strain | The treatment with TLA increased IL12p40 in a dose-dependent manner. | [68] |
| Cyclophilin 18 (TgCyp18) | Recombinant TgCyp18 increased production of IL-12 via CCR5 dependent pathway. | [69] |
| Profilin-like protein in T. gondii (TgPLP) | TgPLP treatment induced the excessive release of IL-12 dependent on MyD88 signaling pathway in BMMs. | [70] |
| Glycosylphosphatidylinositols (TgGPIs) | High IL-12 production in RAW 264.7 or thioglycolate elicited mouse macrophage cells treated with chemically synthesized TgGPIs. | [71,72] |
| Dense granule 15 (TgGRA15) | TgGRA15 is responsible for the induction of IL-12 secretion by infected mouse macrophages evidenced using KO parasites. | [74] |
| TgGRA24 | TgGRA24 is obviously assuming a significant part in inducing IL-12p40 synthesis, as its absence seriously compromises both IL-12 expression and liberation in mouse BMDM. | [76] |
| Profilin (TgPF) | TgPF is required for recognition by macrophage TLR11 and TLR12 and subsequent production of IL-12. | [73,75] |
| Rhoptry protein 16 (TgROP16) | TgROP16 in type I parasites impairs Stat3 activation and decreased IL-12 production as evidenced using wild type and KO parasites. | [77] |
| TgROP18 | IL-12 production was higher in supernatant of RAW264.7 cells infected with TgROP18 mutant or KO parasites than those of wild type associated with inhibition of host NF-kB. | [78] |
| Neospora Antigen | Macrophage Stimulation | References |
|---|---|---|
| N. caninum tachyzoites (Nc-1) | N. caninum triggered the obvious recruitment of macrophages at the scene of infection with concurrent elevated IL-12p40 release in infected murine macrophages in vitro. | [80] |
| Live tachyzoites and soluble extract of N. caninum (Nc-1) | Cultured mouse macrophages showed an elevated IL-12p40 production when stimulated by live tachyzoites and antigen extracts, which was dependent on the p38 MAPK pathway. | [81] |
| N. caninum tachyzoites (Nc-1) | In vitro, IL-12p40 was elevated in N. caninum infected macrophages of Dectin-1−/− mice than WT. | [82] |
| N. caninum tachyzoites (Nc-1) | N. caninum infection induced an obvious increase in IL-12p40 by mouse macrophages in vitro. This level was markedly decreased after abolishing the TLR11/MEK/ERK pathway. | [83] |
| N. caninum tachyzoites (Nc-1) | The treatment of N. caninum infected mice with rIL-12 greatly relieved encephalitis and reduced the parasite burden in the mouse brain. | [84] |
| N. caninum tachyzoites (Nc-Spain7 and Nc-Spain1H) | Cultured bovine macrophages showed higher ROS and IL-12p40 liberations when infected with Nc-Spain1H than those infected with Nc-Spain7. | [23] |
| Glycosylphosphatidylinositols (NcGPIs) | Chemically synthesized NcGPIs induced IL-12, TNF-α, and IL-1β secretion by a mouse cell line of macrophages and natural dendritic cells. | [85] |
| N. caninum 14-3-3 protein (Nc14-3-3) | Recombinant Nc14-3-3 activates the AKT and MAPK and signaling pathways, and elevated IL-12p40 production from mouse peritoneal macrophages. | [86] |
| NcGRA7 | Infection of mouse macrophages with NcGRA7KO parasites induced lower secretion of IL-12p40 than those of parental or complemented parasites. | [87] |
| Vaccine Antigen | Innate Immunity | Humoral Immunity | Cellular Immunity | Protection | References |
|---|---|---|---|---|---|
| TgPrx1 | Stimulated IL-12 production from naturally isolated mouse macrophages and IL-6 from RAW cell line | Generated high IgG1 and low IgG2 levels after the third immunization | Triggered IFN-γ production from splenocytes of immunized mice | Survival rate in immunized mice was higher (66.7%) than the control group that received PBS (27.8%) | [49] |
| TgPrx3 | Stimulated IL-12 production from naturally isolated mouse peritoneal macrophages | Generated high IgG1 and IgG2 levels after the third immunization | Triggered IFN-γ production from splenocytes of immunized mice | Immunized mice showed higher survival (55.6%) than the control group that received PBS (27.8%) | [57] |
| NcGRA6 | Induced excessive IL-12 liberation from naturally isolated mouse peritoneal macrophages | Generated only IgG1 after the third immunization | Triggered IFN-γ production from splenocytes of vaccinated mice | Higher survival rate was observed in vaccinated mice (91.7%) than the control group that received PBS (16.7%) | [88] |
| NcCyp-OML | Induced excessive IL-12 liberation from naturally isolated mouse peritoneal macrophages | Generated only IgG1 after the third immunization | Triggered IFN-γ production from splenocytes of vaccinated mice | Higher survival rate was noticed immunized mice (83.3%) than the control group that received PBS (16.7%) | [89] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fereig, R.M.; Omar, M.A.; Alsayeqh, A.F. Exploiting the Macrophage Production of IL-12 in Improvement of Vaccine Development against Toxoplasma gondii and Neospora caninum Infections. Vaccines 2022, 10, 2082. https://doi.org/10.3390/vaccines10122082
Fereig RM, Omar MA, Alsayeqh AF. Exploiting the Macrophage Production of IL-12 in Improvement of Vaccine Development against Toxoplasma gondii and Neospora caninum Infections. Vaccines. 2022; 10(12):2082. https://doi.org/10.3390/vaccines10122082
Chicago/Turabian StyleFereig, Ragab M., Mosaab A. Omar, and Abdullah F. Alsayeqh. 2022. "Exploiting the Macrophage Production of IL-12 in Improvement of Vaccine Development against Toxoplasma gondii and Neospora caninum Infections" Vaccines 10, no. 12: 2082. https://doi.org/10.3390/vaccines10122082
APA StyleFereig, R. M., Omar, M. A., & Alsayeqh, A. F. (2022). Exploiting the Macrophage Production of IL-12 in Improvement of Vaccine Development against Toxoplasma gondii and Neospora caninum Infections. Vaccines, 10(12), 2082. https://doi.org/10.3390/vaccines10122082







