Global Research Trend in Vaccine Design
Abstract
1. Introduction
1.1. Literature Review
1.2. Major Objectives of the Study
- To study the distribution of year-wise progress of worldwide research publication, total citation, and average citation pattern in vaccine design research.
- To find highly productive authors and authors with a creative life in vaccine design research.
- To know the highly productive organization in the area of vaccine design research.
- To discover the distribution of highly cited research papers
- To evaluate network visualization of co-occurrence of author keywords, citation of countries and co-citation of cited authors in the sphere of vaccine design.
1.3. Data and Methodology
2. Results
2.1. Distribution of Yearwise Progress of Publications, Total Citation and Average Citations
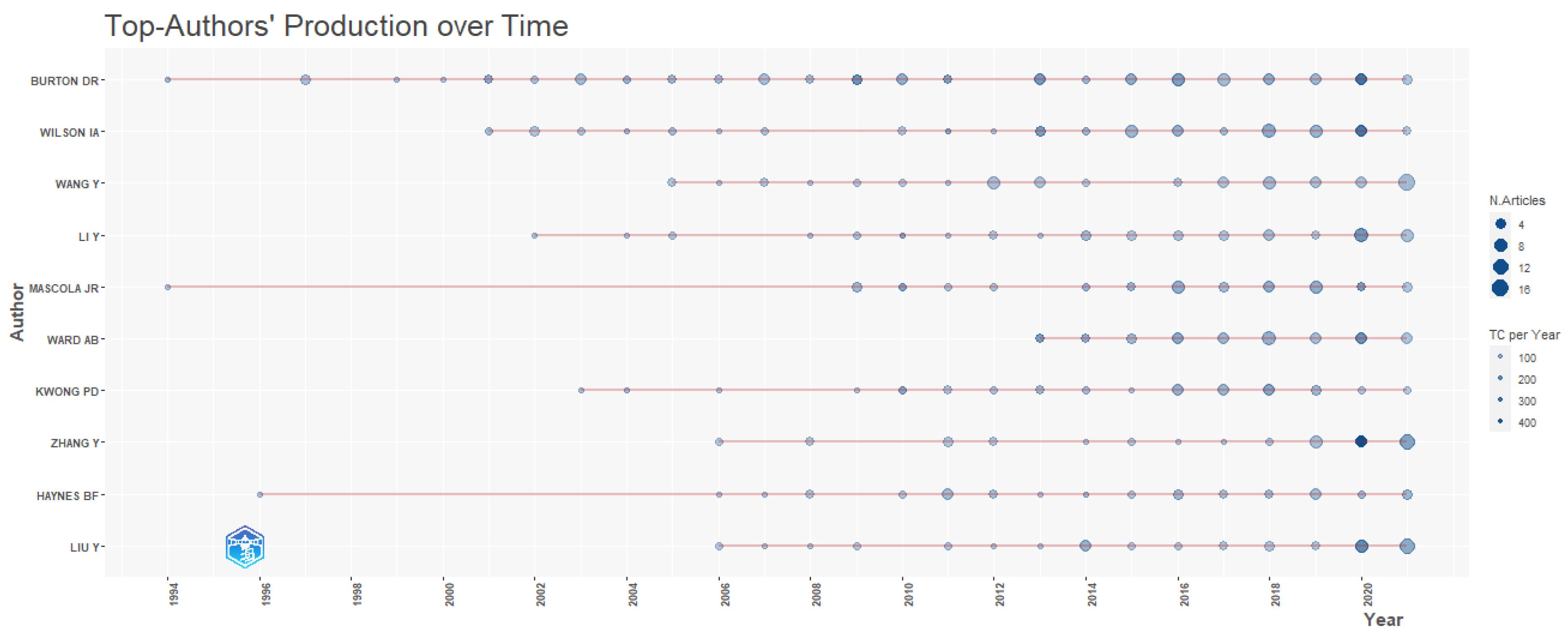
2.2. Distribution of Most Prolific Authors
2.3. Distribution of Highly Productive Organizations
2.4. Distribution of Highly Cited Documents
2.5. Analysis of Bibliometric Visualisation
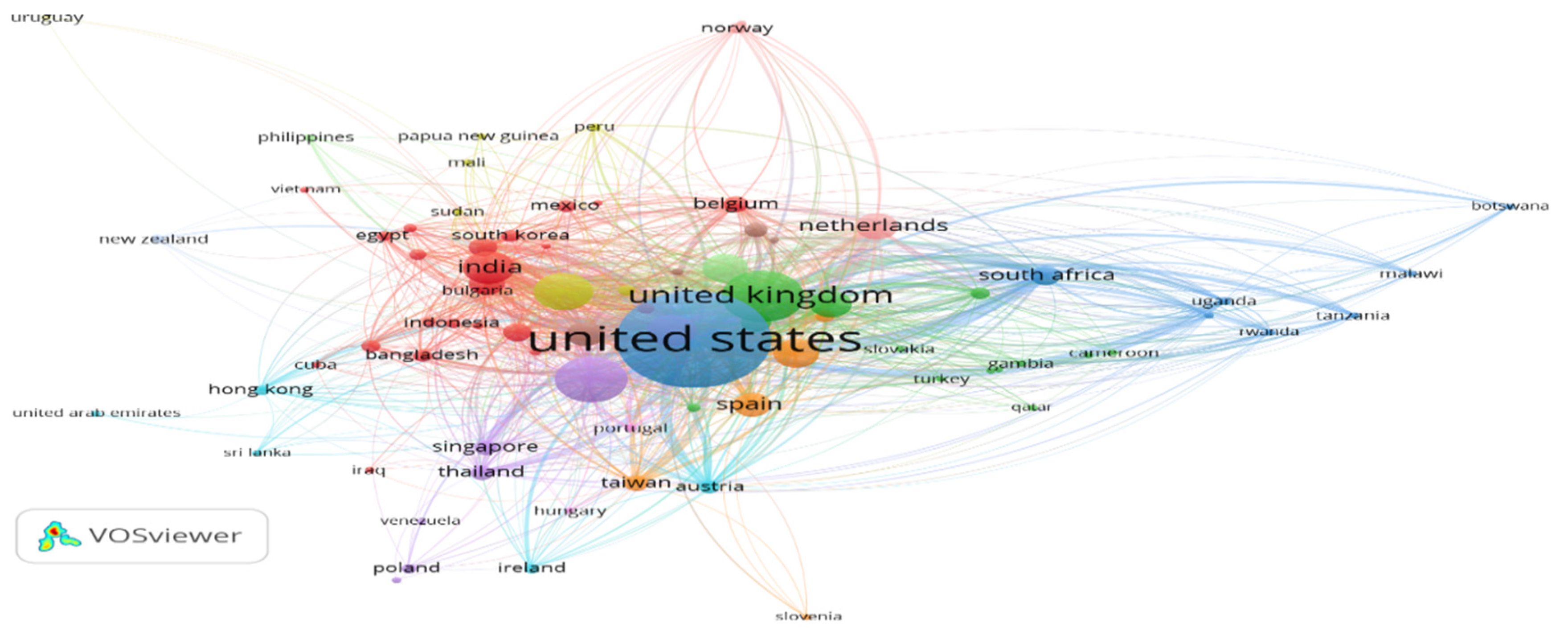
2.5.1. Citation Analysis When Unit of Analysis Is Countries
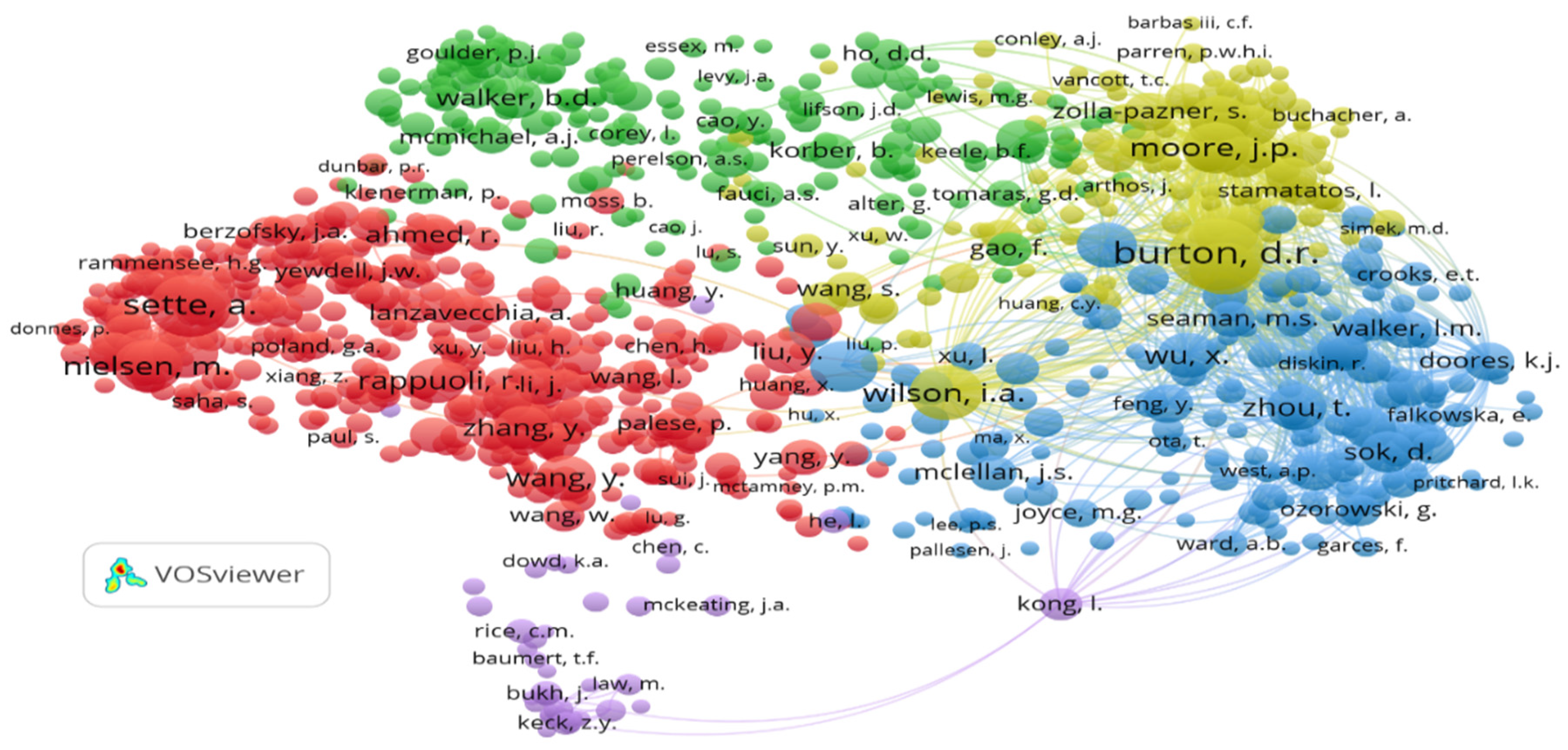
2.5.2. Network Visualisation of Co-Citation of Cited Authors
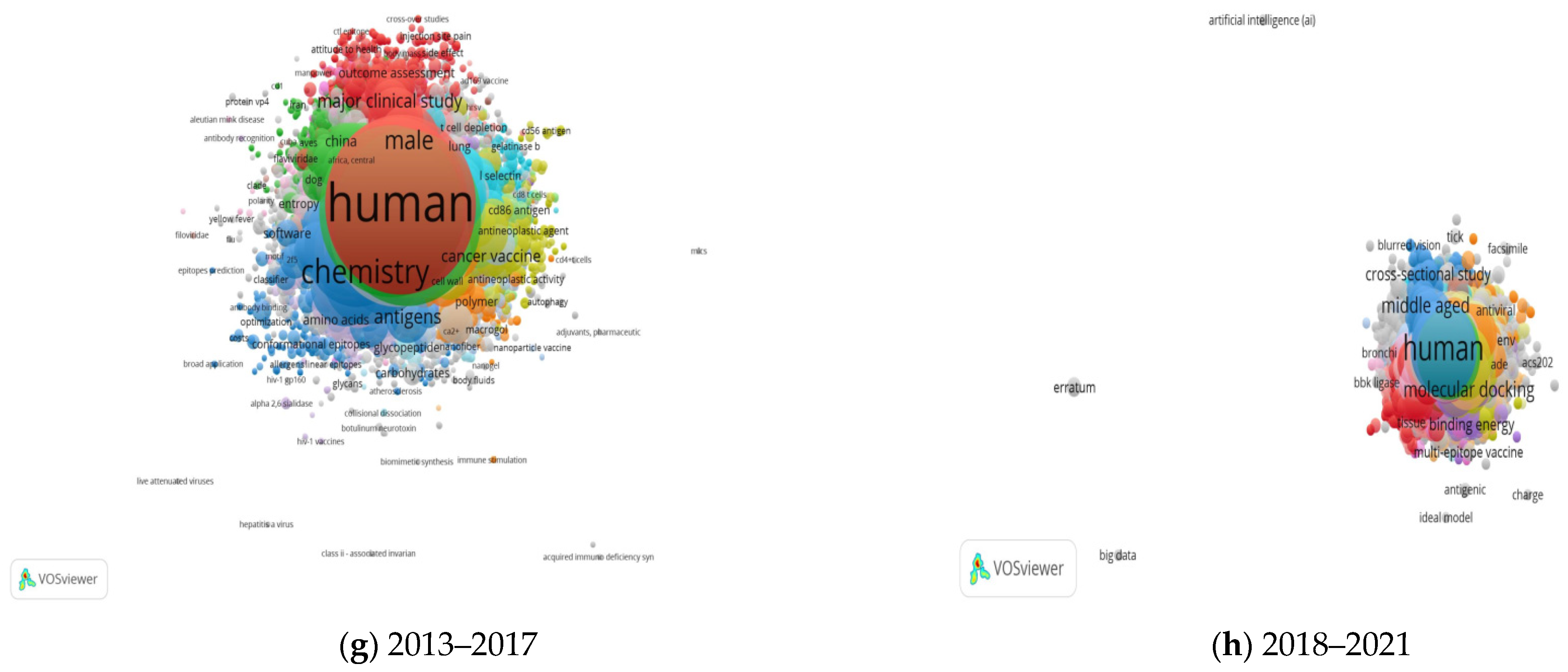
2.5.3. Network Visualization of Co-Occurrence of Keywords
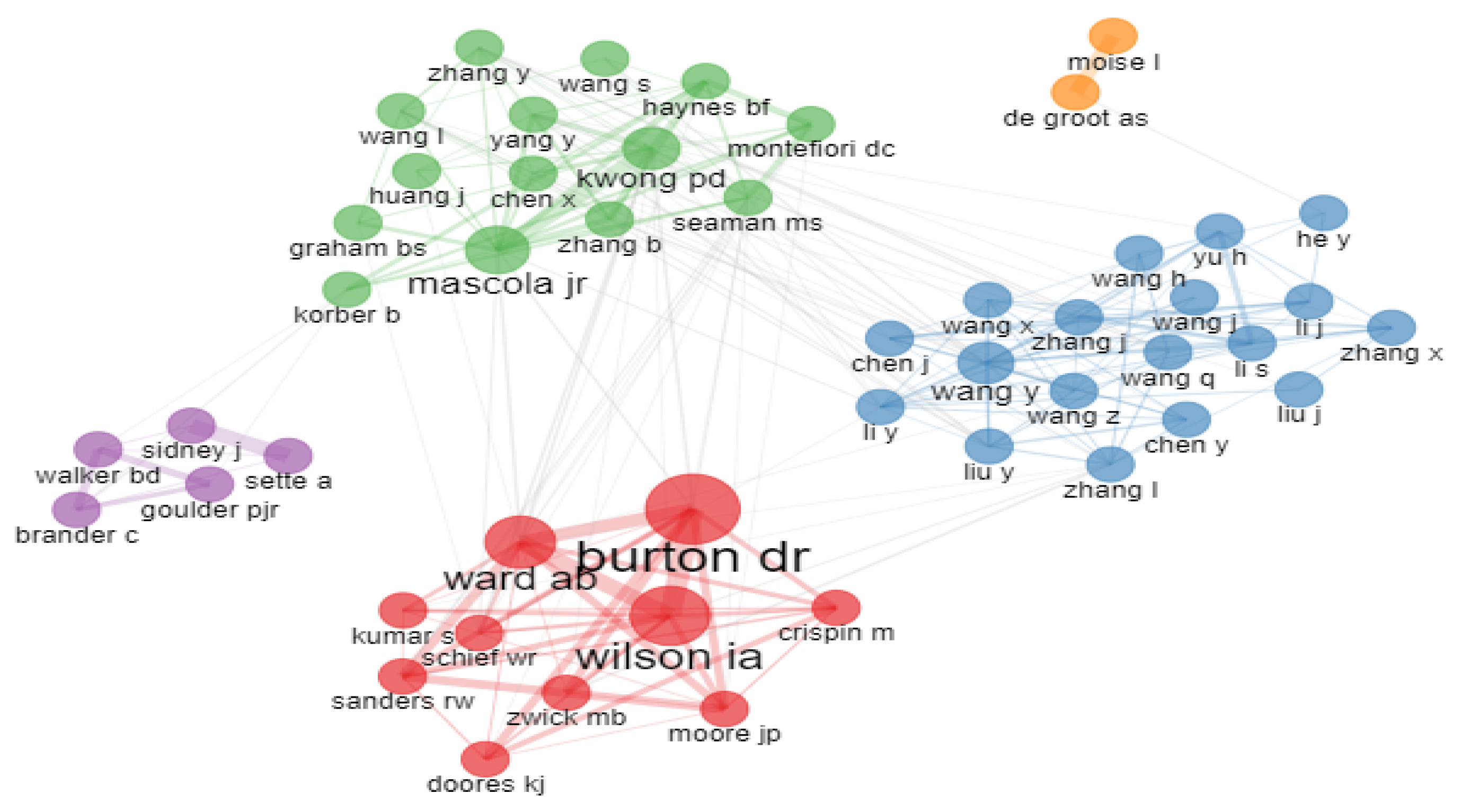
2.5.4. Collaboration Network of Authors
3. Discussion
Research Implications
4. Conclusions and Direction of Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NIH | National Institute of Health |
| HPV | Human Papilloma Virus |
| DNA | Deoxyribonucleic acid |
| IVV | Influenza virus vaccine |
| MMR | Measles, Mumps, Rubella |
| HVTN | HIV Vaccine Trials Network |
| INRAE | National Institute for Agriculture, Food and the Environment (France) |
| TP | Total Publications |
| TC | Total Citations |
| ACPP | Average Citation per Publication |
| SRI | Scripps Research Institute |
| NIAD | National Institute of Allergy and Infectious Diseases |
| CR | Cited Ratio |
| ACPY | Average Citation per year |
References
- Saleh, A.; Qamar, S.; Tekin, A.; Singh, R.; Kashyap, R. Vaccine development throughout history. Cureus 2021, 13, e16635. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.F.; Newman, M.J. Vaccine Design: The Subunit and Adjuvant Approach; Springer: Cham, Switzerland, 2012. [Google Scholar]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Six, A.; Bellier, B.; Thomas-Vaslin, V.; Klatzmann, D. Systems biology in vaccine design: Systems biology in vaccine design. Microb. Biotechnol. 2012, 5, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.V.; Sanz, E.; Sotolongo, G. Bibliometric study on vaccines (1990–1995) part I: Scientific production in Iberian-American countries. Scientometrics 1998, 43, 189–205. [Google Scholar] [CrossRef]
- Garg, K.C.; Kumar, S.; Madhavi, Y.; Bahl, M. Bibliometrics of global malaria vaccine research. Health Inf. Libr. J. 2009, 26, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, Y.; Cheng, Y.; Liu, L.; Yan, Z.; Tao, L.; Guo, X.; Luo, Y.; Yan, A. Technology resource, distribution, and development characteristics of global influenza virus vaccine: A patent bibliometric analysis. PLoS ONE 2015, 10, e0136953. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Yan, Z.; Ye, X. Multilevel analysis of international scientific collaboration network in the influenza virus vaccine field: 2006–2013. Sustainability 2018, 10, 1232. [Google Scholar] [CrossRef]
- Fernandes, S.; Jit, M.; Bozzani, F.; Griffiths, U.K.; Scott, J.A.G.; Burchett, H.E.D. A bibliometric analysis of systematic reviews on vaccines and immunisation. Vaccine 2018, 36, 2254–2261. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.M.; Sánchez, M.V.G.; Rivero, Y.M.; Ramos, R.C.; Díaz, I.Á. Global and Latin American scientific production related to pneumococcal vaccines. Scientometrics 2018, 115, 1549–1559. [Google Scholar] [CrossRef]
- Zhang, Y.; Quan, L.; Xiao, B.; Du, L. The 100 top-cited studies on vaccine: A bibliometric analysis. Hum. Vaccines Immunother. 2019, 15, 3024–3031. [Google Scholar] [CrossRef] [PubMed]
- Sarirete, A. A bibliometric analysis of COVID-19 vaccines and sentiment analysis. Procedia Comput. Sci. 2021, 194, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Murad, M.A.; Baig, M.; Hui, J. Research trends in COVID-19 vaccine: A bibliometric analysis. Hum. Vaccines Immunother. 2021, 17, 2367–2372. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T.; Haroon, H.; Ornos, E.D.B.; Malibary, H.; Hussain, A.; Baig, M.; Santali, E.Y.; Alestad, J.H.; Muzaheed, M.; Rabaan, A.A.; et al. Bibliometric analysis and network visualization mapping of global research in Q fever vaccine. F1000Research 2022, 11, 364. [Google Scholar] [CrossRef]
- Nye, J.; D’Souza, M.P.; Hu, D.; Ghosh, D. Research productivity and collaboration of the NIH-funded HIV vaccine trials network: A bibliometric analysis. Heliyon 2021, 7, e06005. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, M.; Mr, R. Comparative study of scientific articles on MMR vaccines published from India over the last few decades. Indian J. Biochem. Biophys. Available online: http://nopr.niscpr.res.in/handle/123456789/59764 (accessed on 9 June 2022).
- Bhanage, D.A.; Pawar, A.V. Bibliometric survey of IT infrastructure management to avoid failure conditions. Inf. Discov. Deliv. 2021, 49, 45–56. [Google Scholar] [CrossRef]
- Kore, M.; Nilesh Naik, D.; Chaudhari, D. A bibliometric approach to track research trends in computer-aided early detection of cancer using biomedical imaging techniques. J. Sci. Res. 2022, 10, 318–327. [Google Scholar] [CrossRef]
- Sinha, P.K.; Bhushan Sahoo, S.; Gajbe, S.B.; Chakrabory, K.; Mahato, S.S. Altmetrics research progress: A bibliometric analysis and visualization. J. Sci. Res. 2020, 9, 300–309. [Google Scholar] [CrossRef]
- Trivedi, D.; Majumder, N.; Pandya, M.; Bhatt, A.; Chaudhari, S.P. Evaluating the global research productivity on domestic violence: A bibliometric visualisation analysis. Collect. Curation, 2022; ahead of print. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Chhtrapati, D.; Chaudhari, S.P.; Mevada, D.; Bhatt, A.; Trivedi, D. Research productivity and network visualization on digital evidence: A bibliometric study. Sci. Technol. Libr. 2021, 40, 358–372. [Google Scholar] [CrossRef]
- Gangurde, A.; Mankar, P.; Chaudhari, D.; Pawar, A. A systematic bibliometric analysis of hate speech detection on social media sites. J. Sci. Res. 2022, 11, 100–111. [Google Scholar] [CrossRef]
- Mukherjee, B. Analysis of global research trends in coronaviruses: A bibliometric investigation. J. Sci. Res. 2020, 9, 185–194. [Google Scholar] [CrossRef]
- Ellegaard, O.; Wallin, J.A. The bibliometric analysis of scholarly production: How great is the impact? Scientometrics 2015, 105, 1809–1831. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M. Quantitative analysis of international collaboration on covid-19: Indian perspective. Indian J. Biochem. Biophys. 2020, 57, 439–443. [Google Scholar]

| Year | Total Publications (TP) | Total Citations (TC) | Mean | Average Citation Per Publications (ACPP) | h-Index |
|---|---|---|---|---|---|
| 1983–1991 | 84 | 4407 | 62.51 | 52.46 | 26 |
| 1992–2001 | 462 | 29,131 | 60.01 | 63.05 | 88 |
| 2002–2011 | 1570 | 91,249 | 59.96 | 58.12 | 139 |
| 2012 | 235 | 8100 | 3.45 | 34.47 | 47 |
| 2013 | 237 | 10,092 | 4.73 | 42.58 | 50 |
| 2014 | 279 | 9199 | 4.12 | 32.97 | 51 |
| 2015 | 270 | 8007 | 4.24 | 29.66 | 49 |
| 2016 | 271 | 8627 | 5.31 | 31.83 | 46 |
| 2017 | 277 | 6307 | 4.55 | 22.77 | 41 |
| 2018 | 341 | 7552 | 5.54 | 22.15 | 43 |
| 2019 | 331 | 5444 | 5.48 | 16.45 | 36 |
| 2020 | 477 | 14,145 | 14.83 | 29.65 | 45 |
| 2021 | 545 | 3499 | 6.42 | 6.42 | 27 |
| Total/Average | 5379 | 205,759 | 18.55 | 38.25 |
| Author Name | TP | TC | h-Index | ACPP | Affiliated Institution | Country |
|---|---|---|---|---|---|---|
| Burton, D.R. | 86 | 18,449 | 57 | 214.52 | Massachusetts Institute of Technology | USA |
| Wilson, I.A. | 63 | 7926 | 37 | 125.81 | Scripps Research Institute | USA |
| Ward, A.B. | 48 | 4814 | 28 | 100.29 | Scripps Research Institute | USA |
| Sette, A. | 39 | 3267 | 23 | 83.77 | Aligning Science Across Parkinson’s (ASAP) | USA |
| Mascola, J.R. | 38 | 5264 | 31 | 138.53 | National Institute of Allergy and Infectious Diseases (NIAID) | USA |
| De Groot, A.S. | 37 | 952 | 14 | 25.73 | University of Rhode Island | USA |
| Kwong, P.D. | 37 | 5277 | 30 | 142.62 | Columbia University | USA |
| Sanders, R.W. | 34 | 3582 | 18 | 105.35 | Weill Cornell Medicine | USA |
| Walker, B.D. | 32 | 4528 | 26 | 141.5 | Massachusetts Institute of Technology | USA |
| Montefiori, D.C. | 28 | 1993 | 19 | 71.18 | Duke University Medical Centre | USA |
| Affiliations | TP | TC | h-Index | CR | ACPP | Country |
|---|---|---|---|---|---|---|
| National Institutes of Health NIH | 266 | 17,243 | 68 | 96.6 | 64.82 | USA |
| Scripps Research Institute | 213 | 26,434 | 74 | 97.7 | 124.1 | USA |
| National Institute of Allergy and Infectious Diseases NIAID | 197 | 13,612 | 58 | 97 | 69.1 | USA |
| University of Oxford | 163 | 11,081 | 53 | 93.3 | 67.98 | UK |
| Harvard Medical School | 141 | 13,550 | 57 | 97.9 | 96.1 | USA |
| Massachusetts Institute of Technology | 132 | 12,289 | 54 | 99.2 | 93.1 | USA |
| Massachusetts General Hospital | 132 | 12,640 | 58 | 98.5 | 95.76 | USA |
| University of Washington | 110 | 11,309 | 46 | 97.3 | 102.81 | USA |
| University of Melbourne | 95 | 5066 | 34 | 99 | 53.33 | Australia |
| Howard Hughes Medical Institute | 72 | 7782 | 44 | 95.8 | 108.08 | USA |
| Article Title | TC | Name of Source | Year | ACPY |
|---|---|---|---|---|
| Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: Implications for the microbial “pan-genome” by Tettelin, H. et al. | 1398 | Proceedings of the National Academy of Sciences of the United States of America | 2005 | 82.24 |
| Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target by Burton, D.R. et al. | 1350 | Science | 2009 | 103.85 |
| Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1 by Wu, X. et al. | 1280 | Science | 2010 | 106.67 |
| Synthetic peptide vaccine design: Synthesis and properties of a high-density multiple antigenic peptide system by Tam, J.P. | 1184 | Proceedings of the National Academy of Sciences of the United States of America | 1988 | 34.82 |
| Interferons and viruses: An interplay between induction, signaling, antiviral responses and virus countermeasures by Randall, R.E. et al. | 1171 | Journal of General Virology | 2008 | 83.64 |
| T-cell quality in memory and protection: Implications for vaccine design by Seder, R.A. et al. | 1135 | Nature Reviews Immunology | 2008 | 81.07 |
| Broad neutralization coverage of HIV by multiple highly potent antibodies by Walker, L.M. et al. | 1113 | Nature | 2011 | 101.18 |
| Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody by Burton, D.R. et al. | 962 | Science | 1994 | 33.17 |
| A neutralizing antibody selected from plasma cells that bind to group 1 and group 2 influenza A hemagglutinins by Corti, D. et al. | 852 | Science | 2011 | 77.45 |
| CD8+ T-cell responses to different HIV proteins have discordant associations with viral load by Kiepiela, P. et al. | 824 | Nature Medicine | 2007 | 54.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trivedi, D.; Chaudhari, S.P.; Bhatt, A.; Pathak, M. Global Research Trend in Vaccine Design. Vaccines 2022, 10, 2034. https://doi.org/10.3390/vaccines10122034
Trivedi D, Chaudhari SP, Bhatt A, Pathak M. Global Research Trend in Vaccine Design. Vaccines. 2022; 10(12):2034. https://doi.org/10.3390/vaccines10122034
Chicago/Turabian StyleTrivedi, Dharmendra, Shanti P. Chaudhari, Atul Bhatt, and Manohar Pathak. 2022. "Global Research Trend in Vaccine Design" Vaccines 10, no. 12: 2034. https://doi.org/10.3390/vaccines10122034
APA StyleTrivedi, D., Chaudhari, S. P., Bhatt, A., & Pathak, M. (2022). Global Research Trend in Vaccine Design. Vaccines, 10(12), 2034. https://doi.org/10.3390/vaccines10122034







