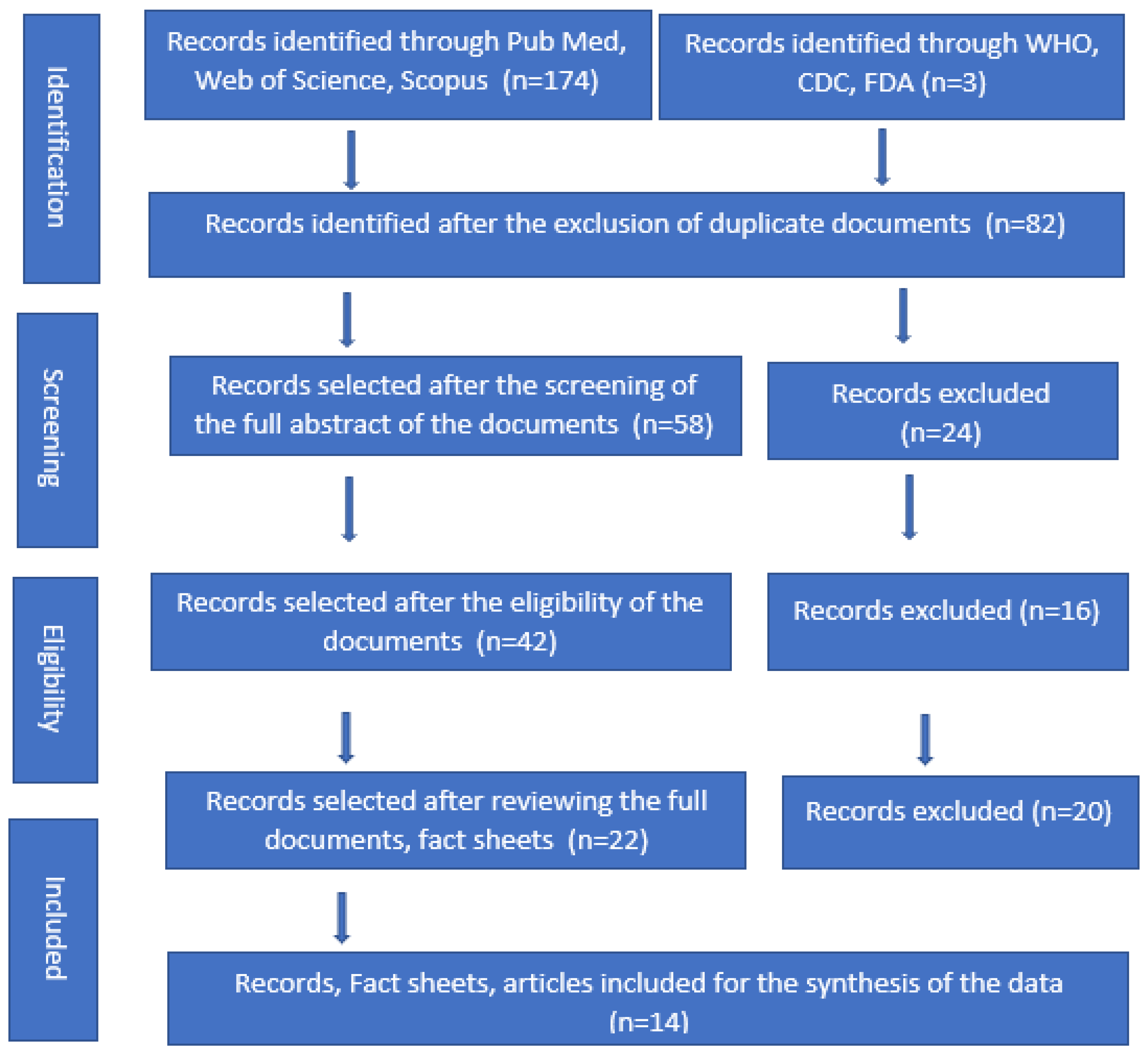
Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications and Adverse Effects of JYNNEOS and ACAM2000 Monkeypox Vaccines
Abstract
1. Introduction
2. Materials and Methods
3. Results
| Characteristics | JYNNEOS Vaccine | ACAM2000 Vaccine |
|---|---|---|
| Immunogenicity/duration of immunity | Two weeks after the second dose of JYNNEOS [20] | Four weeks after the single dose of ACAM2000 [17] |
| Neutralizing antibodies | At day 14, the mean titer of neutralizing antibodies was 16.2 [19]. At week 6, the mean titer of neutralizing antibodies was 153.5 [19] | At day 14, the mean titer of neutralizing antibodies by ACAM2000 was 16.2 [19]. At week 4, the mean titer of neutralizing antibodies was 79.3 [19] |
| Risks in pregnancy, infancy and in children | JYNNEOS may be administered during pregnancy, breastfeeding and infancy [8]. This vaccine is off-label for children and pregnant women [15,20] | ACAM2000 has a risk of adverse effects and is contraindicated during pregnancy, breastfeeding and in infants [8,17] |
| Contraindication | For the emergency uses of JYNNEOS, no contraindications are identified. It is contraindicated for people with a history of atopic dermatitis, an allergy to one of the vaccine components. JYNNEOS is safe in persons with immunocompromising conditions [8,16,20] | Pregnancy, breastfeeding, smoking, hypertension, diabetes mellitus, coronary artery disease, cardiomyopathy, people with allergic reactions and anaphylaxis. ACAM2000 is contraindicated in people with immunosuppression conditions, e.g., “leukaemia, lymphoma, human immunodeficiency virus infection, and acquired immune deficiency syndrome (AIDS)” [16,17]. |
| Common adverse effects | Local: pain, redness, swelling, itching, hyperpigmentation, skin discoloration Systemic: fatigue, headache, myalgias, nausea, chills, fever [15,20] | Local: pain, redness, swelling and itching at the site of injection. Systemic: fatigue, fever, muscle pain, headache, lymph node enlargement, malaise, arm soreness, body aches, skin rash, nausea, vomiting, diarrhea, swelling of the face, dizziness and shortness of breath [16,17] |
| Severe adverse effects | No risk of severe adverse effects such as myopericarditis or cardiomyopathy in recipients of JYNNEOS [15,20] | Increased risk of myopericarditis and cardiomyopathy [16,17] |
4. Discussion
5. Study Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meo, S.A.; Al-Khlaiwi, T.; Aljofan, Z.F.; Alanazi, A.I.; Meo, A.S. Public Perceptions of the Emerging Human Monkeypox Disease and Vaccination in Riyadh, Saudi Arabia: A Cross-Sectional Study. Vaccines 2022, 10, 1534. [Google Scholar] [CrossRef] [PubMed]
- Centres for Disease Control and Prevention (CDC). 2022 Monkeypox Outbreak Global Map. Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html (accessed on 6 November 2022).
- Jezek, Z.; Grab, B.; Szczeniowski, M.V.; Paluku, K.M.; Mutombo, M. Human monkeypox: Secondary attack rates. Bull. World Health Organ. 1988, 66, 465–470. [Google Scholar] [PubMed]
- Kozlov, M. How deadly is monkeypox? What scientists know. Nature 2022, 609, 663. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Monkeypox. Available online: https://www.who.int/news-room/fact-sheets/detail/monkeypox (accessed on 6 November 2022).
- See, K.C. Vaccination for Monkeypox Virus Infection in Humans: A Review of Key Considerations. Vaccines 2022, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration FDA-US. Monkeypox Update: FDA Authorizes Emergency Use of JYNNEOS Vaccine to Increase Vaccine Supply. Available online: https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply (accessed on 4 October 2022).
- Abdelaal, A.; Reda, A.; Lashin, B.I.; Katamesh, B.E.; Brakat, A.M.; Al-Manaseer, B.M.; Kaur, S.; Asija, A.; Patel, N.K.; Basnyat, S.; et al. Preventing the Next Pandemic: Is Live Vaccine Efficacious against Monkeypox, or Is There a Need for Killed Virus and mRNA Vaccines? Vaccines 2022, 10, 1419. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Safety of Smallpox Vaccines–December. 2015. Available online: https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/smallpox-vaccines (accessed on 6 October 2022).
- Food & Drug Administration US (FDA). ACAM2000 (Smallpox Vaccine) Questions and Answers. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/acam2000-smallpox-vaccine-questions-and-answers#acam (accessed on 5 October 2022).
- Centre for Disease Control Prevention (CDC). Vaccine Information Statements (VISs) Smallpox/Monkeypox VIS. Available online: https://www.cdc.gov/vaccines/hcp/vis/vis-statements/smallpox-monkeypox.html#smallpox-vaccines (accessed on 5 October 2022).
- Clarivate Analytics. Available online: https://www.webofscience.com/wos/woscc/basic-search (accessed on 2 October 2022).
- National Library of Medicine. Pub Med. JYNNEOS and ACAM2000 Vaccines. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=JYNNEOS+and+ACAM2000+Vaccines&sort=pubdate (accessed on 1 October 2022).
- Ahmed, S.F.; Sohail, M.S.; Quadeer, A.A.; McKay, M.R. Vaccinia-Virus-Based Vaccines Are Expected to Elicit Highly Cross-Reactive Immunity to the 2022 Monkeypox Virus. Viruses 2022, 14, 1960. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet: For Healthcare Providers Administering Vaccine: Emergency Use Authorization of Jynneos (Smallpox and Monkeypox Vaccine, Live, Non-Replicating). Available online: https://www.fda.gov/media/160773/download (accessed on 2 October 2022).
- Rao, A.K.; Petersen, B.W.; Whitehill, F.; Razeq, J.H.; Isaacs, S.N.; Merchlinsky, M.J.; Campos-Outcalt, D.; Morgan, R.L.; Damon, I.; Sánchez, P.J.; et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 734–742, Erratum in MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 886. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control Prevention (CDC). ACAM2000 Vaccine. Available online: https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/acam2000-vaccine.html (accessed on 4 October 2022).
- de Rouvroit, A.L.; Heegaard, E.D. Total costs associated with replicating and non-replicating smallpox vaccines. Glob. Secur. Health Sci. Policy 2016, 1, 3–9. [Google Scholar] [CrossRef]
- Pittman, P.R.; Hahn, M.; Lee, H.S.; Koca, C.; Samy, N.; Schmidt, D.; Hornung, J.; Weidenthaler, H.; Heery, C.R.; Meyer, T.P.H.; et al. Phase 3 Efficacy Trial of Modified Vaccinia Ankara as a Vaccine against Smallpox. N. Engl. J. Med. 2019, 381, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Control Prevention (CDC). JYNNEOS Vaccine. Available online: https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/jynneos-vaccine.html (accessed on 4 October 2022).
- Petersen, B.W. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses—Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, B.M. Newer Poxvirus Vaccine Is Recommended. JAMA 2022, 328, 123. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.B.; Ray, L.C.; Kugeler, K.J.; Fothergill, A.; White, E.B.; Canning, M.; Farrar, J.L.; Feldstein, L.R.; Gundlapalli, A.V.; Houck, K.; et al. Incidence of Monkeypox Among Unvaccinated Persons Compared with Persons Receiving ≥1 JYNNEOS Vaccine Dose—32 U.S. Jurisdictions, July 31–September 3, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022, 71, 1278–1282. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | JYNNEOS Vaccine | ACAM2000 Vaccine |
|---|---|---|
| Generic name | Smallpox and monkeypox vaccine live non-replicating | Smallpox vaccinia, vaccine, live replicating |
| Brand name | JYNNEOS/Imamune/Imvanex, MVA Vaccinia Ankara | ACAM2000 Emergent Bio Solutions |
| Types of vaccine | Third-generation vaccine [14] | Second-generation vaccine [14] |
| Manufacturer, city and country | Bavarian Nordic, Hørsholm, Denmark | Gaithersburg, MD, USA and Sanofi Pasteur Biologics, LLC [9] |
| Vaccine production | Prepared using weakened live vaccinia virus, cannot cause smallpox or monkeypox. Produced from “strain-modified vaccinia Ankara-Bavarian Nordic (MVA-BN), an attenuated, non-replicating orthopoxvirus” [2] | The vaccine is made from a virus called vaccinia is a “pox”-type virus [7] |
| Replication-competent | A live-attenuated, non-replicating vaccine [8] | A live-attenuated replicating vaccine [8] |
| Produced from | “Strain-modified Vaccinia Ankara-Bavarian Nordic (MVA-BN), an attenuated, non-replicating orthopoxvirus” [7] | ACAM2000 is a “live vaccinia virus derived by plaque purification from a previously calf lymph-produced vaccine (Dryvax) and manufactured in Vero cells” [9] |
| Effective in age groups | Active immunization for the prevention of monkeypox disease in individuals less than 18 years, and 18 years of age and older [15] | Not effective in infants mainly aged <1 year, although vaccination is contraindicated [16] |
| FDA approval | FDA granted an emergency use authorization (EUA) for the emergency use of JYNNEOS/interim authorized, on 9 August 2022 [7], and a supplementary letter was issued on 2 September 2022 | Interim authorized 28 June 2022, for people aged 1 year and older for monkeypox prophylaxis, CDC, ACAM2000 vaccine [17] |
| Dose(s) | Age <18 years: subcutaneous injection, 2 doses (0.5 mL each) 4 weeks (28 days) apart. Age ≥18 years: intradermal injection, two doses (0.1 mL each) 4 weeks (28 days) apart [15] | A single dose of 0.0025 mL droplet of reconstituted vaccine [17] |
| Booster shots | “Booster dose is recommended every two years for people with high-virulent orthopoxvirus, and every ten years for those in contact with low-virulent strains” [8,16] | High risk of exposure, researchers working in laboratories handling “variola virus, and monkeypox virus” should receive a booster dose every three years [16] |
| Route of administration | Subcutaneous injection [15] | Need a trained person, percutaneous route (scarification), prick the skin several times with a droplet of the vaccine. ACAM2000 cannot be given through subcutaneous, intradermal, intramuscular or intravenous routes [17] |
| Vaccination cost | The complete vaccination cost per person is about USD 115 [18] | The complete vaccination cost per person is about USD 139 [18]. |
| Storage | Stored at −15 °C to −25 °C, once thawed the vaccine may be kept at +2 °C to +8 °C for 8 weeks [15] | Stored at −15 °C to −25 °C, shipment at −10 °C. After reconstitution use within 6–8 h at room temperature 20–25 °C. Reconstituted vaccine stored at 2–8 °C no longer than 30 days [17] |
| Mechanism of action | Produces “humoral and cellular immune responses to orthopoxviruses, neutralizing antibody for the prevention of smallpox and monkeypox” [15] | ACAM2000 vaccine stimulates the immune system to develop antibodies and cells in the blood to fight against infection [10] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meo, S.A.; Al-Masri, A.A.; Klonoff, D.C.; Alshahrani, A.N.; Al-khlaiwi, T. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications and Adverse Effects of JYNNEOS and ACAM2000 Monkeypox Vaccines. Vaccines 2022, 10, 1971. https://doi.org/10.3390/vaccines10111971
Meo SA, Al-Masri AA, Klonoff DC, Alshahrani AN, Al-khlaiwi T. Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications and Adverse Effects of JYNNEOS and ACAM2000 Monkeypox Vaccines. Vaccines. 2022; 10(11):1971. https://doi.org/10.3390/vaccines10111971
Chicago/Turabian StyleMeo, Sultan Ayoub, Abeer A. Al-Masri, David C. Klonoff, Abdullah Nasser Alshahrani, and Thamir Al-khlaiwi. 2022. "Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications and Adverse Effects of JYNNEOS and ACAM2000 Monkeypox Vaccines" Vaccines 10, no. 11: 1971. https://doi.org/10.3390/vaccines10111971
APA StyleMeo, S. A., Al-Masri, A. A., Klonoff, D. C., Alshahrani, A. N., & Al-khlaiwi, T. (2022). Comparison of Biological, Pharmacological Characteristics, Indications, Contraindications and Adverse Effects of JYNNEOS and ACAM2000 Monkeypox Vaccines. Vaccines, 10(11), 1971. https://doi.org/10.3390/vaccines10111971






