Impact of COVID-19 Vaccination on Seroprevalence of SARS-CoV-2 among the Health Care Workers in a Tertiary Care Centre, South India
Abstract
:1. Introduction
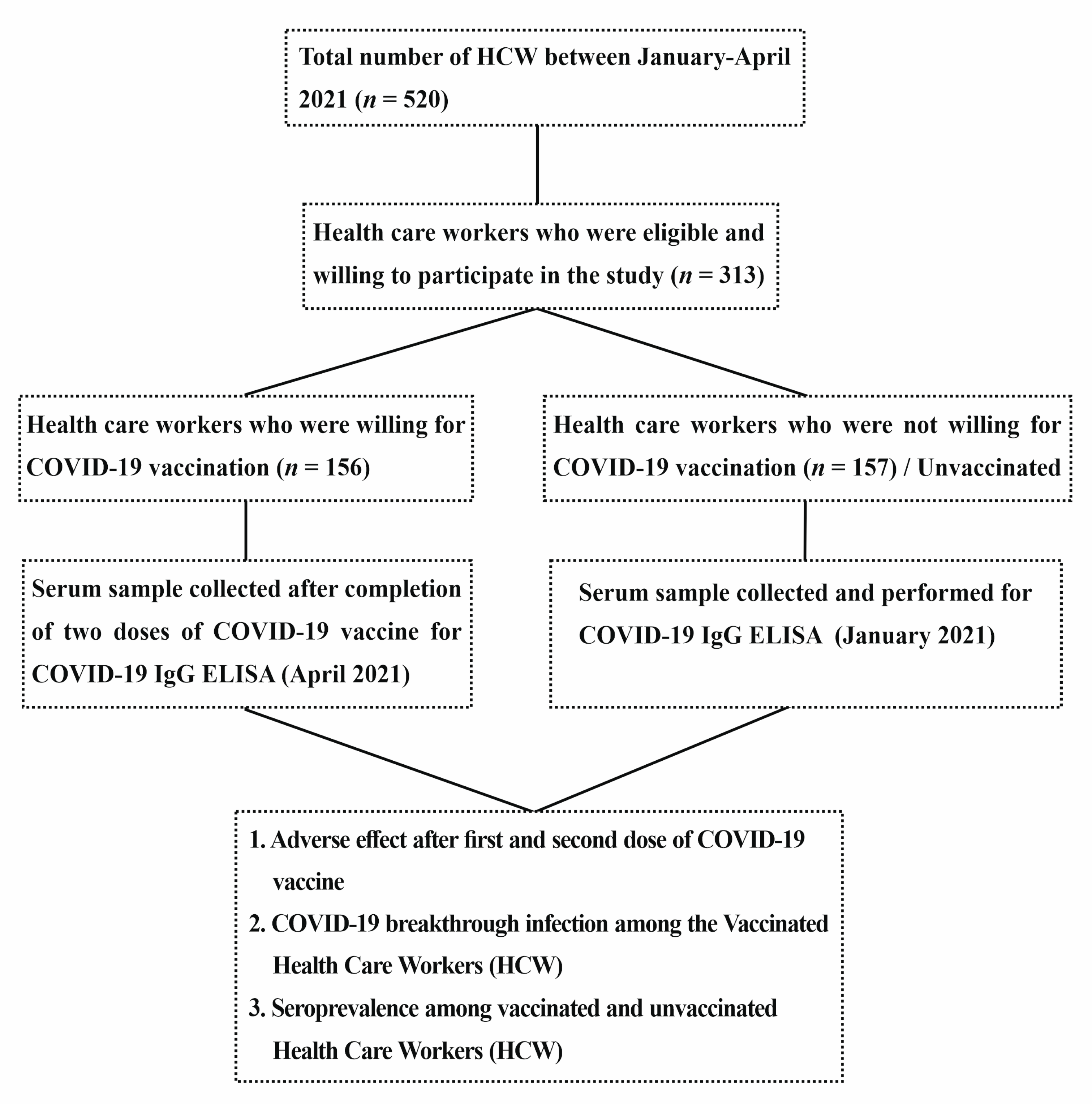
2. Material and Methods
3. Statistical Analysis
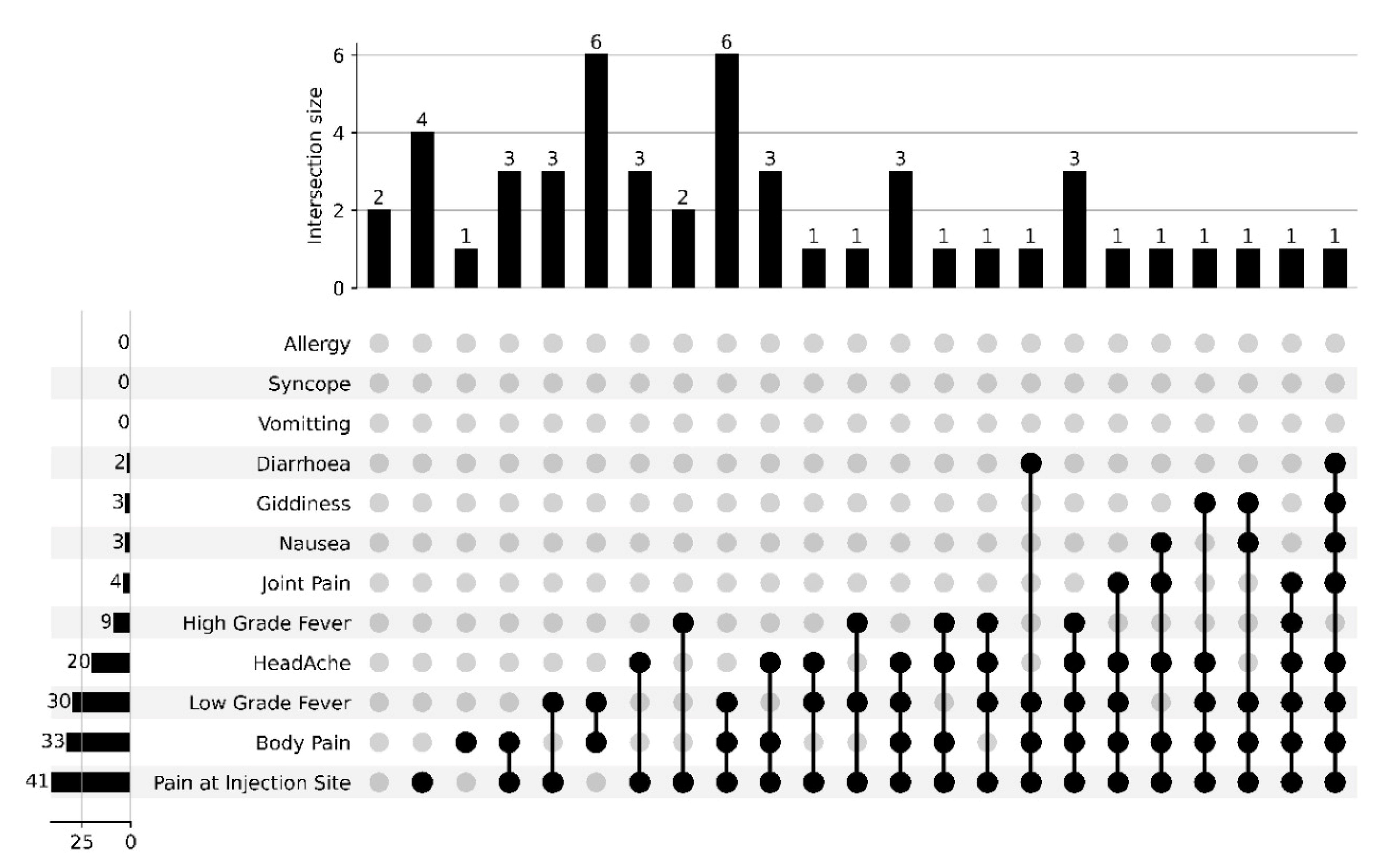
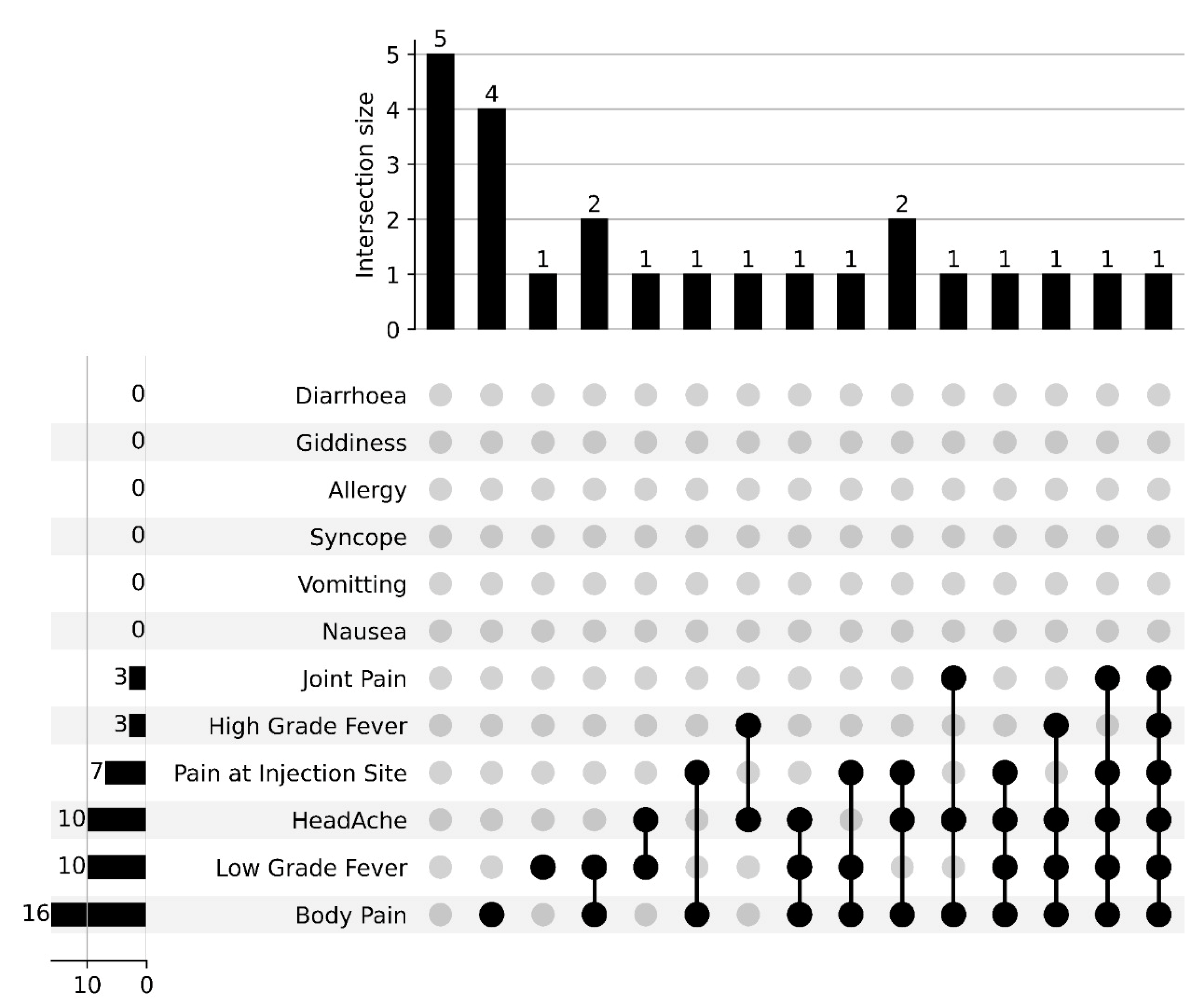
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Corona virus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- COVID-19 Situation Update Worldwide, as of Week 2 2021 Updated January 2021. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 14 April 2022).
- Butt, M.S.; Tharwani, Z.H.; Shaeen, S.K.; Shahzad, A.; Essar, M.Y. Maternal mortality and child malnutrition: Complications of the current crises in Yemen. Clin. Epidemiol. Glob. Health 2022, 15, 101051. [Google Scholar] [CrossRef]
- Costantino, C.; Cannizzaro, E.; Verso, M.G.; Tramuto, F.; Maida, C.M.; Lacca, G.; Alba, D.; Cimino, L.; Conforto, A.; Cirrincione, L.; et al. SARS-CoV-2 Infection in Healthcare Professionals and General Population During “First Wave” of COVID-19 Pandemic: A Cross-Sectional Study Conducted in Sicily, Italy. Front. Public Health 2021, 9, 644008. [Google Scholar] [CrossRef] [PubMed]
- Buresti, G.; Rondinone, B.M.; Gagliardi, D.; Petyx, M.; D’Ancona, F.P.; Pezzotti, P.; Riccardo, F.; Iavicoli, S. The Impact of the First Wave of the COVID-19 Pandemic on Healthcare Workers: An Italian Retrospective Study. Int. J. Environ. Res. Public Health 2022, 19, 5205. [Google Scholar] [CrossRef]
- Park, S.; Chang, S.H.; Lee, J.H.; Lee, J.H.; Ham, J.Y.; Kim, Y.K.; Kim, S.-G.; Ryoo, N.H. Serological evaluation of patients with coronavirus disease-2019 in Daegu, South Korea. PLoS ONE 2022, 17, e0262820. [Google Scholar] [CrossRef]
- Babu, G.R.; Sundaresan, R.; Athreya, S.; Akhtar, J.; Pandey, P.K.; Maroor, P.S.; Padma, M.R.; Lalitha, R.; Shariff, M.; Krishnappa, L.; et al. The burden of active infection and anti-SARS-CoV-2 IgG antibodies in the general population: Re-sults from a statewide sentinel-based population survey in Karnataka, India. Int. J. Infect. Dis. 2021, 108, 27–36. [Google Scholar] [CrossRef]
- Lee, C.; Holroyd, T.A.; Gur-Arie, R.; Sauer, M.; Zavala, E.; Paul, A.M.; Shattuck, D.; Karron, R.A.; Limaye, R.J. COVID-19 vaccine acceptance among Bangladeshi adults: Understanding predictors of vaccine intention to inform vaccine policy. PLoS ONE 2022, 17, e0261929. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Nilsson, A.C.; Holm, D.K.; Justesen, U.S.; Gorm-Jensen, T.; Andersen, N.S.; Øvrehus, A.; Johansen, I.S.; Michelsen, J.; Sprogøe, U.; Lillevang, S.T. Comparison of six commercially available SARS-CoV-2 antibody assays-Choice of assay depends on intended use. Int. J. Infect. Dis. 2021, 103, 381–388. [Google Scholar] [CrossRef]
- Murhekar, M.V.; Bhatnagar, T.; Selvaraju, S.; Saravanakumar, V.; Thangaraj, J.W.V.; Shah, N.; Kumar, M.S.; Rade, K.; Sabarinathan, R.; Asthana, S.; et al. SARS-CoV-2 antibody seroprevalence in India, August–September 2020: Findings from the second nationwide household serosurvey. Lancet Glob Health 2021, 9, e257–e266. [Google Scholar] [CrossRef]
- Inbaraj, L.R.; George, C.E.; Chandrasingh, S. Seroprevalence of COVID-19 infection in a rural district of South India: A population-based seroepidemiologicalstudy. PLoS ONE 2021, 16, e0249247. [Google Scholar] [CrossRef]
- Kumar, D.; Bhota, S.; Gupta, G.; Sood, T.; Kanwal, S.; Jaryal, S.C.; Raina, S.K. Seroprevalence of COVID-19 among health care professionals (HCPs) of tertiary care hospital of northern state of India. J. Fam. Med. Prim. Care 2022, 11, 908–911. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2020, 396, 1979–1993. [Google Scholar] [CrossRef]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J.; et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Remlabeevi, A.; Mathew, T.; Nair, G.H.; Nair, G.L.; Alex, M.R. Adverse events and their association with comorbidities after first and second doses of Covishield vaccination among healthcare workers of Government owned medical colleges in Kerala. MedRxiv 2021. [Google Scholar] [CrossRef]
- Singh, A.K.; Phatak, S.R.; Singh, R.; Bhattacharjee, K.; Singh, N.K.; Gupta, A.; Sharma, A. Antibody response after first and second-dose of ChAdOx1-nCOV (CovishieldTM®) and BBV-152 (CovaxinTM®) among health care workers in India: The final results of cross-sectional coronavirus vaccine-induced antibody titre (COVAT) study. Vaccine 2021, 39, 6492–6509. [Google Scholar] [CrossRef]
- Murugesan, M. Mayo Clinic Proceedings Protective Effect of COVID-19 Vaccine Among Health Care Workers During the Second Wave of the Pandemic in India. Mayo Clin. Proc. 2021, 96, 2493–2494. [Google Scholar]
- Murugesan, M.; Mathews, P.; Paul, H.; Karthik, R.; Mammen, J.J.; Rupali, P. Protective effect conferred by prior infection and vaccination on COVID-19 in a healthcare worker cohort in South India. PLoS ONE 2022, 17, e0268797. [Google Scholar] [CrossRef]
- Whitaker, H.J.; Elgohari, S.; Rowe, C.; Otter, A.D.; Brooks, T.; Linley, E.; Hayden, I.; Ribeiro, S.; Hewson, J.; Lakhani, A.; et al. Impact of COVID-19 vaccination program on seroprevalence in blood donors in England, 2021. J. Infect. 2021, 83, 237–279. [Google Scholar] [CrossRef] [PubMed]
- Herrington, D.M.; Sanders, J.W.; Wierzba, T.F.; Alexander-Miller, M.; Espeland, M.; Bertoni, A.G.; Mathews, A.; Seals, A.L.; Munawar, I.; Runyon, M.S.; et al. Duration of SARS-CoV-2 sero-positivity in a large longitudinal sero-surveillance cohort: The COVID-19 Community Research Partnership. BMC Infect. Dis. 2021, 21, 889. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, Q.; Madhira, V.; Olex, A.L.; Anzalone, A.J.; Vinson, A.; Singh, J.A.; French, E.; Abraham, A.G.; Mathew, J.; et al. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern. Med. 2022, 182, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Tretyn, A.; Szczepanek, J.; Skorupa, M.; Jarkiewicz-Tretyn, J.; Sandomierz, D.; Dejewska, J.; Ciechanowska, K.; Jarkiewicz-Tretyn, A.; Koper, W.; Pałgan, K. Differences in the Concentration of Anti-SARS-CoV-2 IgG Antibodies Post-COVID-19 Recovery or Post-Vaccination. Cells 2021, 10, 1952. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Maeda, K.; Matsuda, K.; Tanaka, A.; Horii, K.; Okudera, K.; Takeuchi, J.; Mizoue, T.; Konishi, M.; Ozeki, M.; et al. COVID-19 breakthrough infections and pre-infection neutralizing antibody. MedRxiv 2021. [Google Scholar] [CrossRef]
- Mandal, S.; Arinaminpathy, N.; Bhargava, B.; Panda, S. India’s pragmatic vaccination strategy against COVID-19: A mathematical modelling-based analysis. BMJ Open 2021, 11, e048874. [Google Scholar] [CrossRef]
- Brochot, E.; Demey, B.; Touzé, A.; Belouzard, S.; Dubuisson, J.; Schmit, J.-L.; Duverlie, G.; Francois, C.; Castelain, S.; Helle, F. Anti-spike, Anti-nucleocapsid and Neutralizing Antibodies in SARS-CoV-2 Inpatients and Asymptomatic Individuals. Front. Microbiol. 2020, 11, 584251. [Google Scholar] [CrossRef]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.P.; Ryan, D.T.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’Aquila, R.T.; Mustanski, B.; et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine 2021, 38, 101018. [Google Scholar] [CrossRef]
- Pang, N.Y.-L.; Pang, A.S.-R.; Chow, V.T.; Wang, D.-Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Mil. Med. Res. 2021, 8, 47. [Google Scholar] [CrossRef]


| Factors | Vaccinated | Unvaccinated | p Value a |
|---|---|---|---|
| 156 (49.8%) | 157 (50.2%) | ||
| Age Group | |||
| 20–25 | 47 (43.5%) | 61 (56.5%) | 0.4250 |
| 26–35 | 63 (52.5%) | 57 (47.5%) | |
| 36–55 | 35 (55.6%) | 28 (44.4%) | |
| >55 | 11 (50.0%) | 11 (50.0%) | |
| Gender | |||
| Male | 52 (48.6%) | 55 (51.4%) | 0.7510 |
| Female | 104 (50.5%) | 102 (49.5%) | |
| Category | |||
| Non-Medical | 29 (28.2%) | 74 (71.8%) | <0.0001 |
| Para-Medical | 80 (63.5%) | 46 (36.5%) | |
| Medical | 47 (56.0%) | 37 (44.0%) | |
| Past History of COVID-19 Infection | |||
| Yes | 22 (71.0%) | 9 (29.0%) | 0.0130 |
| No | 134 (47.5%) | 148 (52.5%) | |
| Type of Masks used for Protection | |||
| Cloth | 8 (11.9) | 59 (88.1%) | <0.0001 |
| Surgical | 96 (52.5) | 87 (47.5%) | |
| N95 | 52 (82.5) | 11 (17.5%) | |
| ELISA Test | |||
| Negative (<1.1) | 13 (11.8%) | 97 (88.2%) | <0.0001 |
| Positive (≥1.1) | 143 (70.4%) | 60 (29.6%) | |
| Factors | Vaccinated | p Value b | |
|---|---|---|---|
| Yes a | No a | ||
| Age Group | |||
| 20–25 | 5.74 (3.83–7.60) | 0.43 (0.14–1.50) | <0.0001 |
| 26–35 | 5.48 (3.49–8.00) | 0.28 (0.14–1.50) | <0.0001 |
| 36–55 | 5.33 (2.23–8.10) | 0.54 (0.12–3.70) | 0.0001 |
| >55 | 5.50 (3.77–8.40) | 1.50 (0.12–3.70) | 0.0326 |
| Gender | |||
| Male | 5.26 (3.82–7.90) | 0.33 (0.10–1.30) | <0.0001 |
| Female | 5.52 (3.39–8.20) | 0.48 (0.15–2.10) | <0.0001 |
| Category | |||
| Non-Medical | 7.44 (4.94–8.60) | 0.50 (0.15–2.00) | <0.0001 |
| Para-Medical | 5.35 (3.36–7.50) | 0.18 (0.15–1.50) | <0.0001 |
| Medical | 5.18 (3.25–8.10) | 0.59 (0.10–2.10) | <0.0001 |
| History of past COVID-19 Infection | |||
| Yes | 6.69 (5.12–8.80) | 3.26 (1.69–3.70) | 0.0014 |
| No | 5.27 (3.28–8.10) | 0.36 (0.13–1.50) | <0.0001 |
| Type of Masks used for Protection | |||
| Cloth | 6.35 (3.24–9.00) | 0.43 (0.10–1.50) | <0.0001 |
| Surgical | 5.68 (3.26–7.90) | 0.42 (0.14–1.70) | <0.0001 |
| N95 | 5.04 (3.79–8.10) | 0.55 (0.13–3.70) | 0.0013 |
| ELISA Test | |||
| Negative (<1.1) | 0.07 (0.05–0.30) | 0.15 (0.10–0.30) | 0.0379 |
| Positive (≥1.1) | 5.87 (4.39–8.40) | 2.71 (1.50–4.20) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elangovan, D.; Hussain, S.M.S.; Virudhunagar Muthuprakash, S.; Devi Periadurai, N.; Viswanath Nalankilli, A.; Volvoikar, H.; Ramani, P.; Sivasubramaniam, J.; Mohanram, K.; Surapaneni, K.M. Impact of COVID-19 Vaccination on Seroprevalence of SARS-CoV-2 among the Health Care Workers in a Tertiary Care Centre, South India. Vaccines 2022, 10, 1967. https://doi.org/10.3390/vaccines10111967
Elangovan D, Hussain SMS, Virudhunagar Muthuprakash S, Devi Periadurai N, Viswanath Nalankilli A, Volvoikar H, Ramani P, Sivasubramaniam J, Mohanram K, Surapaneni KM. Impact of COVID-19 Vaccination on Seroprevalence of SARS-CoV-2 among the Health Care Workers in a Tertiary Care Centre, South India. Vaccines. 2022; 10(11):1967. https://doi.org/10.3390/vaccines10111967
Chicago/Turabian StyleElangovan, Divyaa, Shifa Meharaj Shaik Hussain, Somasunder Virudhunagar Muthuprakash, Nanthini Devi Periadurai, Ashok Viswanath Nalankilli, Harshada Volvoikar, Preethy Ramani, Jayanthi Sivasubramaniam, Kalyani Mohanram, and Krishna Mohan Surapaneni. 2022. "Impact of COVID-19 Vaccination on Seroprevalence of SARS-CoV-2 among the Health Care Workers in a Tertiary Care Centre, South India" Vaccines 10, no. 11: 1967. https://doi.org/10.3390/vaccines10111967
APA StyleElangovan, D., Hussain, S. M. S., Virudhunagar Muthuprakash, S., Devi Periadurai, N., Viswanath Nalankilli, A., Volvoikar, H., Ramani, P., Sivasubramaniam, J., Mohanram, K., & Surapaneni, K. M. (2022). Impact of COVID-19 Vaccination on Seroprevalence of SARS-CoV-2 among the Health Care Workers in a Tertiary Care Centre, South India. Vaccines, 10(11), 1967. https://doi.org/10.3390/vaccines10111967






