COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview
Abstract
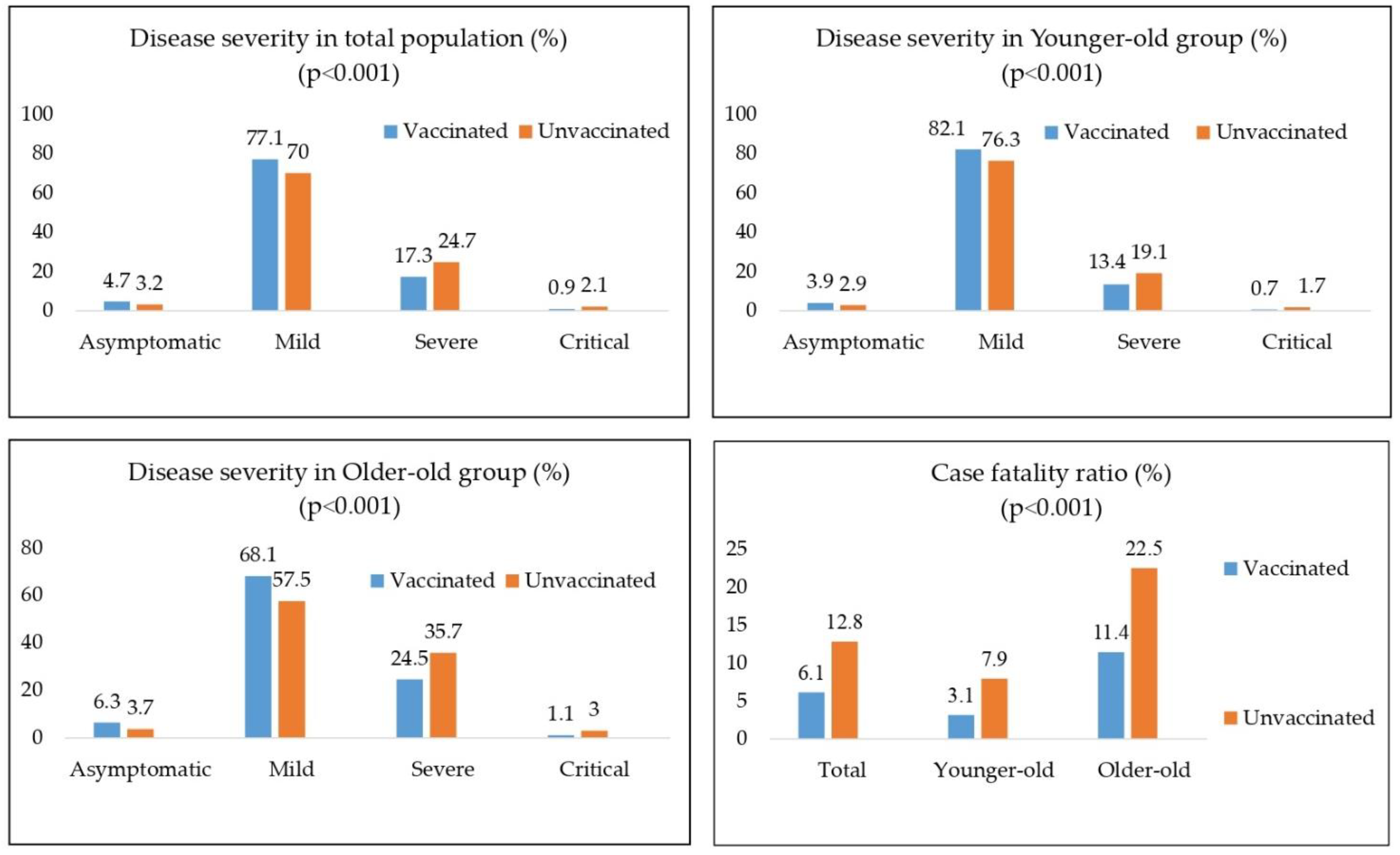
:Highlights
- Case fatality ratio decrease and reduction of severe and critical forms have been noted in fully vaccinated older individuals.
- Increasing age is still an independent factor for disease severity and poorer outcome of COVID-19 unrelated to individual vaccine status.
- 3.
- Providing evidence on health benefits for vaccinated older adults in terms of reducing disease severity and poor outcome.
- 4.
- Focus can be shifted to investment in and implementation of preventive forms of medicine, such as vaccination.
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
4. Discussion
- Lower incidence rate of COVID-19 infection in vaccinated people in the whole >65 years old population
- Among SARS-CoV-2-positive people, we noticed a significantly greater percentage of asymptomatic and mild forms of the diseases in the vaccinated group than in the unvaccinated group
- Vaccination status is an independent predictor of the lethal outcome, with odds ratio of 2.3, as expected in the hypothesis.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Z.; Cai, T.; Fan, L.; Lou, K.; Hua, X.; Huang, Z.; Gao, G. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease 2019. Int. J. Infect. Dis. 2020, 95, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Coomes, E.A.; Haghbayan, H. Interleukin-6 in Covid-19: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151. [Google Scholar] [CrossRef]
- Al-Jayyousi, G.F.; Sherbash, M.A.M.; Ali, L.A.M.; El-Heneidy, A.; Alhussaini, N.W.Z.; Elhassan, M.E.A.; Nazzal, M.A. Factors influencing public attitudes towards COVID-19 vaccination: A scoping review informed by the Socio-ecological model. Vaccines 2021, 9, 548. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- McGill COVID19 Vaccine Tracker Team. Serbia—COVID19 Vaccine Tracker. 2021. Available online: https://covid19.trackvaccines.org/country/serbia/ (accessed on 20 October 2021).
- Petrović, V.; Vuković, V.; Patić, A.; Marković, M.; Ristić, M. Immunogenicity of BNT162b2, BBIBP-CorV and Gam-COVID-Vac vaccines and immunity after natural SARS-CoV-2 infection-A comparative study from Novi Sad, Serbia. PLoS ONE 2022, 17, e0263468. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 breakthrough infections in vaccinated health care workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- Gustafson, C.E.; Kim, C.; Weyand, C.M.; Goronzy, J.J. Influence of immune aging on vaccine responses. J. Allergy Clin. Immunol. 2020, 145, 1309–1321. [Google Scholar] [CrossRef]
- Vokó, Z.; Kiss, Z.; Surján, G.; Surján, O.; Barcza, Z.; Pályi, B.; Formanek-Balku, E.; Molnár, G.A.; Herczeg, R.; Gyenesei, A.; et al. Nationwide effectiveness of five SARS-CoV-2 vaccines in Hungary-the HUN-VE study. Clin. Microbiol. Infect. 2021, 28, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perez, O.; Ribes, I.; Boix, V.; Martinez-García, M.Á.; Otero-Rodriguez, S.; Reus, S.; Sánchez-Martínez, R.; Ramos, J.M.; Chico-Sánchez, P.; Merino, E. Hospitalized patients with breakthrough COVID-19: Clinical features and poor outcome predictors. Int. J. Infect. Dis. 2022, 118, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Nikolić, N.; Čabarkapa, V.; Turkulov, V.; Petrović, V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS ONE 2021, 16, e0247606. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Ghosh, A.; Nair, D.; Dutta, K.; Bhandari, P.S.; Ansari, I.A.; Misra, A. Breakthrough COVID19 infections after vaccinations in healthcare and other workers in a chronic care medical facility in New Delhi, India. Diabetes Metab. Syndr. 2021, 15, 1007–1008. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Oh, J.H.; Park, J.H.; Choi, S.P.; Wee, J.H. Differences in youngest-old, middle-old, and oldest-old patients who visit the emergency department. Clin. Exp. Emerg. Med. 2018, 5, 249–255. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomized controlled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Jain, V.K.; Iyengar, K.P.; Ish, P. Elucidating causes of COVID-19 infection and related deaths after vaccination. Diabetes Metab. Syndr. 2021, 15, 102212. [Google Scholar] [CrossRef]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef]
- Pijls, B.G.; Jolani, S.; Atherley, A.; Derckx, R.T.; Dijkstra, J.I.R.; Franssen, G.H.L.; Hendriks, S.; Richters, A.; Venemans-Jellema, A.; Zalpuri, S.; et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021, 11, e044640. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Cohen, A.A.; Provost, G.; Khalil, A.; Lacombe, G.; Rodrigues, S.; Desroches, M.; Hirokawa, K.; et al. Immunosenescence and altered vaccine efficiency in older subjects: A myth difficult to change. Vaccines 2022, 10, 607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Burns, E.A. Age-related decline in immunity: Implications for vaccine responsiveness. Expert. Rev. Vaccines 2008, 7, 467–479. [Google Scholar] [CrossRef]
- Berman, P.; Hogan, D.B.; Fox, R.A. The atypical presentation of infection in old age. Age Ageing 1987, 16, 201–207. [Google Scholar] [CrossRef]
- Cobre, A.D.F.; Böger, B.; Fachi, M.M.; Vilhena, R.D.O.; Domingos, E.L.; Tonin, F.S.; Pontarolo, R. Risk factors associated with delay in diagnosis and mortality in patients with COVID-19 in the city of Rio de Janeiro, Brazil. Cien. Saude Colet. 2020, 25, 4131–4140. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross-sectional survey. J. Hosp. Infect. 2021, 108, 168–173. [Google Scholar] [CrossRef]
- Paris, C.; Bénézit, F.; Geslin, M.; Polard, E.; Baldeyrou, M.; Turmel, V.; Tadié, É.; Garlantezec, R.; Tattevin, P. COVID-19 vaccine hesitancy among healthcare workers. Infect. Dis. Now 2021, 51, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Petrović, V.; Vuković, V.; Marković, M.; Ristić, M. Early effectiveness of four SARS-CoV-2 vaccines in preventing COVID-19 among adults aged ≥60 years in Vojvodina, Serbia. Vaccines 2022, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect. Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Mori, H.; Obinata, H.; Murakami, W.; Tatsuya, K.; Sasaki, H.; Miyake, Y.; Taniguchi, Y.; Ota, S.; Yamaga, M.; Suyama, Y.; et al. Comparison of COVID-19 disease between young and elderly patients: Hidden viral shedding of COVID-19. J. Infect. Chemother. 2021, 27, 70–75. [Google Scholar] [CrossRef]
- Arregocés-Castillo, L.; Fernández-Niño, J.; Rojas-Botero, M.; Palacios-Clavijo, A.; Galvis-Pedraza, M.; Rincón-Medrano, L.; Pinto-Álvarez, M.; Ruiz-Gómez, F.; Trejo-Valdivia, B. Effectivness of COVID-19 vaccines in older adults in Colombia: A retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022, 3, e242–e252. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Henry, B.M.; Di Piazza, G.; Pighi, L.; De Nitto, S.; Bragantini, D.; Gianfilippi, G.L.; Lippi, G. Anti-SARS-CoV-2 receptor-binding domain total antibodies response in seropositive and seronegative healthcare workers undergoing COVID-19 mRNA BNT162b2 vaccination. Diagnostics 2021, 11, 832. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, P.; Konka, A.; Kasperczyk, J.; Kawa, J.; Lejawa, M.; Maślanka-Seiffert, B.; Zembala-John, J.; Bugdol, M.; Romanik, M.; Bułdak, R.; et al. Immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in older residents of a long-term care facility: Relation with age, frailty and prior infection status. Biogerontology 2022, 23, 53–64. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total Population (n = 11,372) | Younger-Old (n = 7504) | Older-Old (n = 3868) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination coverage (%) | 48.4 | 51.3 | 46.2 | ||||||||||||
| Vaccination status | Yes | No | df | Chi-square test | P | Yes | No | df | Chi-square test | P | Yes | No | df | Chi-square test | P |
| 1975 (17.4) | 9397 (82.6) | 1272 (17.0) | 6232 (83.0) | 703 (18.2) | 3165 (81.8) | ||||||||||
| Gender (m) | 1021 (51.7) | 4082 (43.4) | 1 | 44.977 | <0.001 | 632 (49.7) | 2817 (45.2) | 1 | 8.549 | 0.003 | 389 (55.3) | 1265 (40.0) | 1 | 55.490 | <0.001 |
| Survivors | 1855 (93.9) | 8193 (87.2) | 1 | 71.997 | <0.001 | 1232 (96.9) | 5740 (92.1) | 1 | 36.186 | <0.001 | 623 (88.6) | 2453 (77.5) | 1 | 43.654 | <0.001 |
| Non-survivors | 120 (6.1) | 1204 (12.8) | 40 (3.1) | 492 (7.9) | 80 (11.4) | 712 (22.5) | |||||||||
| Comorbidity | |||||||||||||||
| Hypertension | 641 (32.5) | 3165 (33.7) | 1 | 1.100 | 0.294 | 383 (30.1) | 1864 (29.9) | 1 | 0.020 | 0.887 | 258 (36.7) | 1301 (41.1) | 1 | 4.641 | 0.031 |
| CVD | 136 (6.9) | 767 (8.2) | 1 | 3.636 | 0.057 | 86 (6.8) | 418 (6.7) | 1 | 0.005 | 0.944 | 50 (7.1) | 349 (11.0) | 1 | 9.528 | 0.002 |
| COPD | 38 (1.9) | 151 (1.6) | 1 | 1.004 | 0.316 | 25 (2.0) | 97 (1.6) | 1 | 1.104 | 0.293 | 13 (1.8) | 54 (1.7) | 1 | 0.069 | 0.793 |
| Diabetes mellitus | 156 (7.9) | 705 (7.5) | 1 | 0.366 | 0.545 | 93 (7.3) | 443 (7.1) | 1 | 0.066 | 0.798 | 63 (9.0) | 262 (8.3) | 1 | 0.349 | 0.555 |
| Obesity | 31 (1.6) | 134 (1.4) | 1 | 0.235 | 0.627 | 16 (1.3) | 107 (1.7) | 1 | 1.381 | 0.240 | 15 (2.1) | 27 (0.9) | 1 | 8.784 | 0.003 |
| Malignancy | 14 (0.7) | 110 (1.2) | 1 | 3.226 | 0.072 | 10 (0.8) | 72 (1.2) | 1 | 1.332 | 0.248 | 4 (0.6) | 38 (1.2) | 1 | 2.137 | 0.144 |
| Others | 64 (3.2) | 392 (4.2) | 1 | 3.675 | 0.055 | 39 (3.1) | 231 (3.7) | 1 | 1.250 | 0.264 | 25 (3.6) | 161 (5.1) | 1 | 2.944 | 0.086 |
| Variable | Total Population (n = 11,372) | Younger-Old (n = 7504) | Older-Old (n = 3868) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | S | NS | df | Test | p | S | NS | df | Test | p | S | NS | df | Test | p | |
| Age | N | 10,048 | 1324 | 11370 | −26.366 | <0.001 ** | 6972 | 532 | 7502 | −5.877 | 0.451 ** | 3076 | 792 | 3866 | −12.355 | 0.001 ** |
| Mean ± SD | 72.5 ± 6.2 | 77.4 ± 7.4 | 69.0 ± 2.8 | 69.7 ± 2.8 | 80.4 ± 4.3 | 82.5 ± 4.7 | ||||||||||
| Unvaccinated | 8193 (81.5) | 1204 (90.9) | 1 | 71.997 | <0.001 * | 5740 (82.3) | 492 (92.5) | 1 | 36.186 | <0.001 * | 2453 (79.7) | 712 (89.9) | 1 | 43.654 | <0.001 * | |
| Comorbidity | ||||||||||||||||
| Hypertension | 3196 (31.8) | 610 (46.1) | 1 | 106.911 | <0.001 * | 2035 (29.2) | 212 (39.8) | 1 | 26.783 | <0.001 * | 1161 (37.7) | 398 (50.3) | 1 | 40.959 | <0.001 * | |
| CVD | 685 (6.8) | 218 (16.5) | 1 | 148.965 | <0.001 * | 435 (6.2) | 69 (13.0) | 1 | 35.740 | <0.001 * | 250 (8.1) | 149 (18.8) | 1 | 77.737 | <0.001 * | |
| COPD | 141 (1.4) | 48 (3.6) | 1 | 35.344 | <0.001 * | 100 (1.4) | 22 (4.1) | 1 | 22.547 | <0.001 * | 41 (1.3) | 26 (3.3) | 1 | 14.069 | <0.001 * | |
| Diabetes mellitus | 698 (6.9) | 163 (12.3) | 1 | 48.148 | <0.001 * | 461 (6.6) | 75 (14.1) | 1 | 41.758 | <0.001 * | 237 (7.7) | 88 (11.1) | 1 | 9.495 | 0.002 * | |
| Obesity | 140 (1.4) | 25 (1.9) | 1 | 2.004 | 0.157 * | 112 (1.6) | 11 (2.1) | 1 | 0.652 | 0.419 * | 28 (0.9) | 14 (1.8) | 1 | 4.311 | 0.038 * | |
| Malignancy | 90 (0.9) | 34 (2.6) | 1 | 30.334 | <0.001 * | 59 (0.8) | 23 (4.3) | 1 | 55.291 | <0.001 * | 31 (1.0) | 11 (1.4) | 1 | 0.852 | 0.356 * | |
| Others | 338 (3.4) | 118 (8.9) | 1 | 93.569 | <0.001 * | 218 (3.1) | 52 (9.8) | 1 | 62.973 | <0.001 * | 120 (3.9) | 66 (8.3) | 1 | 27.029 | <0.001 * | |
| Variable | B | S.E. | Wald | df | Sig. | Odds Ratio (OR) | 95% CI for OR | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Unvaccinated | 0.854 | 0.102 | 69.649 | 1 | <0.001 | 2.350 | 1.923 | 2.872 |
| Age | 0.095 | 0.004 | 491.702 | 1 | <0.001 | 1.100 | 1.091 | 1.109 |
| Hypertension | 0.992 | 0.074 | 178.526 | 1 | <0.001 | 2.697 | 2.332 | 3.120 |
| COPD | 1.712 | 0.185 | 85.762 | 1 | <0.001 | 5.541 | 3.857 | 7.961 |
| DM | 1.296 | 0.108 | 144.793 | 1 | <0.001 | 3.655 | 2.959 | 4.514 |
| Obesity | 1.250 | 0.230 | 29.462 | 1 | <0.001 | 3.491 | 2.223 | 5.484 |
| Malignancy | 1.853 | 0.217 | 73.042 | 1 | <0.001 | 6.382 | 4.172 | 9.762 |
| Others | 1.610 | 0.128 | 159.341 | 1 | <0.001 | 5.004 | 3.897 | 6.426 |
| Constant | −10.617 | 0.347 | 937.739 | 1 | <0.001 | 0.000 | ||
| Variable | B | S.E. | Wald | df | Sig. | Odds Ratio (OR) | 95% CI for OR | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Unvaccinated | 1.037 | 0.170 | 37.302 | 1 | <0.001 | 2.820 | 2.022 | 3.933 |
| Age | 0.085 | 0.016 | 27.069 | 1 | <0.001 | 1.088 | 1.054 | 1.123 |
| Hypertension | 1.068 | 0.114 | 88.306 | 1 | <0.001 | 2.910 | 2.329 | 3.635 |
| COPD | 1.840 | 0.254 | 52.533 | 1 | <0.001 | 6.299 | 3.830 | 10.362 |
| DM | 1.522 | 0.153 | 98.912 | 1 | <0.001 | 4.582 | 3.395 | 6.185 |
| Obesity | 1.014 | 0.329 | 9.470 | 1 | <0.001 | 2.755 | 1.445 | 5.254 |
| Malignancy | 2.350 | 0.264 | 79.396 | 1 | <0.001 | 10.482 | 6.252 | 17.575 |
| Others | 1.919 | 0.179 | 115.482 | 1 | <0.001 | 6.816 | 4.803 | 9.673 |
| Constant | −10.129 | 1.152 | 77.253 | 1 | <0.001 | 0.000 | ||
| Variable | B | S.E. | Wald | df | Sig. | Odds Ratio (OR) | 95% CI for OR | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Unvaccinated | 0.744 | 0.130 | 32.661 | 1 | <0.001 | 2.105 | 1.631 | 2.717 |
| Age | 0.096 | 0.009 | 117.361 | 1 | <0.001 | 1.101 | 1.082 | 1.120 |
| Hypertension | 0.926 | 0.099 | 88.108 | 1 | <0.001 | 2.523 | 2.080 | 3.061 |
| COPD | 1.603 | 0.270 | 35.250 | 1 | <0.001 | 4.967 | 2.926 | 8.431 |
| DM | 1.089 | 0.151 | 52.253 | 1 | <0.001 | 2.970 | 2.211 | 3.990 |
| Obesity | 1.613 | 0.347 | 21.647 | 1 | <0.001 | 5.016 | 2.543 | 9.893 |
| Malignancy | 1.113 | 0.367 | 9.183 | 1 | <0.001 | 3.044 | 1.482 | 6.255 |
| Others | 1.324 | 0.177 | 55.847 | 1 | <0.001 | 3.757 | 2.655 | 5.316 |
| Constant | −10.469 | 0.736 | 202.326 | 1 | <0.001 | 0.000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajči, M.P.; Lendak, D.F.; Ristić, M.; Drljača, M.M.; Brkić, S.; Turkulov, V.; Petrović, V. COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines 2022, 10, 1818. https://doi.org/10.3390/vaccines10111818
Bajči MP, Lendak DF, Ristić M, Drljača MM, Brkić S, Turkulov V, Petrović V. COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines. 2022; 10(11):1818. https://doi.org/10.3390/vaccines10111818
Chicago/Turabian StyleBajči, Monika P., Dajana F. Lendak, Mioljub Ristić, Maja M. Drljača, Snežana Brkić, Vesna Turkulov, and Vladimir Petrović. 2022. "COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview" Vaccines 10, no. 11: 1818. https://doi.org/10.3390/vaccines10111818
APA StyleBajči, M. P., Lendak, D. F., Ristić, M., Drljača, M. M., Brkić, S., Turkulov, V., & Petrović, V. (2022). COVID-19 Breakthrough Infections among Patients Aged ≥65 Years in Serbia: Morbidity and Mortality Overview. Vaccines, 10(11), 1818. https://doi.org/10.3390/vaccines10111818





