Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice
Abstract
1. Introduction
2. Material and Methods
2.1. Reagents
2.2. Cells and Virus Stock
2.3. Preparation and Characterization of Nanoparticles
2.4. Cytotoxicity Assay
2.5. Cellular Uptake
2.6. Animal Studies
2.7. Enzyme-Linked Immunosorbent Assay (ELISA) for Antibody Determination
2.8. Immunohistochemistry and Haematoxylin-Eosin Staining
2.9. Neutralization Assay
2.10. Cytokine Release
2.11. Flow Cytometry
2.12. Blood Biochemistry Analysis
2.13. Statistical Analysis
3. Results
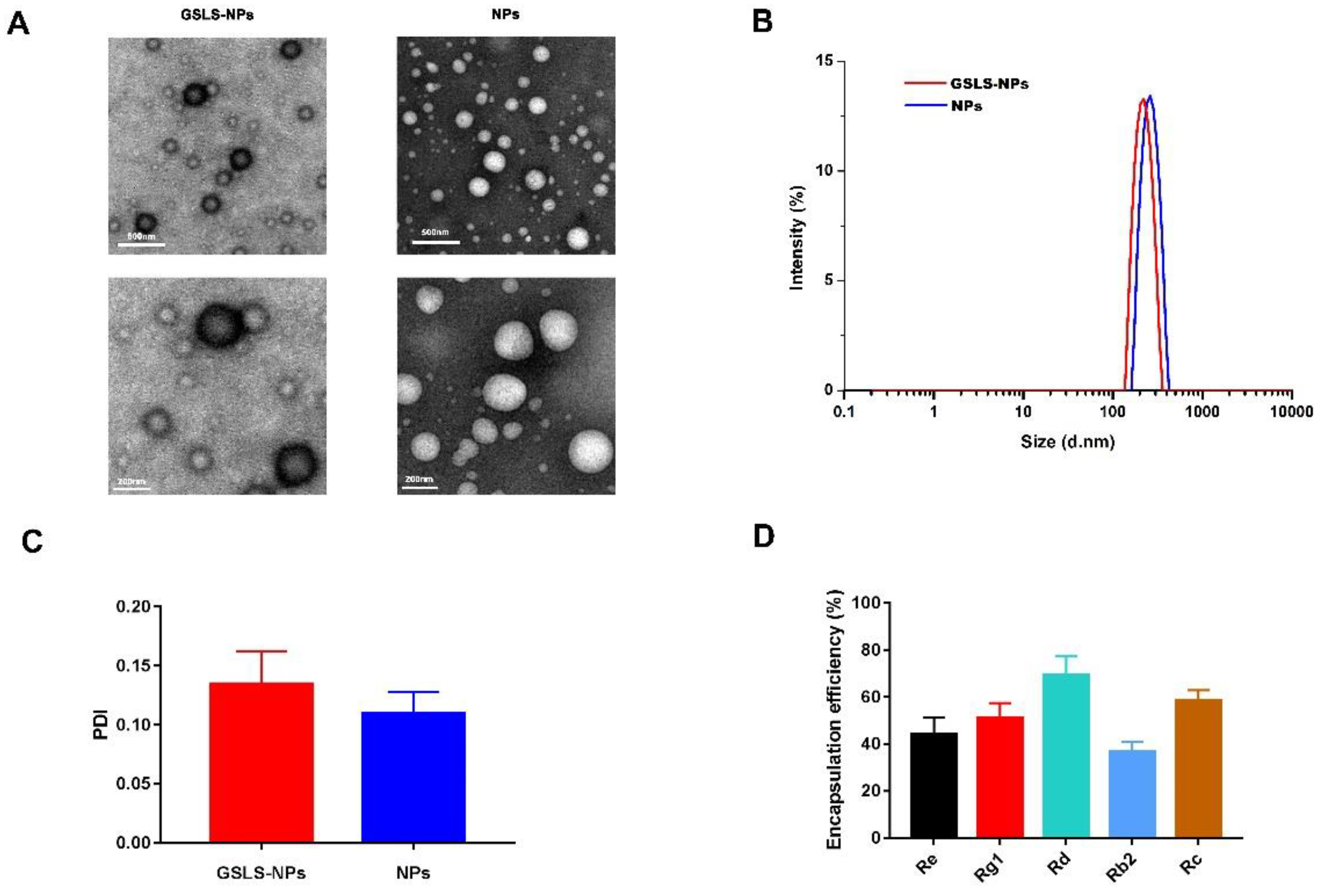
3.1. Synthesis and Characterization of Nanoparticles
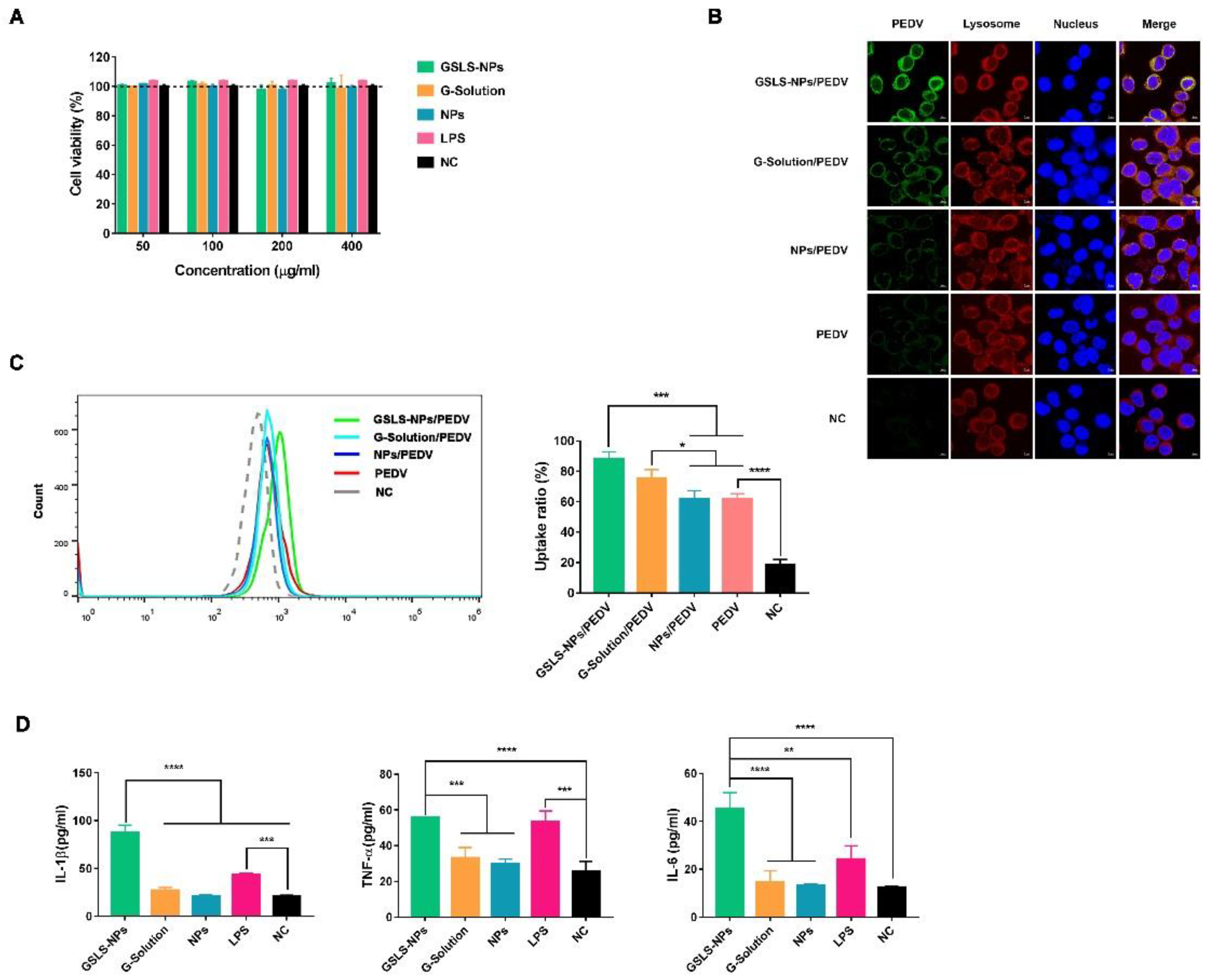
3.2. Viability and Activation of DCs Induced by GSLS-NPs In Vitro
3.3. Oral Administration of GSLS-NPs Enhances PEDV-Specific Antibody Responses in the Serum and Gastrointestinal Tract
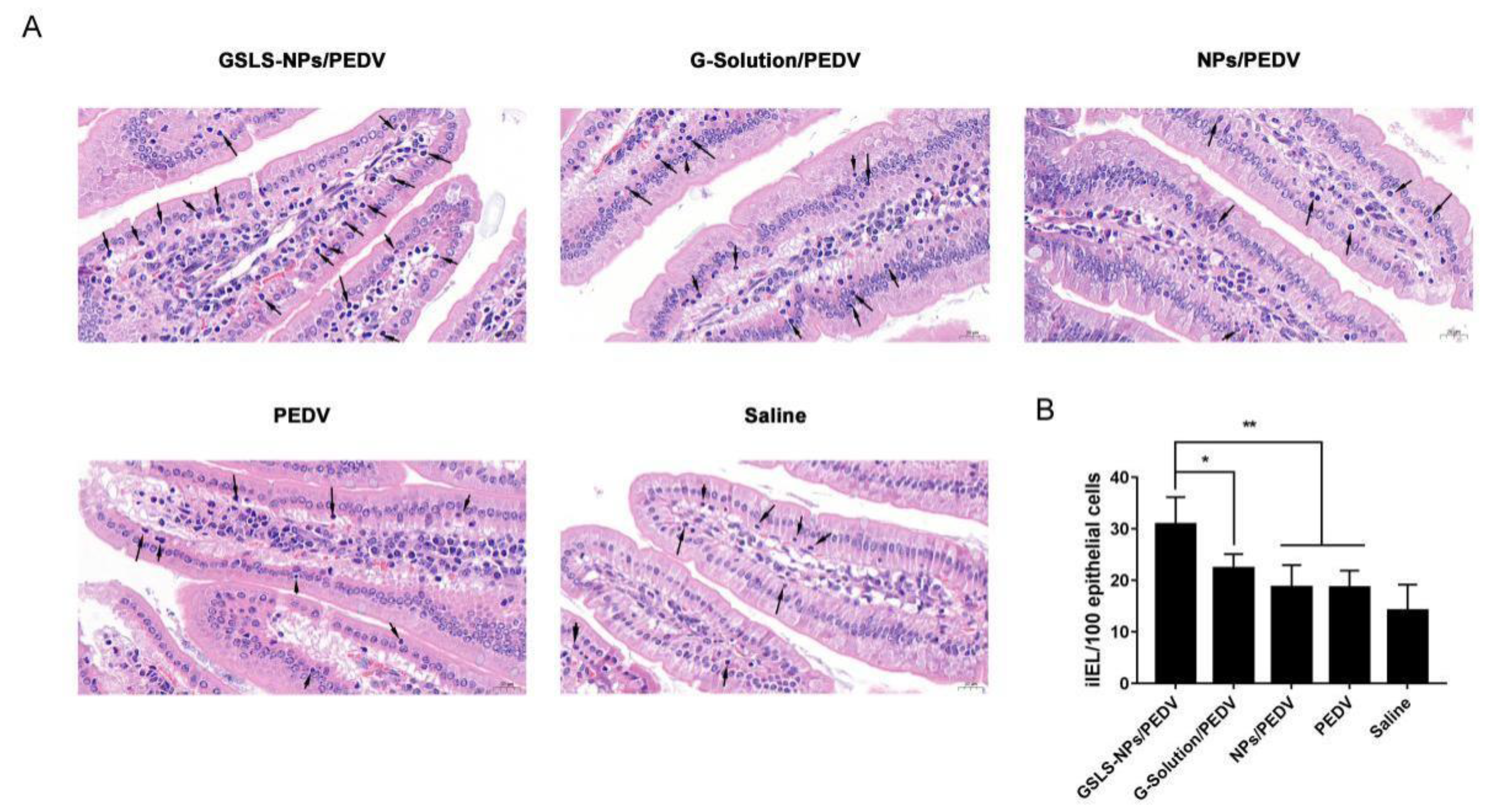
3.4. Oral Administration of GSLS-NPs Increases iIEL Proliferation
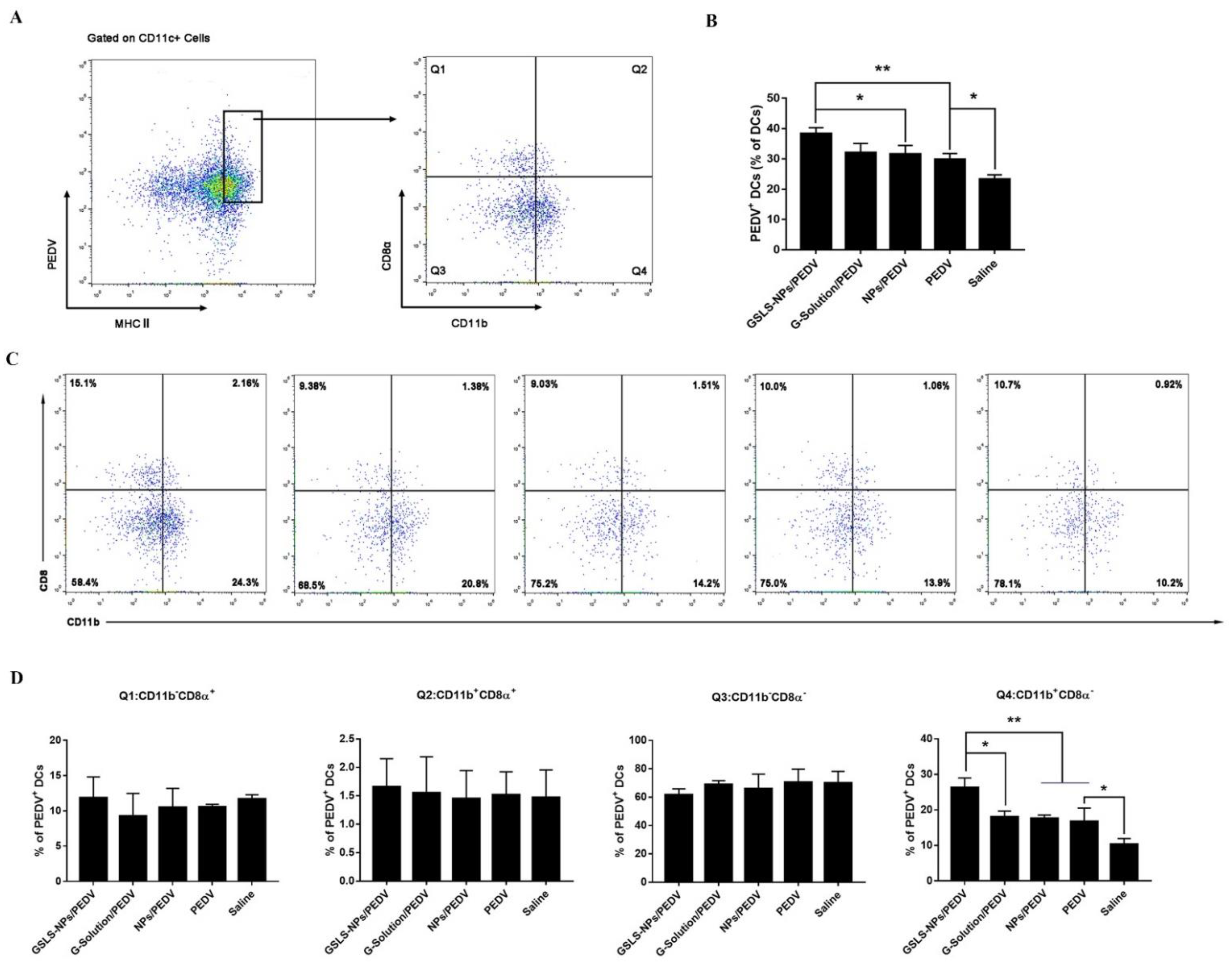
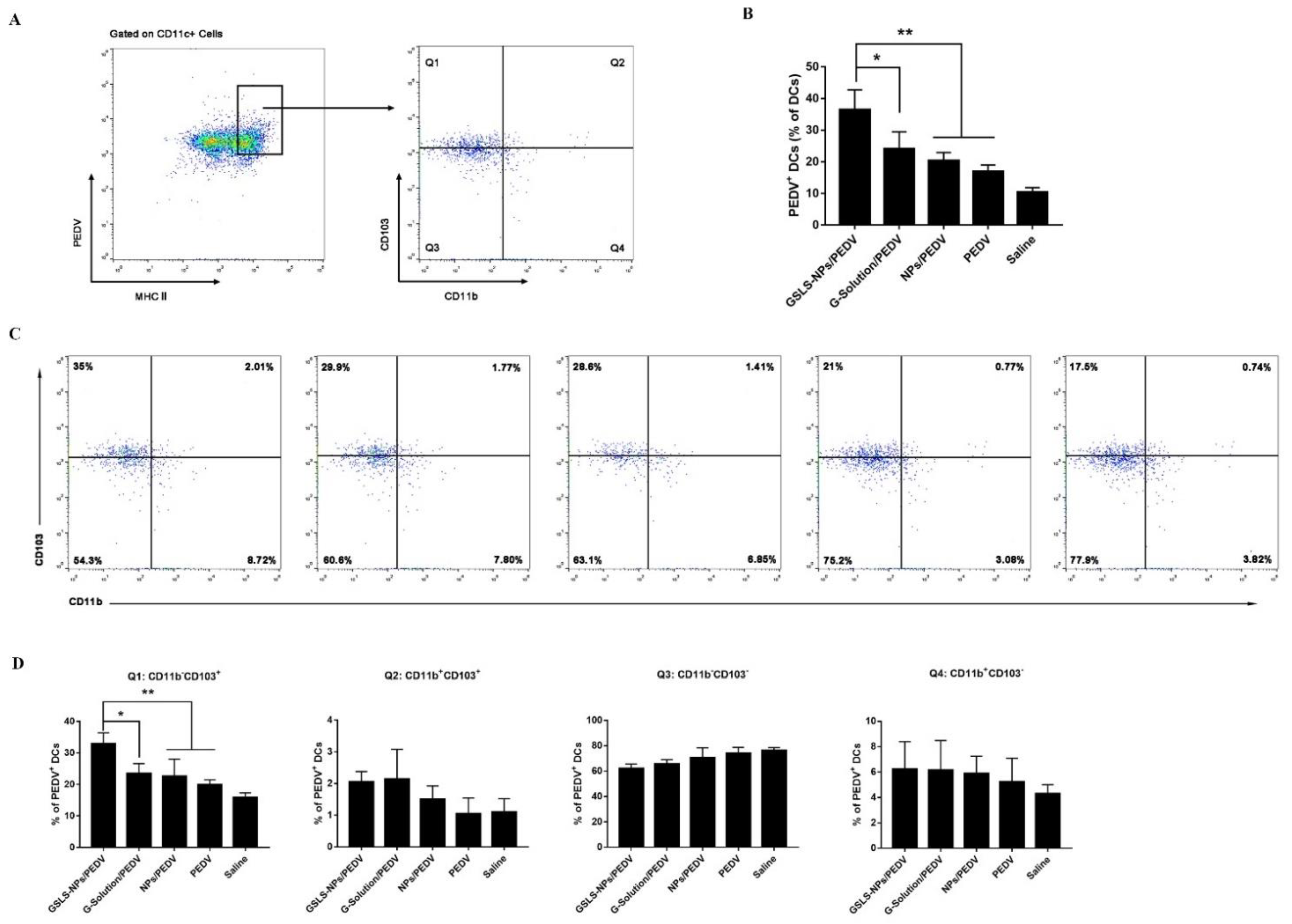
3.5. GSLS-NPs Regulate Distinct Subsets of Splenic and Intestinal DCs
3.6. GSLS-NPs Expand Memory and Effector T-Cell Responses
3.7. Biosafety of GSLS-NPs as an Oral Adjuvant
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Suda, Y.; Miyazaki, A.; Miyazawa, K.; Shibahara, T.; Ohashi, S. Systemic and intestinal porcine epidemic diarrhea virus-specific antibody response and distribution of antibody-secreting cells in experimentally infected conventional pigs. Vet. Res. 2021, 52, 2. [Google Scholar] [CrossRef]
- Zhai, L.; Wang, Y.; Yu, J.; Hu, S. Enhanced immune responses of chickens to oral vaccination against infectious bursal disease by ginseng stem-leaf saponins. Poult. Sci. 2014, 93, 2473–2481. [Google Scholar] [CrossRef]
- Yu, J.; Shi, F.S.; Hu, S. Improved immune responses to a bivalent vaccine of Newcastle disease and avian influenza in chickens by ginseng stem-leaf saponins. Vet. Immunol. Immunopathol. 2015, 167, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ma, Y.; Zhai, L.; Lu, Y.; Chi, X.; Wu, J.; Hu, S. Enhanced immune response to foot-and-mouth disease vaccine by oral administration of ginseng stem-leaf saponins. Immunopharmacol. Immunotoxicol. 2016, 38, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Marciani, D.J. Vaccine adjuvants: Role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today 2003, 8, 934–943. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, M.; Xu, M.; Tang, W.; Zhang, Y.; Lu, L.; Gao, J.; Gao, S. Intranasal Delivery of Microspheres Loaded with 20 (R)-ginsenoside Rg3 Enhances Anti-Fatigue Effect in Mice. Curr. Drug Deliv. 2017, 14, 867–874. [Google Scholar] [CrossRef]
- Canadas, C.; Alvarado, H.; Calpena, A.C.; Silva, A.M.; Souto, E.B.; Garcia, M.L.; Abrego, G. In vitro, ex vivo and in vivo characterization of PLGA nanoparticles loading pranoprofen for ocular administration. Int. J. Pharm. 2016, 511, 719–727. [Google Scholar] [CrossRef]
- Du, G.; Hathout, R.M.; Nasr, M.; Nejadnik, M.R.; Tu, J.; Koning, R.I.; Koster, A.J.; Slutter, B.; Kros, A.; Jiskoot, W.; et al. Intradermal vaccination with hollow microneedles: A comparative study of various protein antigen and adjuvant encapsulated nanoparticles. J. Control. Release 2017, 266, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.; Wusiman, A.; Wang, S.; Zhang, Y.; Liu, Z.; Hu, Y.; Liu, J.; Wang, D. Polyethylenimine-coated PLGA nanoparticles-encapsulated Angelica sinensis polysaccharide as an adjuvant to enhance immune responses. Carbohydr. Polym. 2019, 223, 115128. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, B.; Sun, R.; Liu, W.; Zhu, Q.; Zhang, X.; Wang, R.; Chen, C. PLGA-based biodegradable microspheres in drug delivery: Recent advances in research and application. Drug Deliv. 2021, 28, 1397–1418. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.N. Spreading the load: Antigen transfer between migratory and lymph node-resident dendritic cells promotes T-cell priming. Eur. J. Immunol. 2017, 47, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Wu, Y.; Li, J.; Huang, Y.; Yu, B.; Xu, L.; Xue, Y.; Xiao, C.; Yuan, X. Escherichia coli Heat-Labile Enterotoxin B Subunit Combined with Ginsenoside Rg1 as an Intranasal Adjuvant Triggers Type I Interferon Signaling Pathway and Enhances Adaptive Immune Responses to an Inactivated PRRSV Vaccine in ICR Mice. Vaccines 2021, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Sahu, R.; Dixit, S.; Verma, R.; Duncan, S.A.; Coats, M.T.; Giambartolomei, G.H.; Singh, S.R.; Dennis, V.A. A nanovaccine formulation of Chlamydia recombinant MOMP encapsulated in PLGA 85:15 nanoparticles augments CD4(+) effector (CD44(high) CD62L(low)) and memory (CD44(high) CD62L(high)) T-cells in immunized mice. Nanomedicine 2020, 29, 102257. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.A. The manufacturing techniques of various drug loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices. Biomaterials 2000, 21, 2475–2490. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, L.; Zhang, X.; Xu, J.; Jin, J.O. CD8alpha(-) conventional dendritic cells control Vbeta T-cell immunity in response to Staphylococcus aureus infection in mice. Immunology 2020, 159, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Pang, E.S.; Patton, T.; O’Keeffe, M. Dendritic cell subsets. Semin. Cell Dev. Biol. 2018, 84, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Sengupta, S.; Khoruzhenko, S.; Welsh, R.A.; Kim, A.; Kumar, M.R.; Sonder, S.U.; Sidhom, J.W.; Zhang, H.; Jie, C.; et al. Multiple genetic programs contribute to CD4 T cell memory differentiation and longevity by maintaining T cell quiescence. Cell Immunol. 2020, 357, 104210. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shen, Y.; Luyten, S.; Yang, Y.; Jiang, X. Tissue-resident memory CD8(+) T cells in cancer immunology and immunotherapy. Pharmacol. Res. 2020, 159, 104876. [Google Scholar] [CrossRef] [PubMed]
- Esterhazy, D.; Loschko, J.; London, M.; Jove, V.; Oliveira, T.Y.; Mucida, D. Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance. Nat. Immunol. 2016, 17, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.U.; Brown, S.L.; MacDonald, A.S.; Milling, S.W. Defined Intestinal Regions Are Drained by Specific Lymph Nodes That Mount Distinct Th1 and Th2 Responses Against Schistosoma mansoni Eggs. Front. Immunol. 2020, 11, 592325. [Google Scholar] [CrossRef] [PubMed]
- Tezuka, H.; Ohteki, T. Regulation of IgA Production by Intestinal Dendritic Cells and Related Cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef]
- Guo, H.; Yao, Z.; Chen, L.; Li, L.; Li, Y.; Wang, Y.; Li, T.; Wang, H.; Sun, L.; Hao, D.; et al. Humoral immune responses in piglets experimentally infected with a field strain of porcine epidemic diarrhea virus. Vet. Microbiol. 2020, 246, 108742. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.; Xu, L.; Xue, Y.; Xu, W.; Li, J.; Yu, B.; Ye, S.; Yuan, X. Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice. Vaccines 2022, 10, 1810. https://doi.org/10.3390/vaccines10111810
Su F, Xu L, Xue Y, Xu W, Li J, Yu B, Ye S, Yuan X. Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice. Vaccines. 2022; 10(11):1810. https://doi.org/10.3390/vaccines10111810
Chicago/Turabian StyleSu, Fei, Lihua Xu, Yin Xue, Wei Xu, Junxing Li, Bin Yu, Shiyi Ye, and Xiufang Yuan. 2022. "Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice" Vaccines 10, no. 11: 1810. https://doi.org/10.3390/vaccines10111810
APA StyleSu, F., Xu, L., Xue, Y., Xu, W., Li, J., Yu, B., Ye, S., & Yuan, X. (2022). Immune Enhancement of Nanoparticle-Encapsulated Ginseng Stem-Leaf Saponins on Porcine Epidemic Diarrhea Virus Vaccine in Mice. Vaccines, 10(11), 1810. https://doi.org/10.3390/vaccines10111810




