The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEGylated PLGA-Dtx Nanoparticles
2.3. Characterization of PLGA-Dtx Nanoparticles
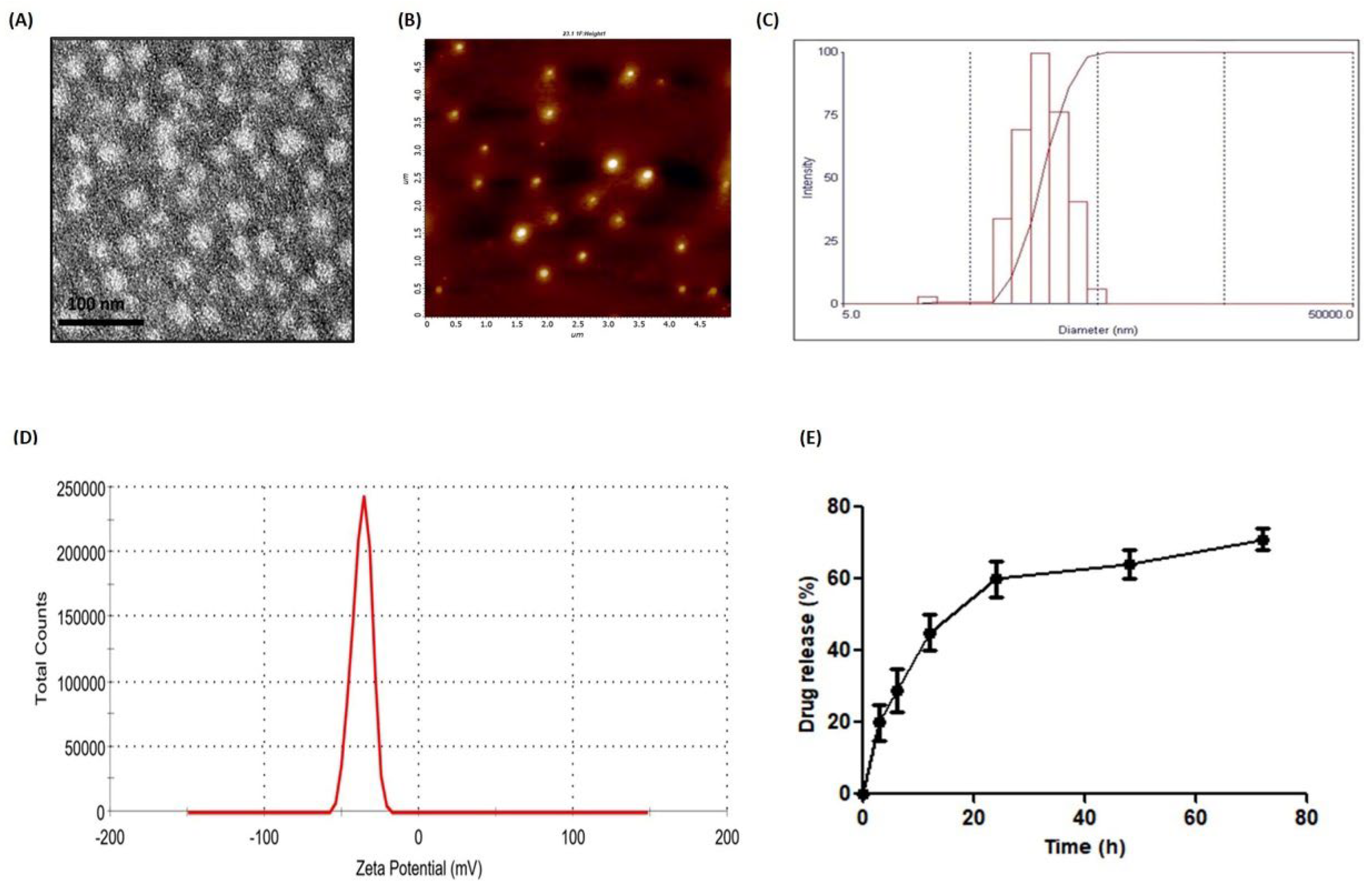
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. Atomic Force Microscopy (AFM)
2.3.3. Transmission Electron Microscopy (TEM)
2.4. Encapsulation Efficiency (%EE) of PLGA-Dtx Nanoparticles
2.5. Release of Dtx from PLGA-Dtx Nanoparticles
2.6. Cell Line and Culture Conditions
2.7. Cell Viability/Death Assay
2.8. Cellular Internalization Assessment
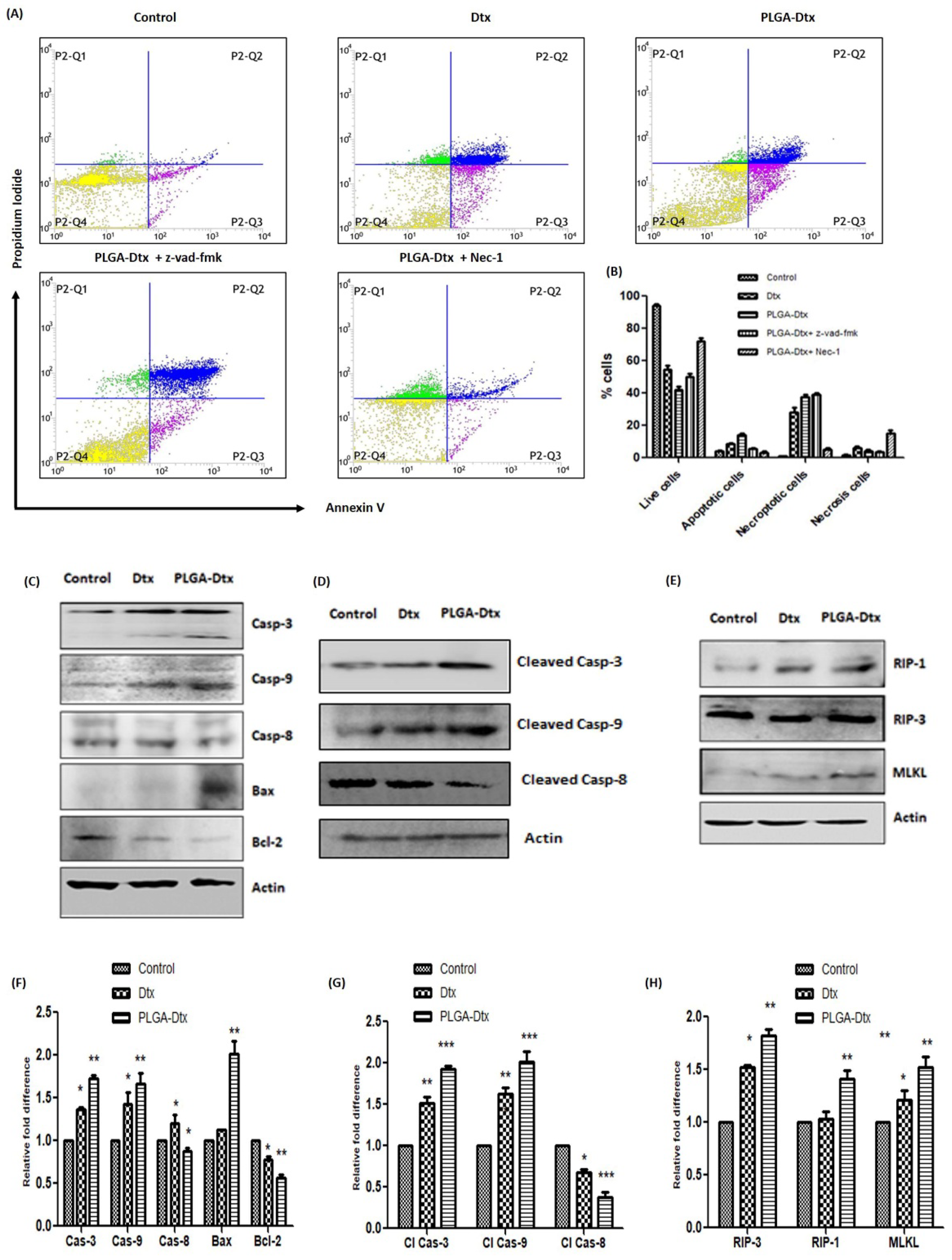
2.9. Annexin V/PI Assay
2.10. Western Blot Analyses
2.11. Measurement of ROS Generation and Oxidative Stress Markers
2.12. Determination of Mitochondrial Membrane Potential (MMP) and Cytosolic-Free Calcium
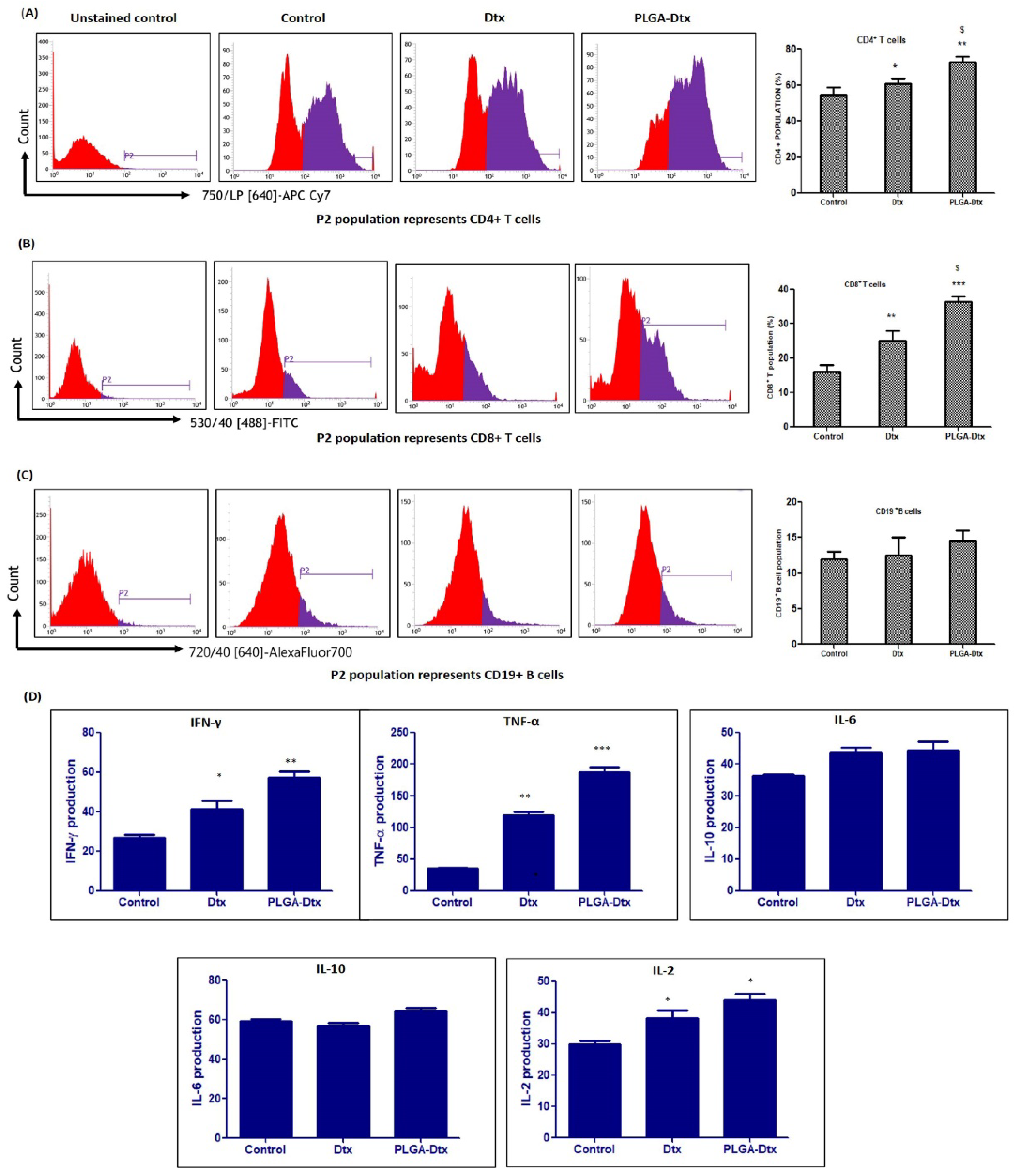
2.13. Immunophenotyping of PBMCs and Cytokine Analysis
2.14. Statistical Analysis
3. Results
3.1. Preparation and Characterizations of PLGA-Dtx
3.2. In Vitro Cytotoxicity Evaluation of PLGA-Dtx Nanoparticles
3.3. Cellular Uptake of PLGA-Dtx NPs
3.4. Assessment of Cell Death Mechanism of PLGA-Dtx
3.5. Involvement of ROS and Calcium Accumulation in PLGA-Dtx-Induced Cell Death
3.6. Immunomodulatory Effects of PLGA-Dtx on Peripheral Blood Mononuclear Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lung Source: Globocan 2020 Number of New Cases in 2020, Both Sexes, All Ages. 2020. Available online: https://gco.iarc.fr/today (accessed on 26 July 2022).
- Sui, X.; Chen, R.; Wang, Z.; Huang, Z.; Kong, N.; Zhang, M.; Han, W.; Lou, F.; Yang, J.; Zhang, Q.; et al. Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 2013, 4, e838. Available online: https://www.nature.com/articles/cddis2013350 (accessed on 26 July 2022). [CrossRef] [PubMed]
- Rathore, R.; McCallum, J.E.; Varghese, E.; Florea, A.-M.; Büsselberg, D. Overcoming chemotherapy drug resistance by targeting inhibitors of apoptosis proteins (IAPs). Apoptosis 2017, 22, 898–919. Available online: https://link.springer.com/article/10.1007/s10495-017-1375-1 (accessed on 26 July 2022). [CrossRef] [PubMed]
- Singh, A.P.; Biswas, A.; Shukla, A.W.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. Available online: https://www.nature.com/articles/s41392-019-0068-314 (accessed on 26 July 2022). [CrossRef] [PubMed]
- ud Din, F.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef] [PubMed]
- Wakelee, H.; Gubens, M. Docetaxel in the treatment of non-small cell lung carcinoma: An update and analysis. Lung Cancer Targets Ther. 2010, 1, 63–76. [Google Scholar] [CrossRef]
- Du, Q.; Jiang, G.; Li, S.; Liu, Y.; Huang, Z. Docetaxel increases the risk of severe infections in the treatment of non-small cell lung cancer: A meta-analysis. Oncoscience 2018, 5, 220–238. [Google Scholar] [CrossRef]
- Seo, Y.G.; Kim, D.W.; Yeo, W.H.; Ramasamy, T.; Oh, Y.K.; Park, Y.J.; Kim, J.-A.; Oh, D.H.; Ku, S.K.; Kim, J.K.; et al. Docetaxel-loaded thermosensitive and bioadhesive nanomicelles as a rectal drug delivery system for enhanced chemotherapeutic effect. Pharm. Res. 2013, 30, 1860–1870. Available online: https://pubmed.ncbi.nlm.nih.gov/23549753/ (accessed on 26 July 2022). [CrossRef]
- Engels, F.K.; Mathot, R.A.A.; Verweij, J. Alternative drug formulations of docetaxel: A review. Anticancer Drugs 2007, 18, 95–103. Available online: https://pubmed.ncbi.nlm.nih.gov/17159596/ (accessed on 26 July 2022). [CrossRef]
- Zhao, P.; Astruc, D. Docetaxel Nanotechnology in Anticancer Therapy. ChemMedChem 2012, 7, 952–972. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/cmdc.201200052 (accessed on 26 July 2022). [CrossRef]
- Zang, X.; Song, J.; Li, Y.; Han, Y. Targeting necroptosis as an alternative strategy in tumor treatment: From drugs to nanoparticles. J. Control. Release 2022, 349, 213–226. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0168365922004023 (accessed on 26 July 2022). [CrossRef]
- Liu, J.; Lu, Y.; Huang, W.; He, Z. Comprehensive Analysis of Inhibitor of Apoptosis Protein Expression and Prognostic Significance in Non–Small Cell Lung Cancer. Front. Genet. 2021, 12, 2323. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Mann, J.; Yang, N.; Montpetit, R.; Kirschenman, R.; Lemieux, H.; Goping, I.S. BAD sensitizes breast cancer cells to docetaxel with increased mitotic arrest and necroptosis. Sci. Rep. 2020, 10, 355. [Google Scholar] [CrossRef]
- Fizazi, K.; Mella, P.G.; Castellano, D.; Minatta, J.N.; Kalebasty, A.R.; Shaffer, D.; Limón, J.C.V.; López, H.M.S.; Armstrong, A.J.; Horvath, L.; et al. Nivolumab plus docetaxel in patients with chemotherapy-naïve metastatic castration-resistant prostate cancer: Results from the phase II CheckMate 9KD trial. Eur. J. Cancer 2021, 160, 61–71. [Google Scholar] [CrossRef]
- Chan, O.T.M.; Yang, L.X. The immunological effects of taxanes. Cancer Immunol. Immunother. 2000, 49, 181–185. Available online: https://pubmed.ncbi.nlm.nih.gov/10941900/ (accessed on 26 July 2022). [CrossRef]
- Millrud, C.R.; Mehmeti, M.; Leandersson, K. Docetaxel promotes the generation of anti-tumorigenic human macrophages. Exp. Cell Res. 2018, 362, 525–531. [Google Scholar] [CrossRef]
- Mahon, K.L.; Lin, H.M.; Castillo, L.; Lee, B.Y.; Lee-Ng, M.; Chatfield, M.D.; Chiam, K.; Breit, S.N.; Brown, D.A.; Molloy, M.P.; et al. Cytokine profiling of docetaxelresistant castration-resistant prostate cancer. Br. J. Cancer 2015, 112, 1340. [Google Scholar] [CrossRef]
- Nars, M.S.; Kaneno, R. Immunomodulatory effects of low dose chemotherapy and perspectives of its combination with immunotherapy. Int. J. Cancer 2012, 132, 2471–2478. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/ijc.27801 (accessed on 26 July 2022). [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 2008, 8, 59–73. Available online: https://www.nature.com/articles/nri2216 (accessed on 26 July 2022). [CrossRef]
- Khalil, D.N.; Smith, E.L.; Brentjens, R.J.; Wolchok, J.D. The future of cancer treatment: Immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 2016, 13, 273. [Google Scholar] [CrossRef]
- Lenders, V.; Koutsoumpou, X.; Sargsian, A.; Manshian, B.B. Biomedical nanomaterials for immunological applications: Ongoing research and clinical trials. Nanoscale Adv. 2020, 2, 5046–5089. Available online: https://pubs.rsc.org/en/content/articlehtml/2020/na/d0na00478b (accessed on 26 July 2022). [CrossRef] [PubMed]
- Chuang, S.T.; Conklin, B.; Stein, J.B.; Pan, G.; Lee, K.B. Nanotechnology-enabled immunoengineering approaches to advance therapeutic applications. Nano Converg. 2022, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Xu, W.; Li, Z.; Song, W.; Ding, J.; Chen, X. Immunomodulatory Nanosystems. Adv. Sci. 2019, 6, 1900101. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, M.; Kumar, R.; Belz, J.; Shanker, R.; Dwivedi, P.D.; Sridhar, S.; Singh, S.P. Synthesis and in vitro studies of PLGADTX nanoconjugate as potential drug delivery vehicle for oral cancer. Int. J. Nanomed. 2018, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Koopaei, M.N.; Khoshayand, M.R.; Mostafavi, S.H.; Amini, M.; Khorramizadeh, M.R.; Tehrani, M.J.; Atyabi, F.; Dinarvand, R. Docetaxel Loaded PEG-PLGA Nanoparticles: Optimized Drug Loading, In-vitro Cytotoxicity and In-vivo Antitumor Effect. Iran. J. Pharm. Res. IJPR 2014, 13, 819. [Google Scholar]
- Roy, R.; Parashar, V.; Chauhan, L.K.S.; Shanker, R.; Das, M.; Tripathi, A.; Dwivedi, P.D. Mechanism of uptake of ZnO nanoparticles and inflammatory responses in macrophages require PI3K mediated MAPKs signaling. Toxicol. Vitr. 2014, 28, 457–467. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967, 118, 29–32. Available online: https://www.sciencedirect.com/science/article/abs/pii/0003986167902731 (accessed on 27 July 2022). [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Moron, M.S.; Depierre, J.W.; Mannervik, B. Levels of glutathione, glutathione reductase and glutathione Stransferase activities in rat lung and liver. Biochim. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef]
- Tripathi, A.; Yadav, A.; Kumar, A.; Das, M. Sunset yellow FCF, a permitted food dye, alters functional responses of splenocytes at non-cytotoxic dose. Toxicol. Lett. 2013, 217, 197–204. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Souza, T.G.F.; Ciminelli, V.S.T.; Mohallem, N.D.S. A comparison of TEM and DLS methods to characterize size distribution of ceramic nanoparticles. J. Physics Conf. Ser. 2016, 733, 012039. Available online: https://iopscience.iop.org/article/10.1088/1742-6596/733/1/012039 (accessed on 27 July 2022). [CrossRef]
- De Jong, W.H.; Borm, P.J.A. Drug delivery and nanoparticles: Applications and hazards. Int. J. Nanomed. 2008, 3, 133. [Google Scholar] [CrossRef]
- Saqr, A.A.; Wani, S.U.D.; Gangadharappa, H.V.; Aldawsari, M.F.; Khafagy, E.S.; Abu Lila, A.S. Enhanced Cytotoxic Activity of Docetaxel-Loaded Silk Fibroin Nanoparticles against Breast Cancer Cells. Polymers 2021, 13, 1416. [Google Scholar] [CrossRef]
- Bowerman, C.J.; Byrne, J.D.; Chu, K.S.; Schorzman, A.N.; Keeler, A.W.; Sherwood, C.A.; Perry, J.L.; Luft, J.C.; Darr, D.B.; Deal, A.M.; et al. Docetaxel-loaded PLGA nanoparticles improve efficacy in taxane-resistant triple-negative breast cancer. Nano Lett. 2017, 17, 242–248. Available online: https://pubs.acs.org/doi/abs/10.1021/acs.nanolett.6b03971 (accessed on 27 July 2022). [CrossRef]
- Feng, X.; Xiong, X.; Ma, S. Docetaxel-Loaded Novel Nano-Platform for Synergistic Therapy of Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 230. [Google Scholar] [CrossRef]
- Lee, G.Y.; Mubasher, M.; Mckenzie, T.S.; Schmitt, N.C.; Sebelik, M.E.; Flanagan, C.E.; Osta, B.E.; Cothran, M.B.; Green, H.N. Assessment of a NanoDocetaxel Combined Treatment for Head and Neck Cancer. Onco 2021, 1, 83–94. Available online: https://www.mdpi.com/2673-7523/1/2/7/htm17 (accessed on 27 July 2022). [CrossRef]
- Wei, X.; Wei, R.; Jiang, G.; Jia, Y.; Lou, H.; Yang, Z.; Luo, D.; Huang, Q.; Xu, S.; Yang, X.; et al. Mechanical cues modulate cellular uptake of nanoparticles in cancer via clathrin-mediated and caveolae-mediated endocytosis pathways. Nanomedicine 2019, 14, 613–626. Available online: https://www.futuremedicine.com/doi/10.2217/nnm-2018-0334 (accessed on 27 July 2022). [CrossRef]
- Wang, Z.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells. ACS Nano 2009, 3, 4110. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-B.; Li, J.; Lai, X.-N.; Jiang, R.; Zhao, R.-C.; Xiong, L.-X. Multifaceted Roles of Caveolin-1 in Lung Cancer: A New Investigation Focused on Tumor Occurrence, Development and Therapy. Cancers 2020, 12, 291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Tan, H.L.; Huang, Q.; Sun, X.J.; Zhu, X.; Shen, H.M. zVAD-induced necroptosis in L929 cells depends on autocrine production of TNFα mediated by the PKC–MAPKs–AP-1 pathway. Cell Death Differ. 2011, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. Available online: https://www.nature.com/articles/onc2008297 (accessed on 27 July 2022). [CrossRef] [PubMed]
- Koike, A.; Hanatani, M.; Fujimori, K. Pan-caspase inhibitors induce necroptosis via ROS-mediated activationof mixed lineage kinase domain-like protein and p38 in classically activated macrophages. Exp. Cell. Res. 2019, 380, 171–179. Available online: https://pubmed.ncbi.nlm.nih.gov/31039349/ (accessed on 27 July 2022). [CrossRef]
- Görlach, A.; Bertram, K.; Hudecova, S.; Krizanova, O. Calcium and ROS: A mutual interplay. Redox Biol. 2015, 6, 260. [Google Scholar] [CrossRef]
- Tian, Q.; Qin, B.; Gu, Y.; Zhou, L.; Chen, S.; Zhang, S.; Zhang, S.; Han, Q.; Liu, Y.; Wu, X. ROS-Mediated Necroptosis Is Involved in Iron Overload-Induced Osteoblastic Cell Death. Oxidative Med. Cell. Longev. 2020, 2020, 1295382. [Google Scholar] [CrossRef]
- Deragon, M.A.; McCaig, W.D.; Patel, P.S.; Haluska, R.J.; Hodges, A.L.; Sosunov, S.A.; Murphy, M.P.; Ten, V.S.; LaRocca, T.J. Mitochondrial ROS prime the hyperglycemic shift from apoptosis to necroptosis. Cell Death Discov. 2020, 6, 132. Available online: https://www.nature.com/articles/s41420-020-00370-3 (accessed on 27 July 2022). [CrossRef]
- Hsu, S.K.; Chang, W.T.; Lin, I.L.; Chen, Y.F.; Padalwar, N.B.; Cheng, K.C.; Teng, Y.-N.; Wang, C.-H.; Chiu, C.-C. The Role of Necroptosis in ROSMediated Cancer Therapies and Its Promising Applications. Cancers 2020, 12, 2185. [Google Scholar] [CrossRef]
- Garnett, C.T.; Schlom, J.; Hodge, J.W. Combination of Docetaxel and Recombinant Vaccine Enhances T-Cell Responses and Antitumor Activity: Effects of Docetaxel on Immune Enhancement. Clin. Cancer Res. 2008, 14, 3536. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, R.; Li, C.; Song, Y.; Liu, G.; Huang, Q.; Yu, L.; Zhu, D.; Lu, C.; Lu, A.; et al. Nab-paclitaxel promotes the cancer-immunity cycle as18 a potential immunomodulator. Am. J. Cancer Res. 2021, 11, 3445. [Google Scholar]
- Tsavaris, N.; Kosmas, C.; Vadiaka, M.; Kanelopoulos, P.; Boulamatsis, D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br. J. Cancer 2002, 87, 21–27. Available online: https://pubmed.ncbi.nlm.nih.gov/12085250/ (accessed on 27 July 2022). [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, P.; Singh, A.; Verma, A.K.; Kant, S.; Pandey, A.K.; Khare, P.; Prakash, V. The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up. Vaccines 2022, 10, 1801. https://doi.org/10.3390/vaccines10111801
Gupta P, Singh A, Verma AK, Kant S, Pandey AK, Khare P, Prakash V. The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up. Vaccines. 2022; 10(11):1801. https://doi.org/10.3390/vaccines10111801
Chicago/Turabian StyleGupta, Parul, Arpita Singh, Ajay Kumar Verma, Surya Kant, Anuj Kumar Pandey, Puneet Khare, and Ved Prakash. 2022. "The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up" Vaccines 10, no. 11: 1801. https://doi.org/10.3390/vaccines10111801
APA StyleGupta, P., Singh, A., Verma, A. K., Kant, S., Pandey, A. K., Khare, P., & Prakash, V. (2022). The Anti-Tumor and Immunomodulatory Effects of PLGA-Based Docetaxel Nanoparticles in Lung Cancer: The Potential Involvement of Necroptotic Cell Death through Reactive Oxygen Species and Calcium Build-Up. Vaccines, 10(11), 1801. https://doi.org/10.3390/vaccines10111801



