Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Serology Assays
2.2. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Our World in Data Website. Coronavirus Pandemic (COVID-19). 2020 (updated 14 June 2022). Available online: https://ourworldindata.org/coronavirus (accessed on 15 September 2022).
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning immunity after the BNT162b2 vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Lustig, Y.; Zuckerman, N.; Nemet, I.; Atari, N.; Kliker, L.; Regev-Yochay, G.; Sapir, E.; Mor, O.; Alroy-Preis, S.; Mendelson, E.; et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Eurosurveillance 2021, 26, 2100557. [Google Scholar] [CrossRef]
- Bar-On, Y.M.; Goldberg, Y.; Mandel, M.; Bodenheimer, O.; Freedman, L.; Kalkstein, N.; Mizrahi, B.; Alroy-Preis, S.; Ash, N.; Milo, R.; et al. Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel. N. Engl. J. Med. 2021, 385, 1393–1400. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef]
- Redjoul, R.; Le Bouter, A.; Beckerich, F.; Fourati, S.; Maury, S. Antibody response after second BNT162b2 dose in allogeneic HSCT recipients. Lancet 2021, 398, 298–299. [Google Scholar] [CrossRef]
- Kamar, N.; Abravanel, F.; Marion, O.; Couat, C.; Izopet, J.; Del Bello, A. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N. Engl. J. Med. 2021, 385, 661–662. [Google Scholar] [CrossRef]
- Falsey, A.R.; Frenck, R.W., Jr.; Walsh, E.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Bailey, R.; Swanson, K.A.; Xu, X.; et al. SARS-CoV-2 neutralization with BNT162b2 vaccine dose 3. N. Engl. J. Med. 2021, 385, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung Transplant. 2022, 41, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Redjoul, R.; Le Bouter, A.; Parinet, V.; Fourati, S.; Maury, S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021, 8, e681–e683. [Google Scholar] [CrossRef]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.F.; Hanafi, L.; Faucon, A.-L.; Housset, P. SARS-CoV-2 antibody response after a third dose of the BNT162b2 vaccine in patients receiving maintenance hemodialysis or peritoneal dialysis. Am. J. Kidney Dis. 2022, 79, 185–192.e1. [Google Scholar] [CrossRef]
- Saiag, E.; Goldshmidt, H.; Sprecher, E.; Ben-Ami, R.; Bomze, D. Immunogenicity of a BNT162b2 vaccine booster in health-care workers. Lancet Microbe 2021, 2, e650. [Google Scholar] [CrossRef]
- Arbel, R.; Hammerman, A.; Sergienko, R.; Friger, M.; Peretz, A.; Netzer, D.; Yaron, S. BNT162b2 vaccine booster and mortality due to Covid-19. N. Engl. J. Med. 2021, 385, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Collie, S.; Champion, J.; Moultrie, H.; Bekker, L.G.; Gray, G. Effectiveness of BNT162b2 vaccine against Omicron variant in South Africa. N. Engl. J. Med. 2022, 386, 494–496. [Google Scholar] [CrossRef]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466.e4. [Google Scholar] [CrossRef]
- Cele, S.; Jackson, L.; Khoury, D.S.; Khan, K.; Moyo-Gwete, T.; Tegally, H.; San, J.E.; Cromer, D.; Scheepers, C.; Amoako, D.G.; et al. Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 2022, 602, 654–656. [Google Scholar] [CrossRef]
- Shachor-Meyouhas, Y.; Hussein, K.; Szwarcwort-Cohen, M.; Weissman, A.; Mekel, M.; Dabaja-Younis, H.; Hyams, G.; Horowitz, N.A.; Kaplan, M.; Halberthal, M. Single BNT162b2 vaccine dose produces seroconversion in under 60 s cohort. Vaccine 2021, 39, 6902–6906. [Google Scholar] [CrossRef]
- Shachor-Meyouhas, Y.; Hussein, K.; Dabaja-Younis, H.; Szwarcwort-Cohen, M.; Almog, R.; Weissman, A.; Mekel, M.; Hyams, G.; Horowitz, N.A.; Gepstein, V.; et al. Immunogenicity trends 1 and 3 months after second BNT162B2 vaccination among healthcare workers in Israel. Clin. Microbiol. Infect. 2022, 28, 450.e1–450.e4. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Andrée, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 coronavirus disease 2019 vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.-A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861.e1–1861.e5. [Google Scholar] [CrossRef] [PubMed]
- Tober-Lau, P.; Schwarz, T.; Vanshylla, K.; Hillus, D.; Gruell, H.; EICOV/COVIM Study Group; Suttorp, N.; Landgraf, I.; Kappert, K.; Seybold, J.; et al. Long-term immunogenicity of BNT162b2 vaccination in older people and younger health-care workers. Lancet Respir. Med. 2021, 9, e104–e105. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2022, 38, e3465. [Google Scholar] [CrossRef]
- Ferrara, P.; Ponticelli, D.; Agüero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, J.V.; Mantovani, L.G.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Gianfredi, V.; Tomaselli, V.; Polosa, R. The effect of smoking on humoral response to COVID-19 vaccines: A systematic review of epidemiological studies. Vaccines 2022, 10, 303. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Kurihara, M.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; et al. Age and smoking predict antibody titres at 3 months after the second dose of the BNT162b2 COVID-19 vaccine. Vaccines 2021, 9, 1042. [Google Scholar] [CrossRef]
- Reddy, R.K.; Charles, W.N.; Sklavounos, A.; Dutt, A.; Seed, P.T.; Khajuria, A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 1045–1056. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
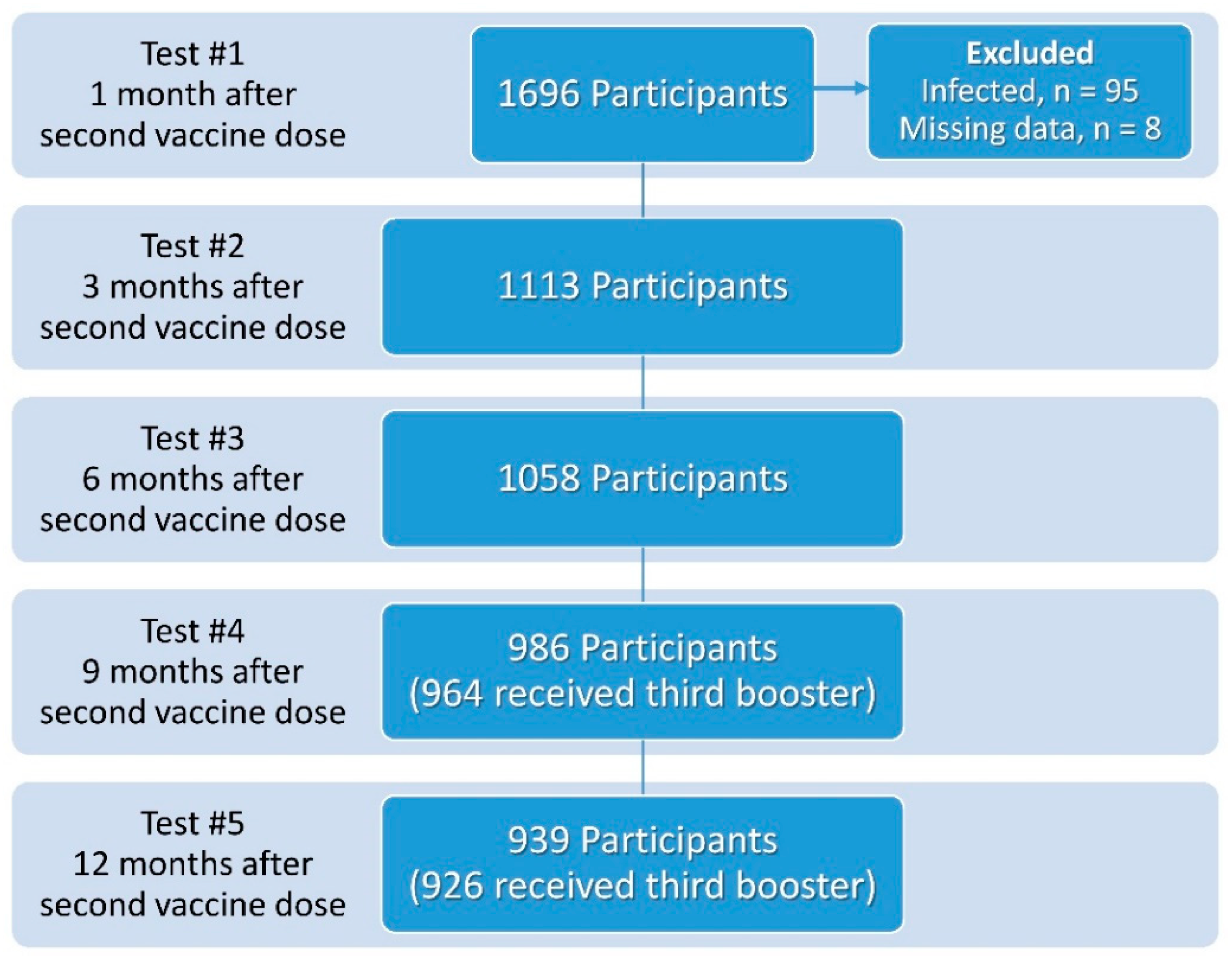
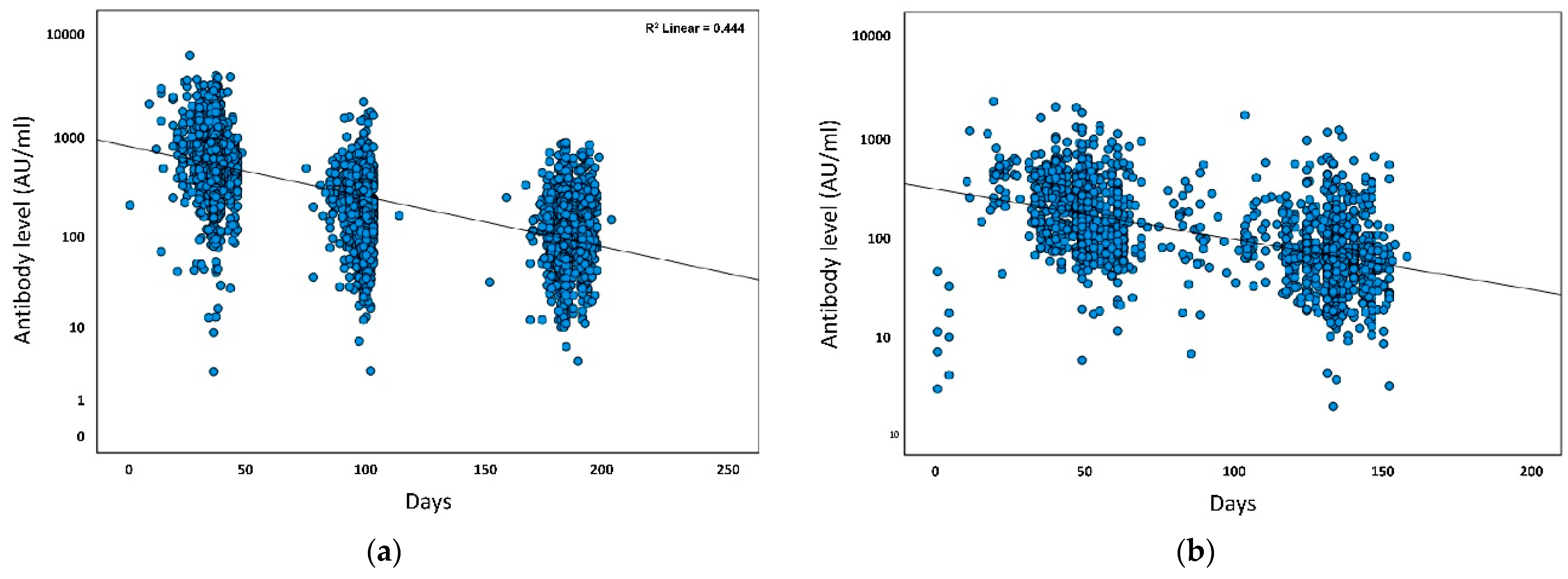
| Parameter | Test 1 (1 Month) n = 1588 | Test 2 (3 Months) n = 1113 | Test 3 (6 Months) n = 1058 | Test 4 (9 Months) n = 964 | Test 5 (12 Months) n = 926 |
|---|---|---|---|---|---|
| Age (mean ± SD) | 47.7 ± 12.6 | 49.2 ± 12.0 | 49.9 ± 12.1 | 50.8 ± 11.7 | 51.4 ± 11.6 |
| Sex | |||||
| Male | 480 (30%) | 297 (27%) | 286 (27%) | 249 (26%) | 252 (27%) |
| Female | 1108 (70%) | 816 (73%) | 772 (73%) | 715 (74%) | 674 (73%) |
| Underlying diseases * | |||||
| Heart disease † | 56 (3.5%) | 39 (3.5%) | 42 (4.0%) | 41 (4.3%) | 35 (3.8%) |
| Malignancy | 22 (1.4%) | 17 (1.5%) | 16 (1.5%) | 17 (1.8%) | 16 (1.7%) |
| Pulmonary disease | 57 (3.6%) | 42 (3.8%) | 45 (4.3%) | 37 (3.8%) | 37 (4.0%) |
| Systemic autoimmune disease | 109 (6.9%) | 95 (8.5%) | 83 (7.8%) | 83 (8.6%) | 78 (8.4%) |
| Immunodeficiency ‡ | 50 (3.1%) | 36 (3.2%) | 34 (3.2%) | 35 (3.6%) | 38 (4.1%) |
| Hypothyroidism | 142 (8.9%) | 119 (10.7%) | 112 (10.6%) | 97 (10.1%) | 92 (9.9%) |
| Chronic renal disease | 15 (0.9%) | 10 (0.9%) | 11 (1.0%) | 12 (1.2%) | 9 (1.0%) |
| Dialysis | 4 (0.3%) | 2 (0.2%) | 2 (0.2%) | 3 (0.3%) | 2 (0.2%) |
| Other | 241 (15.2%) | 182 (16.4%) | 184 (17.4%) | 166 (17.2%) | 160 (17.3%) |
| BMI § | n = 1082 | n = 907 | n = 882 | n = 888 | n = 808 |
| <18.5 | 17 (1.6%) | 12 (1.3%) | 13 (1.5%) | 15 (1.7%) | 12 (1.5%) |
| 18.5–24.9 | 503 (46.5%) | 423 (47%) | 405 (46%) | 404 (45.5%) | 364 (45%) |
| 25.0–29.9 | 363 (33.5%) | 309 (34%) | 301 (34%) | 309 (35%) | 285 (35%) |
| ≥30.0 | 199 (18%) | 163 (18%) | 163 (18.5%) | 160 (18%) | 147 (18%) |
| Smoker | 119 (7.5%) | 94 (8.4%) | 99 (9.4%) | 89 (9.2%) | 75 (8.1%) |
| Parameter | Test 1 (1 Month) n = 1588 | Test 2 (3 Months) n = 1113 | Test 3 (6 Months) n = 1058 | Test 4 (9 Months) n = 964 | Test 5 (12 Months) n = 926 |
|---|---|---|---|---|---|
| Mean | 683.01 | 223.75 | 132.25 | 2268.15 | 924.51 |
| Median | 568.50 | 176.00 | 98.50 | 1600.00 | 629.00 |
| Standard Deviation | 507.81 | 197.38 | 120.17 | 2301.73 | 1167.92 |
| Minimum | 3 | 3 | 4 | 2 | 19 |
| Maximum | 5920 | 2020 | 786 | 21,400 | 15,700 |
| Percentiles | |||||
| 25th | 391.50 | 100.50 | 53.00 | 861.50 | 397.00 |
| 50th | 568.50 | 176.00 | 98.50 | 1600.00 | 629.00 |
| 75th | 792.00 | 291.50 | 165.00 | 2792.50 | 927.00 |
| Parameter | Estimate * | p Value | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Age | −0.004 | <0.001 | −0.005 | −0.004 |
| Female sex | 0.039 | 0.003 | 0.013 | 0.066 |
| Heart disease † | −0.114 | <0.001 | −0.177 | −0.052 |
| Malignancy | −0.011 | 0.820 | −0.104 | 0.082 |
| Systemic autoimmune disease | 0.010 | 0.668 | −0.037 | 0.058 |
| Lung disease | 0.060 | 0.055 | −0.001 | 0.121 |
| Immunodeficiency ‡ | −0.178 | <0.001 | −0.251 | −0.106 |
| Hypothyroidism | 0.005 | 0.824 | −0.035 | 0.044 |
| Chronic kidney disease | −0.053 | 0.366 | −0.168 | 0.062 |
| Glucocorticosteroid treatment | −0.053 | 0.366 | −0.168 | 0.062 |
| Smoking | −0.081 | <0.001 | −0.122 | −0.040 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, K.; Dabaja-Younis, H.; Szwarcwort-Cohen, M.; Almog, R.; Leiba, R.; Weissman, A.; Mekel, M.; Hyams, G.; Horowitz, N.A.; Gepstein, V.; et al. Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers. Vaccines 2022, 10, 1741. https://doi.org/10.3390/vaccines10101741
Hussein K, Dabaja-Younis H, Szwarcwort-Cohen M, Almog R, Leiba R, Weissman A, Mekel M, Hyams G, Horowitz NA, Gepstein V, et al. Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers. Vaccines. 2022; 10(10):1741. https://doi.org/10.3390/vaccines10101741
Chicago/Turabian StyleHussein, Khetam, Halima Dabaja-Younis, Moran Szwarcwort-Cohen, Ronit Almog, Ronit Leiba, Avi Weissman, Michal Mekel, Gila Hyams, Nethanel A. Horowitz, Vardit Gepstein, and et al. 2022. "Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers" Vaccines 10, no. 10: 1741. https://doi.org/10.3390/vaccines10101741
APA StyleHussein, K., Dabaja-Younis, H., Szwarcwort-Cohen, M., Almog, R., Leiba, R., Weissman, A., Mekel, M., Hyams, G., Horowitz, N. A., Gepstein, V., Cohen Saban, H., Tarabeia, J., Halberthal, M., & Shachor-Meyouhas, Y. (2022). Third BNT162b2 Vaccine Booster Dose against SARS-CoV-2-Induced Antibody Response among Healthcare Workers. Vaccines, 10(10), 1741. https://doi.org/10.3390/vaccines10101741







