The Role of Reactive Species on Innate Immunity
Abstract
:1. Introduction
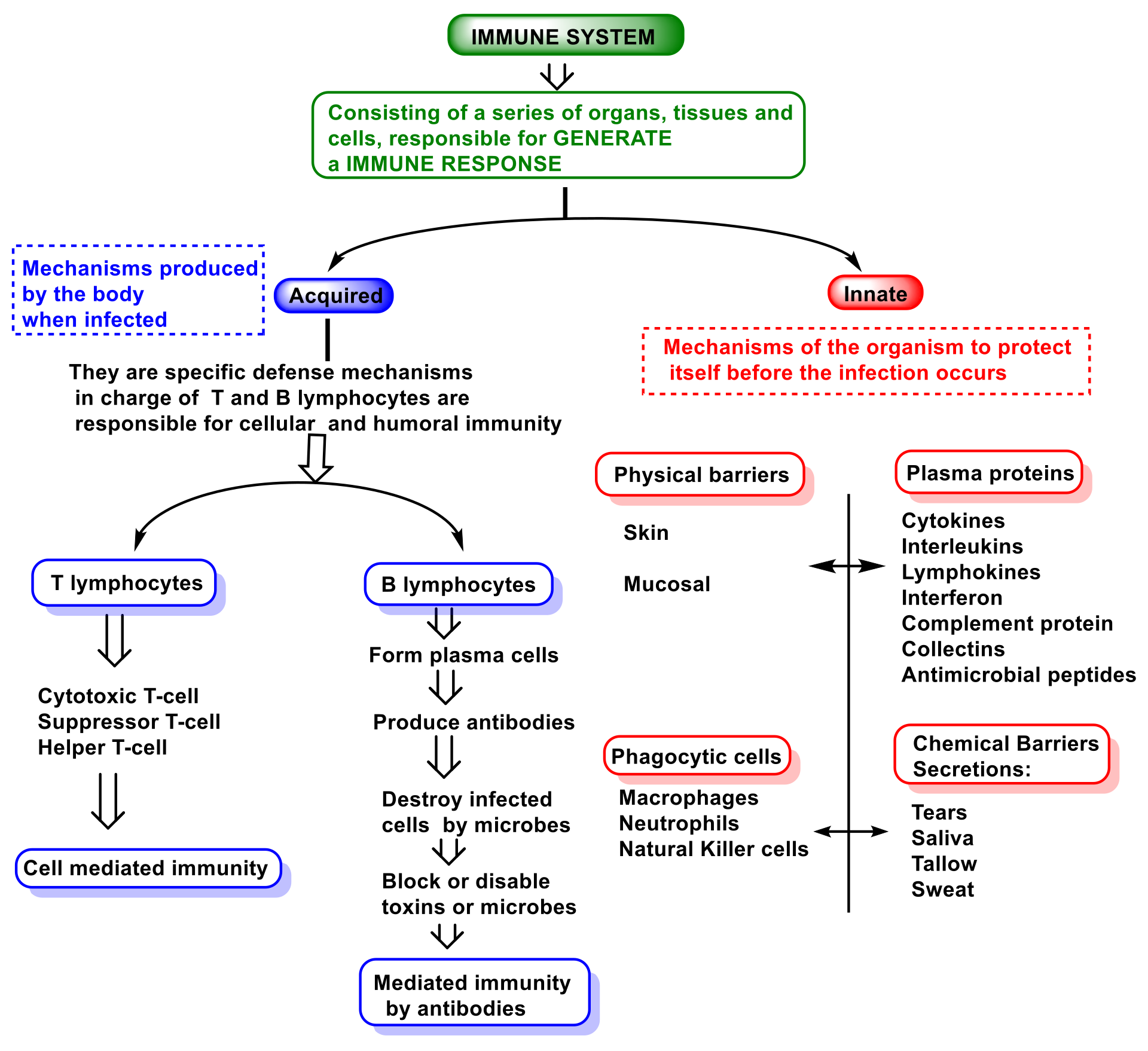
2. Function and Features of Immunity and Innate Immune System
- (i)
- The so-called alternative pathway is activated by the presence of bacterial surfaces that can bind complement protein C3. C3-coated bacteria are rapidly and efficiently phagocytosed and destroyed. C3 can activate other complement components by inducing a protein called C9 to insert itself into the cell walls of bacteria, causing them to rupture;
- (ii)
- A second pathway of complement activation is triggered when bacterial surface carbohydrates bind to a mannose-binding lectin (MBL), collectin 11 (CL-K1), and ficolins (Ficolin-1, Ficolin-2, and Ficolin-3). Its activation leads to C4 and C2 activation by their serine-proteases; or
- (iii)
- The classical complement pathway is initiated by antigen-antibody complexes with the antibody isotypes IgG and IgM. Upon activation, several proteins are recruited to generate C3 convertase, which cleaves the C3 protein. The C3b component of cleaved C3 binds to the C3 convertase to generate the C5 convertase, which cleaves the C5 protein. The cleaved products attract phagocytes to the site of infection and mark target cells for elimination by phagocytosis. C5 convertase initiates the terminal phase of the complement system, resulting in the assembly of the MAC membrane attack complex, creating a pore in the target cell membrane, and inducing its lysis [72].
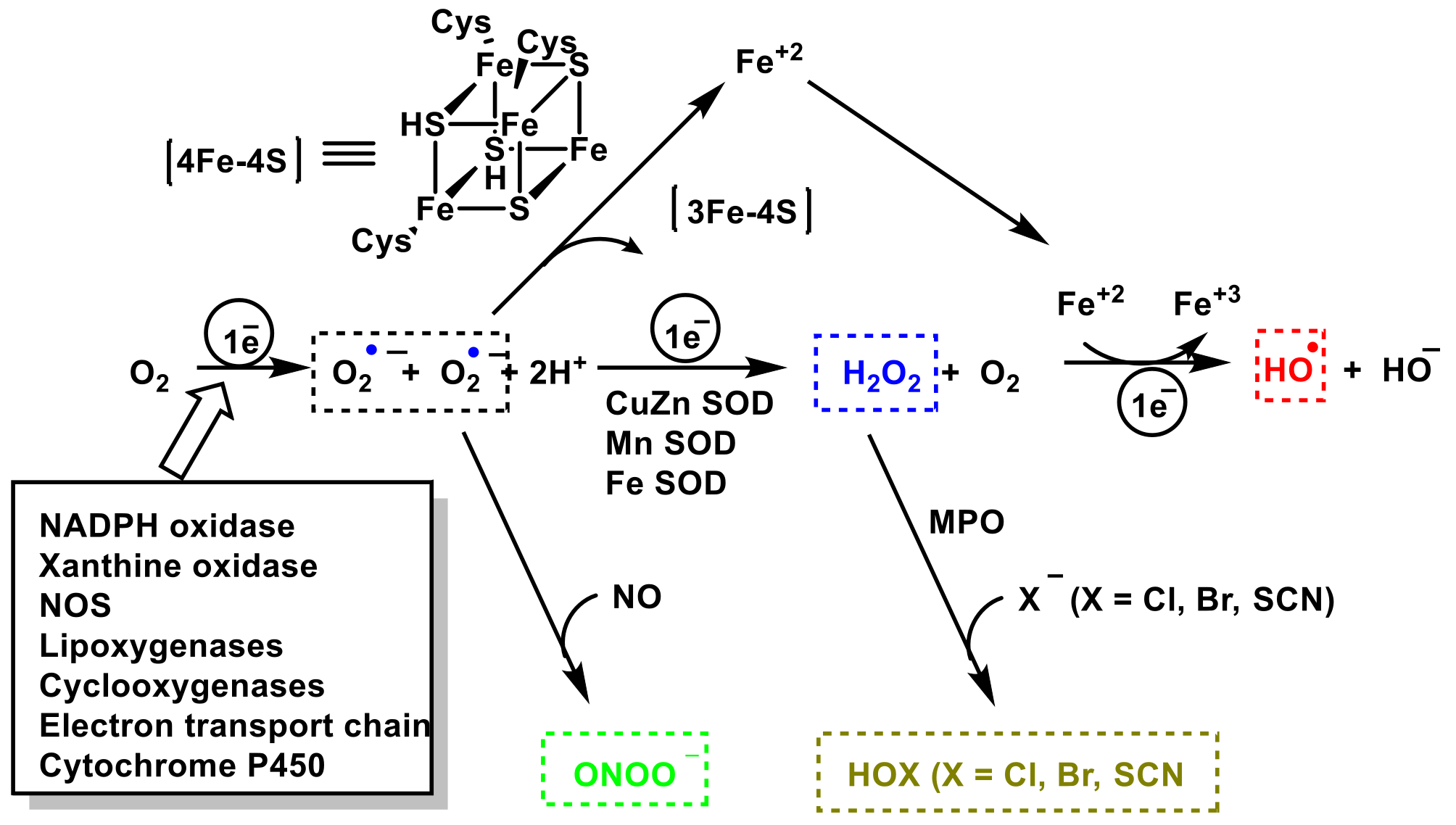
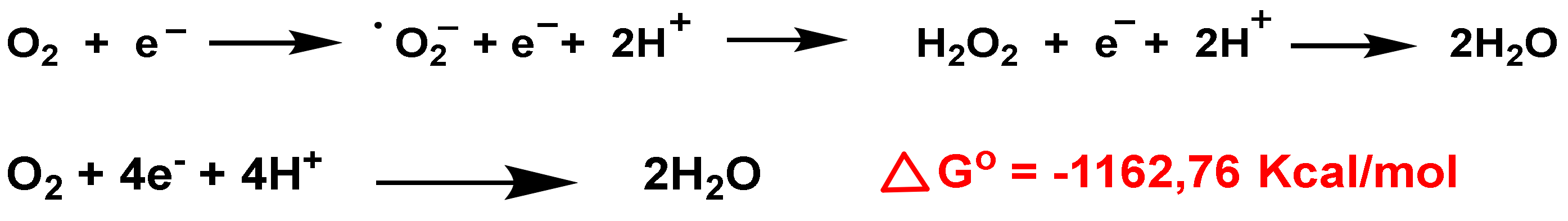
3. Role of Superoxide Anion •O2− and Hydrogen Peroxide H2O2 on Innate Immunity
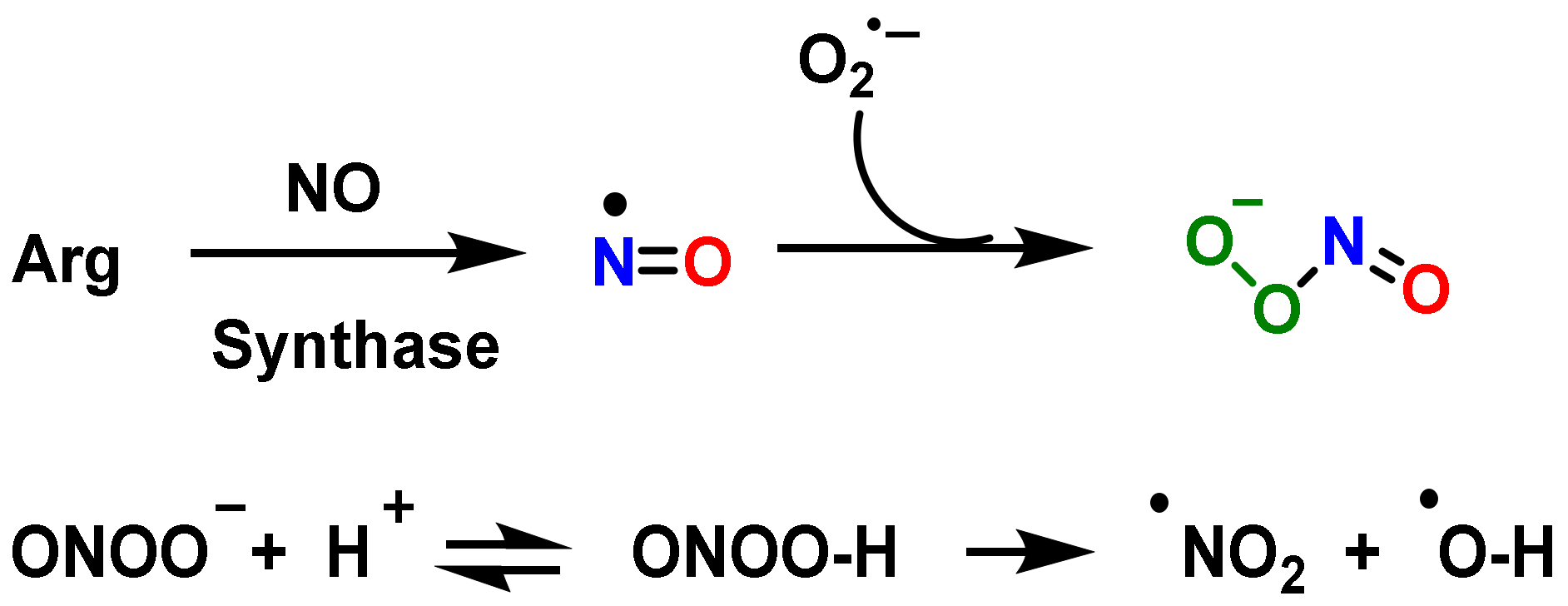
4. Role of Nitric Oxide Radical •NO and Peroxynitrite ONOO— on Innate Immunity
5. Role of Hypochlorous Acid HOCl on Innate Immunity
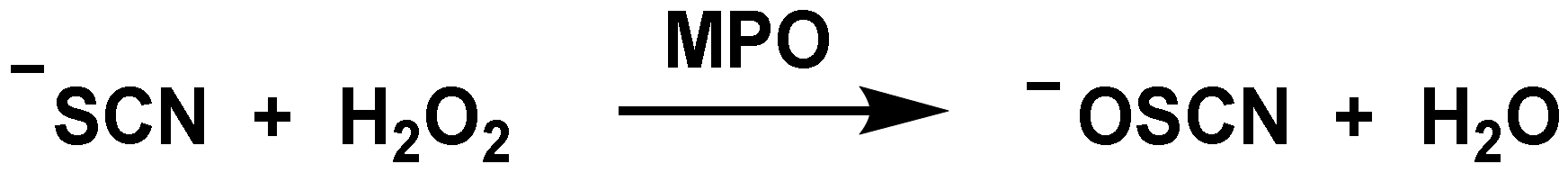
6. Role of Hypothiocyanite OSCN− on Innate Immunity
7. Antimicrobial Peptides and Induction of ROS
8. Mechanisms Developed by Microorganisms to Avoid the Reactive Species
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Methods Mol. Biol 2020, 2087, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Hackett, C.J. Innate immune activation as a broad-spectrum biodefense strategy: Prospects and research challenges. J. Allergy Clin. Immunol. 2003, 112, 686–694. [Google Scholar] [CrossRef]
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive oxygen species in pathogen clearance: The killing mechanisms, the adaption response, and the side effects. Front. Microbiol. 2021, 11, 622534. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regenerat. Res. 2013, 8, 2003. [Google Scholar]
- Fisher, A.B. Redox signaling across cell membranes. Antioxid Redox Signal. 2009, 11, 1349–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Royer-Pokora, B.; Kunkel, L.M.; Monaco, A.P.; Goff, S.C.; Newburger, P.E.; Baehner, R.L.; Cole, F.S.; Curnutte, J.T.; Orkin, S.H. Cloning the gene for an inherited human disorder—chronic granulomatous disease—On the basis of its chromosomal location. Nature 1986, 322, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinauer, M.C.; Orkin, S.H.; Brown, R.; Jesaitis, A.J.; Parkos, C.A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 1987, 327, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Parkos, C.A.; Allen, R.A.; Cochrane, C.G.; Jesaitis, A.J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J. Clin. Investig. 1987, 80, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W. Absence of both cytochrome b−245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature 1987, 326, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Segal, A.W.; Heyworth, P.G.; Cockcroft, S.; Barrowman, M.M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature 1985, 316, 547–549. [Google Scholar] [CrossRef]
- Volpp, B.D.; Nauseef, W.M.; Clark, R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 1988, 242, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Wientjes, F.B.; Hsuan, J.J.; Totty, N.F.; Segal, A.W. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. 1993, 296 (Pt. 3), 557–561. [Google Scholar] [CrossRef] [Green Version]
- Abo, A.; Pick, E. Purification and characterization of a third cytosolic component of the superoxide-generating NADPH oxidase of macrophages. J. Biol. Chem. 1991, 266, 23577–23585. [Google Scholar] [CrossRef]
- Roberts, A.W.; Kim, C.; Zhen, L.; Lowe, J.B.; Kapur, R.; Petryniak, B.; Spaetti, A.; Pollock, J.D.; Borneo, J.B.; Bradford, G.B.; et al. Deficiency of the Hematopoietic Cell-Specific Rho Family GTPase Rac2 Is Characterized by Abnormalities in Neutrophil Function and Host Defense. Immunity 1999, 10, 183–196. [Google Scholar] [CrossRef] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.R.; Frey, C.; Griffith, O.W. l-arginine binding to nitric-oxide synthase: The role of H-bonds to the nonreactive guanidinium nitrogens. J. Biol. Chem. 1999, 274, 25218–25226. [Google Scholar] [CrossRef] [Green Version]
- Radi, R.; Peluffo, G.; Alvarez, M.a.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488. [Google Scholar] [CrossRef]
- Prolo, C.; Álvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophagd-derived oxidizing cytotoxin to combat invading pathogens. Biofactors 2014, 40, 215–225. [Google Scholar] [CrossRef] [Green Version]
- Tobler, A.; Koeffler, H.P. Myeloperoxidase: Localization, structure, and function. In Blood Cell Biochemistry Volume 3; Springer: Berlin/Heidelberg, Germany, 1991; pp. 255–288. [Google Scholar]
- Hurst, J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012, 53, 508–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2010, 48, 8–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Principles of innate and adaptive immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Kiboneka, A. Principals of innate and adaptive immunity. Immunity to microbes & fundamental concepts in immunology. World J. Adv. Res. Rev. 2021, 10, 188–197. [Google Scholar]
- Zimmerman, L.; Vogel, L.; Bowden, R. Understanding the vertebrate immune system: Insights from the reptilian perspective. J. Exp. Biol. 2010, 213, 661–671. [Google Scholar] [CrossRef] [Green Version]
- Alberts, B. Molecular Biology of the Cell; WW Norton & Company: New York, NY, USA, 2017. [Google Scholar]
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139. [Google Scholar] [CrossRef] [PubMed]
- LaRosa, D.F.; Rahman, A.H.; Turka, L.A. The innate immune system in allograft rejection and tolerance. J. Immunol. 2007, 178, 7503–7509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75. [Google Scholar] [CrossRef] [PubMed]
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khovidhunkit, W.; Kim, M.-S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390. [Google Scholar] [CrossRef]
- Finberg, R.W.; Wang, J.P.; Kurt-Jones, E.A. Toll like receptors and viruses. Rev. Med. Virol. 2007, 17, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of miR-223-3p and miR-2909 on inflammatory factors IL-6, IL-1ß, and TNF-α, and the TLR4/TLR2/NF-κB/STAT3 signaling pathway induced by lipopolysaccharide in human adipose stem cells. PLoS ONE 2019, 14, e0212063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 2004, 25, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef] [PubMed]
- Muller, W. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, P.Z. Cytokines & their physiologic and pharmacologic functions in inflammation: A review. Int. J. Pharm. Life Sci. 2011, 2, 212599524. [Google Scholar]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113. [Google Scholar] [CrossRef]
- Favaro, R.R.; Phillips, K.; Delaunay-Danguy, R.; Ujčič, K.; Markert, U.R. Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction. Front. Immunol. 2022, 13, 824263. [Google Scholar] [CrossRef]
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 1–19. [Google Scholar] [CrossRef]
- Ochel, A.; Tiegs, G.; Neumann, K. Type 2 innate lymphoid cells in liver and gut: From current knowledge to future perspectives. Int. J. Mol. Sci. 2019, 20, 1896. [Google Scholar] [CrossRef] [Green Version]
- Maazi, H.; Akbari, O. Type two innate lymphoid cells: The Janus cells in health and disease. Immunol. Rev. 2017, 278, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Withers, D.R.; Hepworth, M.R. Group 3 innate lymphoid cells: Communications hubs of the intestinal immune system. Front. Immunol. 2017, 8, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Withers, D.R. Lymphoid tissue inducer cells. Curr. Biol. 2011, 21, R381–R382. [Google Scholar] [CrossRef]
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801. [Google Scholar] [CrossRef] [Green Version]
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021, 7, 1–10. [Google Scholar] [CrossRef]
- Huang, S.U.-S.; O’Sullivan, K.M. The expanding role of extracellular traps in inflammation and autoimmunity: The new players in casting dark webs. Int. J. Mol. Sci. 2022, 23, 3793. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.; Prikhodko, A.; Galkin, I.; Pletjushkina, O.; Zinovkin, R.; Sud’ina, G.; Chernyak, B.; Pinegin, B. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 2017, 96, 254–265. [Google Scholar] [CrossRef]
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165664. [Google Scholar] [CrossRef] [PubMed]
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood J. Am. Soc. Hematol. 2013, 122, 2784–2794. [Google Scholar] [CrossRef] [PubMed]
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry (Mosc) 2020, 85, 1178–1190. [Google Scholar] [CrossRef]
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermöhlen, O.; Krönke, M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Allan, E.R.; Tailor, P.; Balce, D.R.; Pirzadeh, P.; McKenna, N.T.; Renaux, B.; Warren, A.L.; Jirik, F.R.; Yates, R.M. NADPH oxidase modifies patterns of MHC class II–restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 2014, 192, 4989–5001. [Google Scholar] [CrossRef] [Green Version]
- Metz-Boutigue, M.-H.; Shooshtarizadeh, P.; Prevost, G.; Haikel, Y.; Chich, J.-F. Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Curr. Pharm. Design 2010, 16, 1024–1039. [Google Scholar] [CrossRef]
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235. [Google Scholar] [CrossRef]
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef] [Green Version]
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740. [Google Scholar] [CrossRef]
- Collard, C.D.; Väkevä, A.; Morrissey, M.A.; Agah, A.; Rollins, S.A.; Reenstra, W.R.; Buras, J.A.; Meri, S.; Stahl, G.L. Complement activation after oxidative stress: Role of the lectin complement pathway. Am. J. Pathol. 2000, 156, 1549–1556. [Google Scholar] [CrossRef]
- Tabassum, N.; Kheya, I.S.; Asaduzzaman, S.; Maniha, S.; Fayz, A.H.; Zakaria, A.; Noor, R. A review on the possible leakage of electrons through the electron transport chain within mitochondria. Life Sci. 2020, 6, 105–113. [Google Scholar] [CrossRef]
- Wittmann, C.; Chockley, P.; Singh, S.K.; Pase, L.; Lieschke, G.J.; Grabher, C. Hydrogen peroxide in inflammation: Messenger, guide, and assassin. Adv. Hematol. 2012, 2012, 541471. [Google Scholar] [CrossRef]
- Schoonbroodt, S.; Ferreira, V.; Best-Belpomme, M.; Boelaert, J.R.; Legrand-Poels, S.; Korner, M.; Piette, J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of IκBα in NF-κB activation by an oxidative stress. J. Immunol. 2000, 164, 4292–4300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Sansonetti, P.J. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 2004, 173, 4197–4206. [Google Scholar] [CrossRef] [Green Version]
- Filimon, A.; Preda, I.A.; Boloca, A.F.; Negroiu, G. Interleukin-8 in Melanoma Pathogenesis, Prognosis and Therapy—An Integrated View into Other Neoplasms and Chemokine Networks. Cells 2021, 11, 120. [Google Scholar] [CrossRef]
- He, H.-Q.; Ye, R.D. The formyl peptide receptors: Diversity of ligands and mechanism for recognition. Molecules 2017, 22, 455. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.D.; DeLeo, F.R. Role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscp. Rev. Syst. Biol. Med. 2009, 1, 309–333. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, G.; Green, E.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell Infect. Microbiol. 2017, 7, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsbach, P.; Weiss, J. Oxygen-dependent and oxygen-independent mechanisms of microbicidal activity of neutrophils. Immunol. Lett. 1985, 11, 159–163. [Google Scholar] [CrossRef]
- Davies, M.J. Reactivity of Peroxidase-Derived Oxidants with Proteins, Glycoproteins and Proteoglycans. In Mammalian Heme Peroxidases; CRC Press: Boca Raton, FL, USA, 2021; pp. 53–77. [Google Scholar]
- Scieszka, D.; Lin, Y.-H.; Li, W.; Choudhury, S.; Yu, Y.; Freire, M. NETome: The molecular characterization of neutrophil extracellular traps (NETs). Cold Spring Harbor Lab. 2020. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Demaurex, N.; Petheö, G.L. Electron and proton transport by NADPH oxidases. Philos. Trans. Royal Soc. B Biol. Sci. 2005, 360, 2315–2325. [Google Scholar] [CrossRef] [Green Version]
- Waghela, B.N.; Vaidya, F.U.; Agrawal, Y.; Santra, M.K.; Mishra, V.; Pathak, C. Molecular insights of NADPH oxidases and its pathological consequences. Cell Biochem. Funct. 2021, 39, 218–234. [Google Scholar] [CrossRef]
- Purushothaman, D.; Sarin, A. Cytokine-dependent regulation of NADPH oxidase activity and the consequences for activated T cell homeostasis. J. Exp. Med. 2009, 206, 1515–1523. [Google Scholar] [CrossRef] [Green Version]
- Manea, S.-A.; Constantin, A.; Manda, G.; Sasson, S.; Manea, A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015, 5, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil extracellular trap formation: Physiology, pathology, and pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef] [Green Version]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50. [Google Scholar] [CrossRef] [Green Version]
- Mócsai, A.; Zhou, M.; Meng, F.; Tybulewicz, V.L.; Lowell, C.A. Syk is required for integrin signaling in neutrophils. Immunity 2002, 16, 547–558. [Google Scholar] [CrossRef] [Green Version]
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402. [Google Scholar] [CrossRef]
- Kerrigan, A.M.; Brown, G.D. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011, 32, 151–156. [Google Scholar] [CrossRef]
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridovich, I. The biology of oxygen radicals: The superoxide radical is an agent of oxygen toxicity; superoxide dismutases provide an important defense. Science 1978, 201, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [Green Version]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Richard, G.; Salvador, M. Nitric oxide synthase in mammals. Biochem. J. 1994, 298, 249–258. [Google Scholar]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, T.; Sershen, C.L.; May, E.E. Investigating the role of TNF-α and IFN-γ activation on the dynamics of iNOS gene expression in LPS stimulated macrophages. PLoS ONE 2016, 11, e0153289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vásquez-Vivar, J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009, 47, 1108–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oess, S.; Icking, A.; Fulton, D.; Govers, R.; Müller-Esterl, W. Subcellular targeting and trafficking of nitric oxide synthases. Biochem. J. 2006, 396, 401–409. [Google Scholar] [CrossRef]
- Cronin, S.J.; Woolf, C.J.; Weiss, G.; Penninger, J.M. The role of iron regulation in immunometabolism and immune-related disease. Front. Mol. Biosci. 2019, 6, 116. [Google Scholar] [CrossRef] [Green Version]
- Delano, M.J.; Ward, P.A. The immune system’s role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016, 274, 330–353. [Google Scholar] [CrossRef] [Green Version]
- Geissmann, F.; Manz, M.G.; Jung, S.; Siewene, M.H.; Merad, M.; Ley, K. Development of Monocytes, Macrophages, and Dendritic Cells. Science 2010, 327, 656Y656. [Google Scholar] [CrossRef] [Green Version]
- Kröncke, K.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Viallard, J.F.; Pellegrin, J.; Ranchin, V.; Schaeverbeke, T.; Dehais, J.; Longy-Boursier, M.; Ragnaud, J.; Leng, B.; Moreau, J. Th1 (IL-2, interferon-gamma (IFN-γ)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin. Exp. Immunol. 1999, 115, 189–195. [Google Scholar] [CrossRef]
- Niedbala, W.; Alves-Filho, J.C.; Fukada, S.Y.; Vieira, S.M.; Mitani, A.; Sonego, F.; Mirchandani, A.; Nascimento, D.C.; Cunha, F.Q.; Liew, F.Y. Regulation of type 17 helper T-cell function by nitric oxide during inflammation. Proc. Natl. Acad. Sci. USA 2011, 108, 9220–9225. [Google Scholar] [CrossRef] [Green Version]
- Gerdes, H.J.; Yang, M.; Heisner, J.S.; Camara, A.K.; Stowe, D.F. Modulation of peroxynitrite produced via mitochondrial nitric oxide synthesis during Ca2+ and succinate-induced oxidative stress in cardiac isolated mitochondria. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148290. [Google Scholar] [CrossRef] [PubMed]
- Hagman, S.; Mäkinen, A.; Ylä-Outinen, L.; Huhtala, H.; Elovaara, I.; Narkilahti, S. Effects of inflammatory cytokines IFN-γ, TNF-α and IL-6 on the viability and functionality of human pluripotent stem cell-derived neural cells. J. Neuroimmunol. 2019, 331, 36–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, Y.; Marshall, H.E. S-nitrosylation in the regulation of gene transcription. Biochim. Biophys. Acta Gener. Subj. 2012, 1820, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Z.; Gibson, S.A.; Buckley, J.A.; Qin, H.; Benveniste, E.N. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol. 2018, 189, 4–13. [Google Scholar] [CrossRef]
- Wo, Y.; Brisbois, E.J.; Bartlett, R.H.; Meyerhoff, M.E. Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: Just say yes to nitric oxide (NO). Biomater. Sci. 2016, 4, 1161–1183. [Google Scholar] [CrossRef] [Green Version]
- Adler, B.L.; Friedman, A.J. Nitric oxide therapy for dermatologic disease. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Herb, M.; Schramm, M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, B.; Radi, R. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 2003, 25, 295–311. [Google Scholar] [CrossRef]
- Kobayashi, Y. The regulatory role of nitric oxide in proinflammatory cytokine expression during the induction and resolution of inflammation. J. Leukoc. Biol. 2010, 88, 1157–1162. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raulli, R.; McElhaney-Feser, G.; Hrabie, J.; Cihlar, R. Antimicrobial properties of nitric oxide using diazeniumdiolates as the nitric oxide donor. Recent Res. Dev. Microbiol 2002, 6, 177–183. [Google Scholar]
- Shreshtha, S.; Sharma, P.; Kumar, P.; Sharma, R.; Singh, S. Nitric Oxide: It’s Role in Immunity. J.Clin. Diagn. Res. 2018, 12, 1–5. [Google Scholar] [CrossRef]
- Ip, W.E.; Sokolovska, A.; Charriere, G.M.; Boyer, L.; Dejardin, S.; Cappillino, M.P.; Yantosca, L.M.; Takahashi, K.; Moore, K.J.; Lacy-Hulbert, A. Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to Staphylococcus aureus. J. Immunol. 2010, 184, 7071–7081. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Feura, E.S.; Ahonen, M.J.R.; Schoenfisch, M.H. Nitric oxide–releasing macromolecular scaffolds for antibacterial applications. Adv. Healthc. Mater. 2018, 7, 1800155. [Google Scholar] [CrossRef] [PubMed]
- Schnappinger, C.F.N.; Ehrt, S. Acid-Susceptible Mutants of. J. Bacteriol 2009, 191, 625. [Google Scholar]
- Torres, D.; Barrier, M.; Bihl, F.; Quesniaux, V.J.; Maillet, I.; Akira, S.; Ryffel, B.; Erard, F. Toll-like receptor 2 is required for optimal control of Listeria monocytogenes infection. Infect. Immun. 2004, 72, 2131–2139. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zheng, S.; Dweik, R.A.; Erzurum, S.C. Role of epithelial nitric oxide in airway viral infection. Free Radic. Biol. Med. 2006, 41, 19–28. [Google Scholar] [CrossRef]
- Abdul-Cader, M.S.; Amarasinghe, A.; Abdul-Careem, M.F. Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses. Arch. Virol. 2016, 161, 2075–2086. [Google Scholar] [CrossRef]
- Saura, M.; Zaragoza, C.; McMillan, A.; Quick, R.A.; Hohenadl, C.; Lowenstein, J.M.; Lowenstein, C.J. An antiviral mechanism of nitric oxide: Inhibition of a viral protease. Immunity 1999, 10, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Pigott, D.C. Hemorrhagic fever viruses. Crit. Care Clin. 2005, 21, 765–783. [Google Scholar] [CrossRef] [PubMed]
- Kun, J.F.; Mordmüller, B.; Perkins, D.J.; May, J.; Mercereau-Puijalon, O.; Alpers, M.; Weinberg, J.B.; Kremsner, P.G. Nitric oxide synthase 2Lambaréné (G-954C), increased nitric oxide production, and protection against malaria. J. Infect. Dis. 2001, 184, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho, N.; Bombaça, A.C.; Wiśniewski, J.R.; Dias-Lopes, G.; Saboia-Vahia, L.; Cupolillo, E.; de Jesus, J.B.; de Almeida, R.P.; Padrón, G.; Menna-Barreto, R. Nitric Oxide Resistance in Leishmania (Viannia) braziliensis Involves Regulation of Glucose Consumption, Glutathione Metabolism and Abundance of Pentose Phosphate Pathway Enzymes. Antioxidants 2022, 11, 277. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, C.; Martinez, R.; Figueiredo, R.T.; Bozza, M.T.; Lima, F.R.; Pires, A.L.; Silva, P.M.; Bonomo, A.; Lannes-Vieira, J.; De Souza, W. Soluble factors released by Toxoplasma gondii-infected astrocytes down-modulate nitric oxide production by gamma interferon-activated microglia and prevent neuronal degeneration. Infect. Immun. 2003, 71, 2047–2057. [Google Scholar] [CrossRef] [Green Version]
- Zarebavani, M.; Dargahi, D.; Einollahi, N.; Dashti, N.; Safari, F.; Rezaeian, M. Significance of nitric oxide level in giardiasis. Clin. Lab. 2017, 63, 47–52. [Google Scholar] [CrossRef]
- Beal, M.F.; Ferrante, R.J.; Browne, S.E.; Matthews, R.T.; Kowall, N.W.; Brown, R.H., Jr. Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1997, 42, 644–654. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Shinobu, L.A.; Schulz, J.B.; Matthews, R.T.; Thomas, C.E.; Kowall, N.W.; Gurney, M.E.; Beal, M.F. Increased 3-nitrotyrosine and oxidative damage in mice with a human copper/zinc superoxide dismutase mutation. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1997, 42, 326–334. [Google Scholar] [CrossRef]
- Liberatore, G.T.; Jackson-Lewis, V.; Vukosavic, S.; Mandir, A.S.; Vila, M.; McAuliffe, W.G.; Dawson, V.L.; Dawson, T.M.; Przedborski, S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999, 5, 1403–1409. [Google Scholar] [CrossRef]
- Schulz, J.B.; Matthews, R.T.; Muqit, M.M.; Browne, S.E.; Beal, M.F. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J. Neurochem. 1995, 64, 936–939. [Google Scholar] [CrossRef]
- Yoshida, T.; Limmroth, V.; Irikura, K.; Moskowitz, M.A. The NOS inhibitor, 7-nitroindazole, decreases focal infarct volume but not the response to topical acetylcholine in pial vessels. J. Cerebral Blood Flow Metab. 1994, 14, 924–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siraki, A.G. The many roles of myeloperoxidase: From inflammation and immunity to biomarkers, drug metabolism and drug discovery. Redox Biol. 2021, 46, 102109. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food Sci. Nutr. 2021, 62, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Malle, E. Halogenation Activity of Mammalian Heme Peroxidases. Antioxidants 2022, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.M.; Davies, K. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem. J. 1988, 254, 685–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storkey, C.; Davies, M.J.; Pattison, D.I. Reevaluation of the rate constants for the reaction of hypochlorous acid (HOCl) with cysteine, methionine, and peptide derivatives using a new competition kinetic approach. Free Radic. Biol. Med. 2014, 73, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Hypochlorous Acid Chemistry in Mammalian Cells-Influence on Infection and Role in Various Pathologies. Int. J. Mol. Sci. 2022, 23, 735. [Google Scholar] [CrossRef]
- Schuller-Levis, G.B.; Park, E. Taurine and its chloramine: Modulators of immunity. Neurochem. Res. 2004, 29, 117–126. [Google Scholar] [CrossRef]
- Wang, L.; Bassiri, M.; Najafi, R.; Najafi, K.; Yang, J.; Khosrovi, B.; Hwong, W.; Barati, E.; Belisle, B.; Celeri, C. Hypochlorous acid as a potential wound care agent: Part I. Stabilized hypochlorous acid: A component of the inorganic armamentarium of innate immunity. J. Burns Wounds 2007, 6, e5. [Google Scholar] [PubMed]
- Robson, M.C.; Payne, W.G.; Ko, F.; Mentis, M.; Donati, G.; Shafii, S.M.; Culverhouse, S.; Wang, L.; Khosrovi, B.; Najafi, R. Hypochlorous acid as a potential wound care agent: Part II. Stabilized hypochlorous acid: Its role in decreasing tissue bacterial bioburden and overcoming the inhibition of infection on wound healing. J. Burns Wounds 2007, 6, e6. [Google Scholar]
- Snell, J.A.; Jandova, J.; Wondrak, G.T. Hypochlorous Acid: From Innate Immune Factor and Environmental Toxicant to Chemopreventive Agent Targeting Solar UV-Induced Skin Cancer. Front. Oncol. 2022, 12, 887220. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhou, F.; Wang, Y.; Feng, H.; Meng, Q.; Zhang, Z.; Zhang, R. A redox-switchable colorimetric probe for “naked-eye” detection of hypochlorous acid and glutathione. Molecules 2019, 24, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colon, S.; Page-McCaw, P.; Bhave, G. Role of hypohalous acids in basement membrane homeostasis. Antioxid. Redox Signal. 2017, 27, 839–854. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Network 2018, 18, e27. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyoshi-Koshio, T.; Utsuki, Y.; Mizuno, S.; Suzuki, K. Virucidal activity and viral protein modification by myeloperoxidase: A candidate for defense factor of human polymorphonuclear leukocytes against influenza virus infection. J. Infect. Dis. 1991, 164, 8–14. [Google Scholar] [CrossRef]
- Strandin, T.; Mäkelä, S.; Mustonen, J.; Vaheri, A. Neutrophil activation in acute hemorrhagic fever with renal syndrome is mediated by hantavirus-infected microvascular endothelial cells. Front. Immunol. 2018, 9, 2098. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, S.; Chelluboina, S.; Jedge, P.; Doke, P.; Palkar, S.; Mishra, A.C.; Arankalle, V.A. Elevated levels of neutrophil activated proteins, alpha-defensins (DEFA1), calprotectin (S100A8/A9) and myeloperoxidase (MPO) are associated with disease severity in COVID-19 patients. Front. Cell. Infec. Microbiol. 2021, 2021, 1056. [Google Scholar] [CrossRef]
- Goud, P.T.; Bai, D.; Abu-Soud, H.M. A multiple-hit hypothesis involving reactive oxygen species and myeloperoxidase explains clinical deterioration and fatality in COVID-19. Int. J. Biol. Sci. 2021, 17, 62. [Google Scholar] [CrossRef]
- Remucal, C.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Khazaei, A.; Sarmasti, N.; Seyf, J.Y.; Merati, Z. Anchoring N-Halo (sodium dichloroisocyanurate) on the nano-Fe3O4 surface as “chlorine reservoir”: Antibacterial properties and wastewater treatment. Arab. J. Chem. 2020, 13, 2219–2232. [Google Scholar] [CrossRef]
- Thomas, E.; Pruitt, K.; Tenovuo, J. The Lactoperoxidase System, Chemsistry and Biological Significance; CRC Press: Boca Raton, FL, USA, 1985. [Google Scholar]
- Klebanoff, S.J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 1980, 93, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.M.; LaMar, A.; He, X.; Braverman, L.E.; Pearce, E.N. Iodine status and thyroid function of Boston-area vegetarians and vegans. J. Clin. Endocrinol. Metab. 2011, 96, E1303–E1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senthilmohan, R.; Kettle, A.J. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch. Biochem. Biophys. 2006, 445, 235–244. [Google Scholar] [CrossRef]
- Van Dalen, J.C.; Whitehouse, W.M.; Winterbourn, C.C.; Kettle, J.A. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem. J. 1997, 327, 487–492. [Google Scholar] [CrossRef] [Green Version]
- Furtmüller, P.G.; Zederbauer, M.; Jantschko, W.; Helm, J.; Bogner, M.; Jakopitsch, C.; Obinger, C. Active site structure and catalytic mechanisms of human peroxidases. Arch. Biochem. Biophys. 2006, 445, 199–213. [Google Scholar] [CrossRef]
- Cegolon, L.; Javanbakht, M.; Mastrangelo, G. Nasal disinfection for the prevention and control of COVID-19: A scoping review on potential chemo-preventive agents. Int. J. Hyg. Environ. Health 2020, 230, 113605. [Google Scholar] [CrossRef]
- Cegolon, L.; Mirandola, M.; Salaris, C.; Salvati, M.V.; Mastrangelo, G.; Salata, C. Hypothiocyanite and hypothiocyanite/lactoferrin mixture exhibit virucidal activity in vitro against SARS-CoV-2. Pathogens 2021, 10, 233. [Google Scholar] [CrossRef]
- Boto, A.; Pérez de la Lastra, J.M.; González, C.C. The road from host-defense peptides to a new generation of antimicrobial drugs. Molecules 2018, 23, 311. [Google Scholar] [CrossRef] [Green Version]
- Otazo-Pérez, A.; Asensio-Calavia, P.; González-Acosta, S.; Baca-González, V.; López, M.R.; Morales-delaNuez, A.; Pérez de la Lastra, J.M. Antimicrobial Activity of Cathelicidin-Derived Peptide from the Iberian Mole Talpa occidentalis. Vaccines 2022, 10, 1105. [Google Scholar] [CrossRef] [PubMed]
- Braff, M.H.; Di Nardo, A.; Gallo, R.L. Keratinocytes store the antimicrobial peptide cathelicidin in lamellar bodies. J. Investig. Dermatol. 2005, 124, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, G.M. Secretion from myeloid cells: Secretory lysosomes. Microbiol. Spectr. 2016, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, M.; Gennaro, R.; Skerlavaj, B.; Tomasinsig, L.; Circo, R. Cathelicidin peptides as candidates for a novel class of antimicrobials. Curr. Pharm. Design 2002, 8, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Shinnar, A.E.; Butler, K.L.; Park, H.J. Cathelicidin family of antimicrobial peptides: Proteolytic processing and protease resistance. Bioorg. Chem. 2003, 31, 425–436. [Google Scholar] [CrossRef]
- Zaiou, M.; Nizet, V.; Gallo, R.L. Antimicrobial and protease inhibitory functions of the human cathelicidin (hCAP18/LL-37) prosequence. J. Investig. Dermatol. 2003, 120, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Rowe-Magnus, D.A.; Kao, A.Y.; Prieto, A.C.; Pu, M.; Kao, C. Cathelicidin peptides restrict bacterial growth via membrane perturbation and induction of reactive oxygen species. MBio 2019, 10, e02019–e02021. [Google Scholar] [CrossRef]
- Yang, B.; Good, D.; Mosaiab, T.; Liu, W.; Ni, G.; Kaur, J.; Liu, X.; Jessop, C.; Yang, L.; Fadhil, R. Significance of LL-37 on immunomodulation and disease outcome. BioMed Res. Int. 2020, 2020, 8349712. [Google Scholar] [CrossRef]
- Dong, X.; Wu, D.; Zhang, Y.; Jia, L.; Pan, X.; Sun, J.; Pan, L.-L. Cathelicidin modulates vascular smooth muscle cell phenotypic switching through ROS/IL-6 pathway. Antioxidants 2020, 9, 491. [Google Scholar] [CrossRef]
- Memariani, H.; Memariani, M. Anti-fungal properties and mechanisms of melittin. Appl. Microbiol. Biotechnol. 2020, 104, 6513–6526. [Google Scholar] [CrossRef]
- Kim, S.R.; Park, S.-W. Papiliocin, an antimicrobial peptide, rescues hyperoxia-induced intestinal injury. Int. J. Ind. Entomol. 2021, 43, 94–98. [Google Scholar]
- Lee, J.; Lee, D.; Choi, H.; Kim, H.H.; Kim, H.; Hwang, J.S.; Lee, D.G.; Kim, J.I. Structure-activity relationships of the intramolecular disulfide bonds in coprisin, a defensin from the dung beetle. BMB Rep. 2014, 47, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hwang, J.-S.; Hwang, I.-s.; Cho, J.; Lee, E.; Kim, Y.; Lee, D.G. Coprisin-induced antifungal effects in Candida albicans correlate with apoptotic mechanisms. Free Radic. Biol. Med. 2012, 52, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Wei, L.; Che, H.; Shen, Y.; Yang, J.; Mi, K.; Liu, J.; Wu, J.; Yang, H.; Mu, L. A Frog Peptide Ameliorates Skin Photoaging Through Scavenging Reactive Oxygen Species. Front. Pharmacol. 2021, 12, 761011. [Google Scholar] [CrossRef] [PubMed]
- Reniere, M.L.; Whiteley, A.T.; Hamilton, K.L.; John, S.M.; Lauer, P.; Brennan, R.G.; Portnoy, D.A. Glutathione activates virulence gene expression of an intracellular pathogen. Nature 2015, 517, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Juttukonda, L.J.; Green, E.R.; Lonergan, Z.R.; Heffern, M.C.; Chang, C.J.; Skaar, E.P. Acinetobacter baumannii OxyR Regulates the Transcriptional Response to Hydrogen Peroxide. Infect. Immun. 2019, 87, e00413–00418. [Google Scholar] [CrossRef] [Green Version]
- Chiang, S.M.; Schellhorn, H.E. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch. Biochem. Biophys. 2012, 525, 161–169. [Google Scholar] [CrossRef]
- Troxell, B.; Hassan, H.M. Transcriptional regulation by Ferric Uptake Regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 59. [Google Scholar] [CrossRef]
- Dolan, S.K.; Welch, M. The glyoxylate shunt, 60 years on. Annu. Rev. Microbiol. 2018, 72, 309–330. [Google Scholar] [CrossRef]
- Christodoulou, D.; Link, H.; Fuhrer, T.; Kochanowski, K.; Gerosa, L.; Sauer, U. Reserve Flux Capacity in the Pentose Phosphate Pathway Enables Escherichia coli’s Rapid Response to Oxidative Stress. Cell. Syst. 2018, 6, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Garai, P.; Marathe, S.; Chakravortty, D. Effectors of Salmonella pathogenicity island 2: An island crucial to the life of Salmonella. Virulence 2011, 2, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Vulić, M.; Keren, I.; Lewis, K. Role of oxidative stress in persister tolerance. Antimicrob. Agents Chemother. 2012, 56, 4922–4926. [Google Scholar] [CrossRef] [PubMed]


| Bacteria | MBC | Bacteria | MBC |
|---|---|---|---|
| Escherichia coli | 0.7 | Pseudomonas aeruginosa | 0.35 |
| Staphylococcus aureus | 0.173 | Staphylococcus epidermidis | 0.338 |
| Micrococcus luteus | 2.77 | Corynebacterium amycolatum | 0.169 |
| Haemophilus influenzae | 0.338 | Proteus mirabilis | 0.340 |
| Staphylococcus hominis | 1.4 | Staphylococcus haemolyticus | 0.338 |
| Staphylococcus saprophyticus | 0.35 | Candida albicans | 2.7 |
| Klebsiella pneumoniae | 0.7 | Serratia marcescens | 0.169 |
| Streptococcus pyogenes | 0.169 | Enterobacter aerogenes | 0.676 |
| Methicillin-resistant Staphylococcus aureus | 0.682 | Vancomycin-resistant Enterococcus faecium | 2.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. https://doi.org/10.3390/vaccines10101735
Andrés CMC, Pérez de la Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. The Role of Reactive Species on Innate Immunity. Vaccines. 2022; 10(10):1735. https://doi.org/10.3390/vaccines10101735
Chicago/Turabian StyleAndrés, Celia María Curieses, José Manuel Pérez de la Lastra, Celia Andrés Juan, Francisco J. Plou, and Eduardo Pérez-Lebeña. 2022. "The Role of Reactive Species on Innate Immunity" Vaccines 10, no. 10: 1735. https://doi.org/10.3390/vaccines10101735
APA StyleAndrés, C. M. C., Pérez de la Lastra, J. M., Juan, C. A., Plou, F. J., & Pérez-Lebeña, E. (2022). The Role of Reactive Species on Innate Immunity. Vaccines, 10(10), 1735. https://doi.org/10.3390/vaccines10101735







