Equine Influenza Virus: An Old Known Enemy in the Americas
Abstract
:1. Introduction
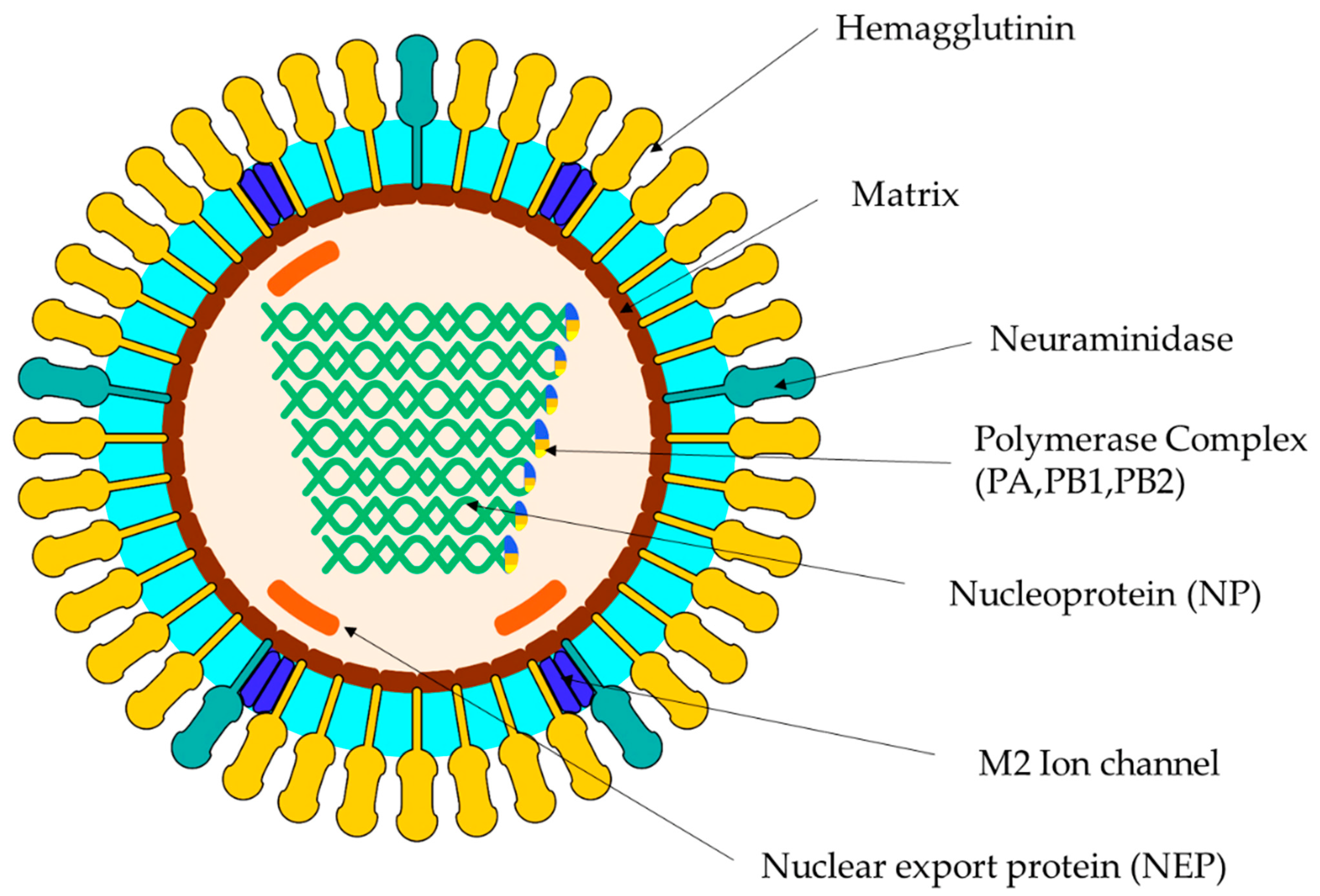
2. Virus Classification
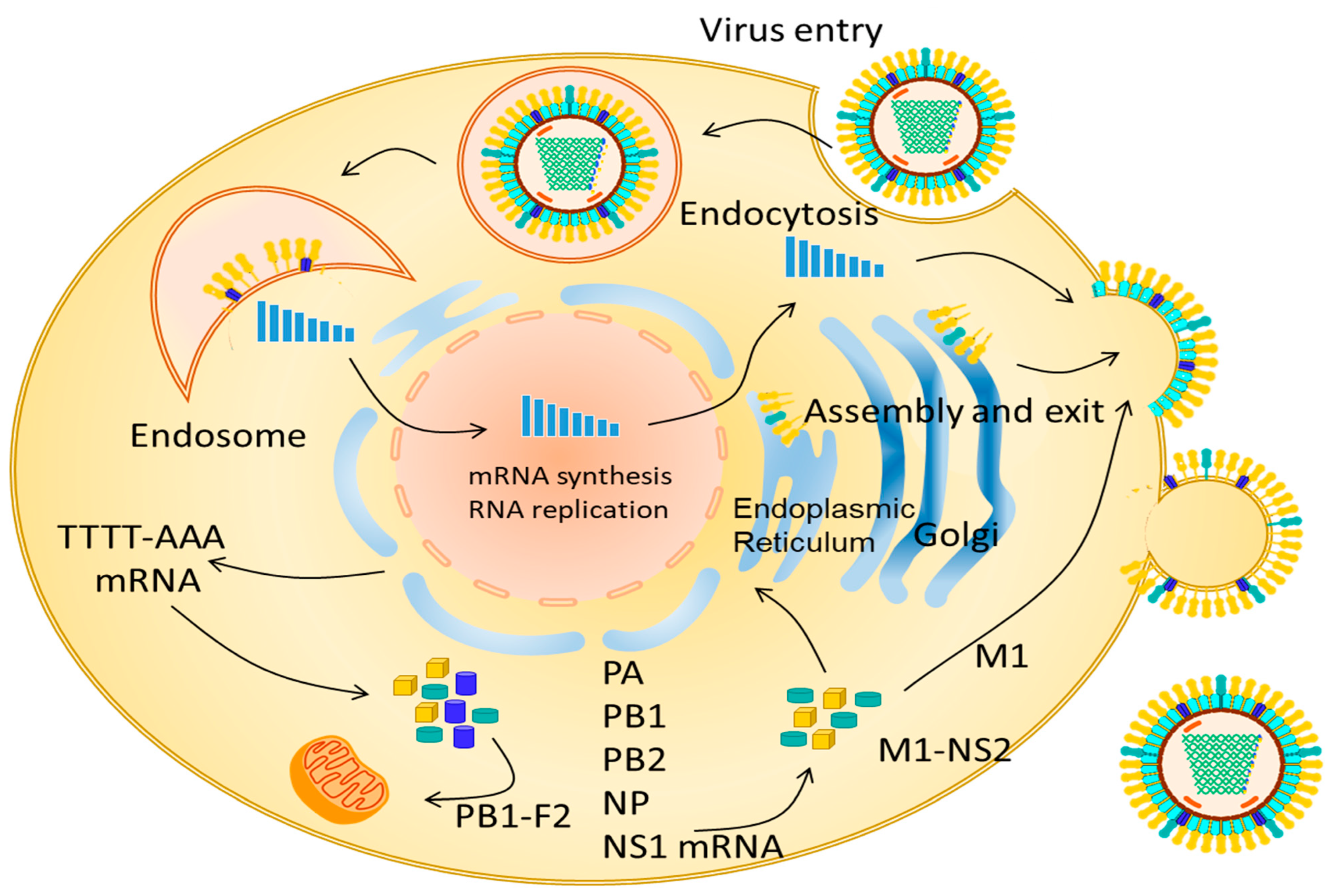
3. Replication EIV
4. Pathogenesis
4.1. Clinical
4.2. Molecular
5. Epidemiology
6. Molecular Epidemiology
7. EIV Risk Factors in the Americas
8. Studies on EIV Occurrence and Reported Outbreaks
9. Prevention and Control
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Khurana, S.K.; Chakraborty, S.; Malik, Y.S.; Virmani, N.; Singh, R.; et al. A Comprehensive Review on Equine Influenza Virus: Etiology, Epidemiology, Pathobiology, Advances in Developing Diagnostics, Vaccines, and Control Strategies. Front. Microbiol. 2018, 9, 1941. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.R.L.; Heil, G.L.; Chambers, T.M.; Capuano, A.; White, S.K.; Gray, G.C. Serological evidence of equine influenza infections among persons with horse exposure, Iowa. J. Clin. Virol. 2015, 67, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Newton, J.R. Equine influenza—A global perspective. Vet. Microbiol. 2013, 167, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Rosanowski, S.; Cogger, N.; Rogers, C.; Stevenson, M. Evaluating the effectiveness of strategies for the control of equine influenza virus in the New Zealand equine population. Transbound. Emerg. Dis. 2016, 63, 321–332. [Google Scholar] [CrossRef]
- Garner, M.; Scanlan, W.; Cowled, B.; Carroll, A. Regaining Australia’s equine influenza-free status: A national perspective. Aust. Vet. J. 2011, 89, 169–173. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Oseni, S.O.; Martinez-Sobrido, L.; Chambers, T.M. Equine Influenza Virus and Vaccines. Viruses 2021, 13, 1657. [Google Scholar] [CrossRef]
- Amat, J.A.R.; Patton, V.; Chauché, C.; Goldfarb, D.; Crispell, J.; Gu, Q.; Coburn, A.M.; Gonzalez, G.; Mair, D.; Tong, L.; et al. Long-term adaptation following influenza A virus host shifts results in increased within-host viral fitness due to higher replication rates, broader dissemination within the respiratory epithelium and reduced tissue damage. PLoS Pathog. 2021, 17, e1010174. [Google Scholar] [CrossRef]
- Sovinova, O.; Tumova, F.; Pouska, J.; Nemec, J. Isolation of a virus causing respiratory diseases in horses. Acta Virol. 1958, 1, 52–61. [Google Scholar]
- Waddell, G.; Teigland, M.; Sigel, M. A new influenza virus associated with equine respiratory disease. J. Am. Vet. Med. Assoc. 1963, 143, 587–590. [Google Scholar]
- Kwasnik, M.; Gora, I.M.; Zmudzinski, J.F.; Rola, J.; Polak, M.P.; Rozek, W. Genetic Analysis of the M Gene of Equine Influenza Virus Strains Isolated in Poland, in the Context of the Asian-like Group Formation. J. Vet. Res. 2018, 62, 405–412. [Google Scholar] [CrossRef] [Green Version]
- Krammer, F.; Smith, G.J.D.; Fouchier, R.A.M.; Peiris, M.; Kedzierska, K.; Doherty, P.C.; Palese, P.; Shaw, M.L.; Treanor, J.; Webster, R.G.; et al. Influenza. Nat. Rev. Dis. Primers 2018, 4, 3. [Google Scholar] [CrossRef]
- Baigent, S.J.; McCauley, J.W. Influenza type A in humans, mammals and birds: Determinants of virus virulence, host-range and interspecies transmission. Bioessays 2003, 25, 657–671. [Google Scholar] [CrossRef]
- Cauldwell, A.V.; Long, J.S.; Moncorgé, O.; Barclay, W.S. Viral determinants of influenza A virus host range. J. Gen. Virol. 2014, 95, 1193–1210. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Li, F.; Wang, D. The first decade of research advances in influenza D virus. J. Gen. Virol. 2021, 102, jgv001529. [Google Scholar] [CrossRef]
- Skelton, R.M.; Huber, V.C. Comparing Influenza Virus Biology for Understanding Influenza D Virus. Viruses 2022, 14, 1036. [Google Scholar] [CrossRef]
- Nedland, H.; Wollman, J.; Sreenivasan, C.; Quast, M.; Singrey, A.; Fawcett, L.; Christopher-Hennings, J.; Nelson, E.; Kaushik, R.S.; Wang, D.; et al. Serological evidence for the co-circulation of two lineages of influenza D viruses in equine populations of the Midwest United States. Zoonoses Public Health 2018, 65, e148–e154. [Google Scholar] [CrossRef]
- Boukharta, M.; Kasmi, Y.; Zakham, F.; El Amri, H.; Ennaji, M.M. Prediction of genetic mutations of equine influenza virus related to adaptive determination nuclear export ribonucleoprotein complex. J. Basic Appl. Zool. 2019, 80, 25. [Google Scholar] [CrossRef]
- Szewczyk, B.; Bieńkowska-Szewczyk, K.; Król, E. Introduction to molecular biology of influenza a viruses. Acta Biochim. Pol. 2014, 61, 397–401. [Google Scholar] [CrossRef] [Green Version]
- Scholtens, R.G.; Steele, J.H.; Dowdle, W.R.; Wilma, B.Y.; Robinson, R.Q. U.S. Epizootic of Equine Influenza, 1963. Public Health Rep. 1964, 79, 393–402. [Google Scholar] [CrossRef]
- Daly, J.M.; MacRae, S.; Newton, J.R.; Wattrang, E.; Elton, D.M. Equine influenza: A review of an unpredictable virus. Vet. J. 2011, 189, 7–14. [Google Scholar] [CrossRef]
- Tůmová, B. Equine influenza—A segment in influenza virus ecology. Comp. Immunol. Microbiol. Infect. Dis. 1980, 3, 45–59. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The Pathology of Influenza Virus Infections. Annu. Rev. Pathol. Mech. Dis. 2008, 3, 499–522. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Matsumae, H.; Katoh, M.; Eisfeld, A.J.; Neumann, G.; Hase, T.; Ghosh, S.; Shoemaker, J.E.; Lopes, T.J.S.; Watanabe, T.; et al. A comprehensive map of the influenza A virus replication cycle. BMC Syst. Biol. 2013, 7, 97. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Xia, H.; Huang, J.; Zheng, Y.; Liu, C.; Su, J.; Ping, J. Features of Nuclear Export Signals of NS2 Protein of Influenza D Virus. Viruses 2020, 12, 1100. [Google Scholar] [CrossRef]
- Cao, S.; Liu, X.; Yu, M.; Li, J.; Jia, X.; Bi, Y.; Sun, L.; Gao, G.F.; Liu, W. A Nuclear Export Signal in the Matrix Protein of Influenza A Virus Is Required for Efficient Virus Replication. J. Virol. 2012, 86, 4883–4891. [Google Scholar] [CrossRef] [Green Version]
- van Maanen, C.; Cullinane, A. Equine influenza virus infections: An update. Vet. Q. 2002, 24, 79–94. [Google Scholar] [CrossRef]
- Vollmer, A.H.; Gebre, M.S.; Barnard, D.L. Serum amyloid A (SAA) is an early biomarker of influenza virus disease in BALB/c, C57BL/2, Swiss-Webster, and DBA.2 mice. Antivir. Res. 2016, 133, 196–207. [Google Scholar] [CrossRef] [Green Version]
- Muranaka, M.; Yamanaka, T.; Katayama, Y.; Niwa, H.; Oku, K.; Matsumura, T.; Oyamada, T. Time-related Pathological Changes in Horses Experimentally Inoculated with Equine Influenza A Virus. J. Equine Sci. 2012, 23, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Chambers, T.M. A Brief Introduction to Equine Influenza and Equine Influenza Viruses. In Animal Influenza Virus: Methods and Protocols; Spackman, E., Ed.; Springer: New York, NY, USA, 2020; pp. 355–360. [Google Scholar]
- Jurado-Tarifa, E.; Daly, J.M.; Pérez-Écija, A.; Barba-Recreo, M.; Mendoza, F.J.; Al-Shuwaikh, A.M.; García-Bocanegra, I. Epidemiological survey of equine influenza in Andalusia, Spain. Prev. Vet. Med. 2018, 151, 52–56. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Cullinane, A. A comparative antibody study of the potential susceptibility of Thoroughbred and non-Thoroughbred horse populations in Ireland to equine influenza virus. Influenza Other Respir. Viruses 2010, 4, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Watson, J.; Daniels, P.; Kirkland, P.; Carroll, A.; Jeggo, M. The 2007 outbreak of equine influenza in Australia: Lessons learned for international trade in horses. Rev. Sci. Tech. 2011, 30, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landolt, G.A. Equine Influenza Virus. Vet. Clin. North Am. Equine Pract. 2014, 30, 507–522. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, V.S.; Olsen, C.W.; Dybdahl-Sissoko, N.; Evans, D. Apoptosis: A mechanism of cell killing by influenza A and B viruses. J. Virol. 1994, 68, 3667–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.; Holland, R.E.; Donofrio, J.C.; McCoy, M.H.; Tudor, L.R.; Chambers, T.M. Caspase activation in equine influenza virus induced apoptotic cell death. Vet. Microbiol. 2002, 84, 357–365. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Goraya, M.U.; Maarouf, M.; Huang, S.; Chen, J.-L. Host immune response to influenza A virus infection. Front. Immunol. 2018, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- World Organisation for Animal Health. Equine influenza (infection with equine influenza virus). In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals; World Organisation for Animal Health: Paris, France, 2019. [Google Scholar]
- Firestone, S.M.; Schemann, K.A.; Toribio, J.-A.L.; Ward, M.P.; Dhand, N.K. A case-control study of risk factors for equine influenza spread onto horse premises during the 2007 epidemic in Australia. Prev. Vet. Med. 2011, 100, 53–63. [Google Scholar] [CrossRef]
- Spence, K.L.; O’Sullivan, T.L.; Poljak, Z.; Greer, A.L. Using a computer simulation model to examine the impact of biosecurity measures during a facility-level outbreak of equine influenza. Can. J. Vet. Res. 2018, 82, 89–96. [Google Scholar]
- Mena, J.; Brito, B.; Moreira, R.; Tadich, T.; Gonzalez, I.; Cruces, J.; Ortega, R.; van Bakel, H.; Rathnasinghe, R.; Pizarro-Lucero, J.; et al. Reemergence of H3N8 Equine Influenza A virus in Chile. Transbound. Emerg. Dis. 2018, 65, 1408–1415. [Google Scholar] [CrossRef]
- Favaro, P.F.; Reischak, D.; Brandao, P.; Villalobos, E.; Cunha, E.; Lara, M.; Benvenga, G.; Dias, R.; Mori, E.; Richtzenhain, L. Comparison among three different serological methods for the detection of equine influenza virus infection. Rev. Sci. Tech. 2017, 36, 789–798. [Google Scholar] [CrossRef]
- Favaro, P.F.; Fernandes, W.R.; Reischak, D.; Brandão, P.E.; Silva, S.O.d.S.; Richtzenhain, L.J. Evolution of equine influenza viruses (H3N8) during a Brazilian outbreak. Braz. J. Microbiol. 2018, 49, 336–346. [Google Scholar]
- Castro, E.; Perez, R.; Rodriguez, S.; Bassetti, L.; Negro, R.; Vidal, R. Epidemiological and virological findings during an outbreak of equine influenza in Uruguay in 2018. Rev. Sci. Tech. 2019, 38, 737–749. [Google Scholar] [CrossRef]
- Perglione, C.O.; Gildea, S.; Rimondi, A.; Miño, S.; Vissani, A.; Carossino, M.; Cullinane, A.; Barrandeguy, M. Epidemiological and virological findings during multiple outbreaks of equine influenza in S outh A merica in 2012. Influenza Other Respir. Viruses 2016, 10, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Mendez, A.; Viel, L.; Hewson, J.; Doig, P.; Carman, S.; Chambers, T.; Tiwari, A.; Dewey, C. Surveillance of equine respiratory viruses in Ontario. Can. J. Vet. Res. 2010, 74, 271–278. [Google Scholar]
- Olguin Perglione, C.; Golemba, M.D.; Torres, C.; Barrandeguy, M. Molecular Epidemiology and Spatio-Temporal Dynamics of the H3N8 Equine Influenza Virus in South America. Pathogens 2016, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Olguin-Perglione, C.; Vissani, M.A.; Alamos, F.; Tordoya, M.S.; Barrandeguy, M. Multifocal outbreak of equine influenza in vaccinated horses in Argentina in 2018: Epidemiological aspects and molecular characterisation of the involved virus strains. Equine Vet. J. 2020, 52, 420–427. [Google Scholar] [CrossRef]
- Gaidamaka, M.; Vaganov, G.; Dromashko, A.; Shvetskava, B.; Fyadina, D. Disease of the upper respiratory tract in horses following the human influenza epidemic of 1957. Bull. World Health Organ. 1959, 20, 505. [Google Scholar]
- Xie, T.; Anderson, B.D.; Daramragchaa, U.; Chuluunbaatar, M.; Gray, G.C. A Review of Evidence that Equine Influenza Viruses Are Zoonotic. Pathogens 2016, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Pusterla, N.; Kass, P.H.; Mapes, S.; Wademan, C.; Akana, N.; Barnett, C.; MacKenzie, C.; Vaala, W. Voluntary surveillance program for equine influenza virus in the United States from 2010 to 2013. J. Vet. Intern. Med. 2015, 29, 417–422. [Google Scholar] [CrossRef]
- Khurelbaatar, N.; Krueger, W.S.; Heil, G.L.; Darmaa, B.; Ulziimaa, D.; Tserennorov, D.; Baterdene, A.; Anderson, B.D.; Gray, G.C. Little evidence of avian or equine influenza virus infection among a cohort of Mongolian adults with animal exposures, 2010–2011. PLoS ONE 2014, 9, e85616. [Google Scholar] [CrossRef] [Green Version]
- Khurelbaatar, N.; Krueger, W.S.; Heil, G.L.; Darmaa, B.; Ulziimaa, D.; Tserennorov, D.; Baterdene, A.; Anderson, B.D.; Gray, G.C. Sparse evidence for equine or avian influenza virus infections among Mongolian adults with animal exposures. Influenza Other Respir. Viruses 2013, 7, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Burnell, F.J.; Holmes, M.A.; Roiko, A.H.; Lowe, J.B.; Heil, G.L.; White, S.K.; Gray, G.C. Little evidence of human infection with equine influenza during the 2007 epizootic, Queensland, Australia. J. Clin. Virol. 2014, 59, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Berríos, E.P. Influenza equina en Chile (1963–1992): Un posible caso en un ser humano. Rev. Chil. De Infectología 2005, 22, 47–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuchipudi, S.V.; Nelli, R.K.; Gontu, A.; Satyakumar, R.; Surendran Nair, M.; Subbiah, M. Sialic acid receptors: The key to solving the enigma of zoonotic virus spillover. Viruses 2021, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, F.; Murcia, P.R.; Newton, J.R. A Review on Equine Influenza from a Human Influenza Perspective. Viruses 2022, 14, 1312. [Google Scholar] [CrossRef]
- Payungporn, S.; Crawford, P.C.; Kouo, T.S.; Chen, L.-m.; Pompey, J.; Castleman, W.L.; Dubovi, E.J.; Katz, J.M.; Donis, R.O. Influenza A virus (H3N8) in dogs with respiratory disease, Florida. Emerg. Infect. Dis. 2008, 14, 902. [Google Scholar] [CrossRef]
- Daly, J.M.; Blunden, A.S.; MacRae, S.; Miller, J.; Bowman, S.J.; Kolodziejek, J.; Nowotny, N.; Smith, K.C. Transmission of equine influenza virus to English foxhounds. Emerg. Infect. Dis. 2008, 14, 461. [Google Scholar] [CrossRef]
- Gonzalez, G.; Marshall, J.F.; Morrell, J.; Robb, D.; McCauley, J.W.; Perez, D.R.; Parrish, C.R.; Murcia, P.R. Infection and Pathogenesis of Canine, Equine, and Human Influenza Viruses in Canine Tracheas. J. Virol. 2014, 88, 9208–9219. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Damdinjav, B.; Gonzalez, G.; Patron, L.V.; Ramirez-Mendoza, H.; AmatI, J.A.R.; Crispell, J.; Parr, Y.A.; Hammond, T.-a.; Shiilegdamba, E.; et al. Absence of adaptive evolution is the main barrier against influenza emergence in horses in Asia despite frequent virus interspecies transmission from wild birds. PLoS Pathog. 2019, 15, e1007531. [Google Scholar] [CrossRef] [Green Version]
- Kirkland, P.D.; Finlaison, D.S.; Crispe, E.; Hurt, A.C. Influenza virus transmission from horses to dogs, Australia. Emerg. Infect. Dis. 2010, 16, 699. [Google Scholar] [CrossRef]
- Sreenivasan, C.C.; Jandhyala, S.S.; Luo, S.; Hause, B.M.; Thomas, M.; Knudsen, D.E.; Leslie-Steen, P.; Clement, T.; Reedy, S.E.; Chambers, T.M. Phylogenetic analysis and characterization of a sporadic isolate of equine influenza A H3N8 from an unvaccinated horse in 2015. Viruses 2018, 10, 31. [Google Scholar] [CrossRef] [Green Version]
- Aguirre-Ezkauriatza, E.J.; Karlsson, E.A.; Freiden, P.; Schultz-Cherry, S.; Flores-Gutierrez, S.G.H.; Zertuche-Guerra, M.I. Seroepidemiological survey of equine influenza a H3N8 in horses from the eastern region of the United States-Mexico border. J. Anim. Vet. Adv. 2012, 11, 2250–2255. [Google Scholar] [CrossRef]
- Aharonson-Raz, K.; Davidson, I.; Porat, Y.; Altory, A.; Klement, E.; Steinman, A. Seroprevalence and rate of infection of equine influenza virus (H3N8 and H7N7) and equine herpesvirus (1 and 4) in the horse population in Israel. J. Equine Vet. Sci. 2014, 34, 828–832. [Google Scholar] [CrossRef]
- Perkins, G.A.; Wagner, B. The development of equine immunity: Current knowledge on immunology in the young horse. Equine Vet. J. 2015, 47, 267–274. [Google Scholar] [CrossRef]
- Williams, C.A. Horse Species Symposium: The effect of oxidative stress during exercise in the horse1. J. Anim. Sci. 2016, 94, 4067–4075. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, M.; Münstermann, S.; de Guindos, I.; Timoney, P. Equine disease events resulting from international horse movements: Systematic review and lessons learned. Equine Vet. J. 2016, 48, 641–653. [Google Scholar] [CrossRef] [Green Version]
- Alves Beuttemmüller, E.; Woodward, A.; Rash, A.; dos Santos Ferraz, L.E.; Fernandes Alfieri, A.; Alfieri, A.A.; Elton, D. Characterisation of the epidemic strain of H3N8 equine influenza virus responsible for outbreaks in South America in 2012. Virol. J. 2016, 13, 45. [Google Scholar] [CrossRef]
- Woodward, A.L.; Rash, A.S.; Blinman, D.; Bowman, S.; Chambers, T.M.; Daly, J.M.; Damiani, A.; Joseph, S.; Lewis, N.; McCauley, J.W. Development of a surveillance scheme for equine influenza in the UK and characterisation of viruses isolated in Europe, Dubai and the USA from 2010–2012. Vet. Microbiol. 2014, 169, 113–127. [Google Scholar] [CrossRef] [Green Version]
- Reemers, S.; Sonnemans, D.; Horspool, L.; van Bommel, S.; Cao, Q.; van de Zande, S. Determining Equine Influenza Virus Vaccine Efficacy—The Specific Contribution of Strain Versus Other Vaccine Attributes. Vaccines 2020, 8, 501. [Google Scholar] [CrossRef]
- Chambers, T.M. Equine Influenza. Cold Spring Harb. Perspect. Med. 2022, 12, 257–282. [Google Scholar] [CrossRef]
- Murcia, P.R.; Wood, J.L.N.; Holmes, E.C. Genome-Scale Evolution and Phylodynamics of Equine H3N8 Influenza A Virus. J. Virol. 2011, 85, 5312–5322. [Google Scholar] [CrossRef] [Green Version]
- Paillot, R.; Marcillaud Pitel, C.; D’Ablon, X.; Pronost, S. Equine Vaccines: How, When and Why? Report of the Vaccinology Session, French Equine Veterinarians Association, 2016, Reims. Vaccines 2017, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- May, T. Vaccines as community-focused therapy. Expert Rev. Vaccines 2003, 2, 341–343. [Google Scholar] [CrossRef] [Green Version]
- Moreira, R.; García, A.; Ahumada, C.; Badía, C.; Suárez, P.; Yangari, B.; Aguayo, C.; Herrera, J.; Espejo, G.; Pinto, E. Report of 2018 equine influenza outbreak in Chile. Austral. J. Vet. Sci. 2019, 51, 1. [Google Scholar] [CrossRef] [Green Version]
- Plata-Hipólito, C.B.; Cedillo-Rosales, S.; Obregón-Macías, N.; Hernández-Luna, C.E.; Rodríguez-Padilla, C.; Tamez-Guerra, R.S.; Contreras-Cordero, J.F. Genetic and serologic surveillance of canine (CIV) and equine (EIV) influenza virus in Nuevo León State, México. PeerJ 2019, 7, e8239. [Google Scholar] [CrossRef]
- Fretz, P.; Babiuk, L.; McLaughlin, B. Equine respiratory disease on the Western Canadian racetracks. Can. Vet. J. 1979, 20, 58. [Google Scholar]
- Morley, P.S.; Townsend, H.G.; Bogdan, J.R.; Haines, D.M. Descriptive epidemiologic study of disease associated with influenza virus infections during three epidemics in horses. J. Am. Vet. Med. Assoc. 2000, 216, 535–544. [Google Scholar] [CrossRef]
- Berríos Etchegaray, P. Influenza Equina: Actualización; Boletin Vaterinario Oficial: Santiago, Chile, 2012; p. 8. [Google Scholar]
- Karlsson, E.A.; Ciuoderis, K.; Freiden, P.J.; Seufzer, B.; Jones, J.C.; Johnson, J.; Parra, R.; Gongora, A.; Cardenas, D.; Barajas, D. Prevalence and characterization of influenza viruses in diverse species in Los Llanos, Colombia: Prevalence of influenza viruses in Colombia. Emerg. Microbes Infect. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Loroño-Pino, M.A.; Farfan-Ale, J.A.; Garcia-Rejon, J.E.; Lin, M.; Rosado-Paredes, E.; Puerto, F.I.; Bates, A.; Root, J.J.; Franklin, A.B.; Sullivan, H.J.; et al. Antibodies to influenza and West Nile viruses in horses in Mexico. Vet. Rec. 2010, 166, 22–23. [Google Scholar] [CrossRef] [Green Version]
- Brister, H.; Barnum, S.M.; Reedy, S.; Chambers, T.M.; Pusterla, N. Validation of two multiplex real-time PCR assays based on single nucleotide polymorphisms of the HA1 gene of equine influenza A virus in order to differentiate between clade 1 and clade 2 Florida sublineage isolates. J. Vet. Diagn. Investig. 2019, 31, 137–141. [Google Scholar] [CrossRef] [Green Version]
- Pusterla, N.; James, K.; Barnum, S.; Bain, F.; Barnett, D.C.; Chappell, D.; Gaughan, E.; Craig, B.; Schneider, C.; Vaala, W. Frequency of Detection and Prevalence Factors Associated with Common Respiratory Pathogens in Equids with Acute Onset of Fever and/or Respiratory Signs (2008–2021). Pathogens 2022, 11, 759. [Google Scholar] [CrossRef]
- Lee, K.; Pusterla, N.; Barnum, S.M.; Lee, D.-H.; Martínez-López, B. Genome-informed characterisation of antigenic drift in the haemagglutinin gene of equine influenza strains circulating in the United States from 2012 to 2017. Transbound. Emerg. Dis. 2022, 69, e52–e63. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Pusterla, N.; Barnum, S.M.; Lee, D.H.; Martinez-Lopez, B. Investigation of cross-regional spread and evolution of equine influenza H3N8 at US and global scales using Bayesian phylogeography based on balanced subsampling. Transbound. Emerg. Dis. 2022, 69, e1734–e1748. [Google Scholar] [CrossRef] [PubMed]
- Vallone, E.; Canto, R.; Bauza, C.; Somma, R.; Tosi, H. Localization of the Aequi/Uruguay/540/1963 influenza virus in the chorioatlantoic membranes by the immunofluorescence method. An. Fac. Med. Univ. Repub. Montev. Urug. 1965, 50, 107–113. [Google Scholar] [PubMed]
- Equine influenza outbreaks in the UK: A practical approach to prevention. Vet. Rec. 2019, 185, 198–200. [CrossRef]
- Gildea, S.; Lyons, P.; Lyons, R.; Gahan, J.; Garvey, M.; Cullinane, A. Annual booster vaccination and the risk of equine influenza to Thoroughbred racehorses. Equine Vet. J. 2020, 52, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Karagianni, A.E.; Kurian, D.; Cillán-Garcia, E.; Eaton, S.L.; Wishart, T.M.; Pirie, R.S. Training associated alterations in equine respiratory immunity using a multiomics comparative approach. Sci. Rep. 2022, 12, 427. [Google Scholar] [CrossRef]
- Miller, A.B.; Harris, P.A.; Barker, V.D.; Adams, A.A. Short-term transport stress and supplementation alter immune function in aged horses. PLoS ONE 2021, 16, e0254139. [Google Scholar]
- Milwid, R.M.; O’Sullivan, T.L.; Poljak, Z.; Laskowski, M.; Greer, A.L. Comparing the effects of non-homogenous mixing patterns on epidemiological outcomes in equine populations: A mathematical modelling study. Sci. Rep. 2019, 9, 3227. [Google Scholar] [CrossRef]
- Garner, M.; Cowled, B.; East, I.; Moloney, B.; Kung, N. Evaluating the effectiveness of early vaccination in the control and eradication of equine influenza—A modelling approach. Prev. Vet. Med. 2011, 99, 15–27. [Google Scholar] [CrossRef]
- Fougerolle, S.; Legrand, L.; Garrett, D.; Birand, I.; Foursin, M.; D’ablon, X.; Bayssat, P.; Newton, R.J.; Pronost, S.; Paillot, R. Influential factors inducing suboptimal humoral response to vector-based influenza immunisation in thoroughbred foals. Vaccine 2016, 34, 3787–3795. [Google Scholar] [CrossRef]
- Dionísio, L.; Medeiros, F.; Pequito, M.; Faustino-Rocha, A.I. Equine influenza: A comprehensive review from etiology to treatment. Anim. Health Res. Rev. 2021, 22, 56–71. [Google Scholar] [CrossRef]
- Paillot, R. A Systematic Review of Recent Advances in Equine Influenza Vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef] [Green Version]
- Allkofer, A.; Garvey, M.; Ryan, E.; Lyons, R.; Ryan, M.; Lukaseviciute, G.; Walsh, C.; Venner, M.; Cullinane, A. Primary vaccination in foals: A comparison of the serological response to equine influenza and equine herpesvirus vaccines administered concurrently or 2 weeks apart. Arch. Virol. 2021, 166, 571–579. [Google Scholar] [CrossRef]
- Wilson, A.; Pinchbeck, G.; Dean, R.; McGowan, C. Equine influenza vaccination in the UK: Current practices may leave horses with suboptimal immunity. Equine Vet. J. 2021, 53, 1004–1014. [Google Scholar] [CrossRef]
- Schemann, K.; Firestone, S.M.; Taylor, M.R.; Toribio, J.A.L.M.L.; Ward, M.P.; Dhand, N.K. Horse owners’/managers’ perceptions about effectiveness of biosecurity measures based on their experiences during the 2007 equine influenza outbreak in Australia. Prev. Vet. Med. 2012, 106, 97–107. [Google Scholar] [CrossRef]
- Slater, J.; Borchers, K.; Chambers, T.; Cullinane, A.; Duggan, V.; Elton, D.; Legrand, L.; Paillot, R.; Fortier, G. Report of the International Equine Influenza Roundtable Expert Meeting at Le Touquet, Normandy, February 2013. Equine Vet. J. 2014, 46, 645–650. [Google Scholar] [CrossRef]
- Gildea, S.; Garvey, M.; Lyons, P.; Lyons, R.; Gahan, J.; Walsh, C.; Cullinane, A. Multifocal equine influenza outbreak with vaccination breakdown in thoroughbred racehorses. Pathogens 2018, 7, 43. [Google Scholar] [CrossRef]

| Country | Year | Subtype | Clade | Reference |
|---|---|---|---|---|
| USA | 1963 | H3N8 | Predivergent | Sight, et al., 2018 [1] |
| Brazil | 1980 | H3N8 | ND | Favaro, et al., 2017 [41] |
| Canada | 2005 | H3N8 | ND | Diaz, et al., 2010 [45] |
| Uruguay | 2012 | H3N8 | FC1 | Perglione, et al., 2016 [46] |
| Argentina | 2012 | H3N8 | FC1 | Perglione, et al., 2016 [46] |
| Brazil | 2015 | H3N8 | FC1 | Favaro, et al., 2017 [41] |
| Argentina | 2018 | H3N8 | FC1 | Perglione, et al., 2020 [47] |
| Chile | 2018 | H3N8 | FC1 | Mena, et al., 2018 [40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalez-Obando, J.; Forero, J.E.; Zuluaga-Cabrera, A.M.; Ruiz-Saenz, J. Equine Influenza Virus: An Old Known Enemy in the Americas. Vaccines 2022, 10, 1718. https://doi.org/10.3390/vaccines10101718
Gonzalez-Obando J, Forero JE, Zuluaga-Cabrera AM, Ruiz-Saenz J. Equine Influenza Virus: An Old Known Enemy in the Americas. Vaccines. 2022; 10(10):1718. https://doi.org/10.3390/vaccines10101718
Chicago/Turabian StyleGonzalez-Obando, Juliana, Jorge Eduardo Forero, Angélica M Zuluaga-Cabrera, and Julián Ruiz-Saenz. 2022. "Equine Influenza Virus: An Old Known Enemy in the Americas" Vaccines 10, no. 10: 1718. https://doi.org/10.3390/vaccines10101718
APA StyleGonzalez-Obando, J., Forero, J. E., Zuluaga-Cabrera, A. M., & Ruiz-Saenz, J. (2022). Equine Influenza Virus: An Old Known Enemy in the Americas. Vaccines, 10(10), 1718. https://doi.org/10.3390/vaccines10101718







