Maintenance of Antibody Response in Egyptian Healthcare Workers Vaccinated with ChAdOx1 nCoV-19 Vaccine during Delta and Omicron Variants Pandemic: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Blood Sampling
2.3. Measurement of Anti-Spike IgG Serum Level
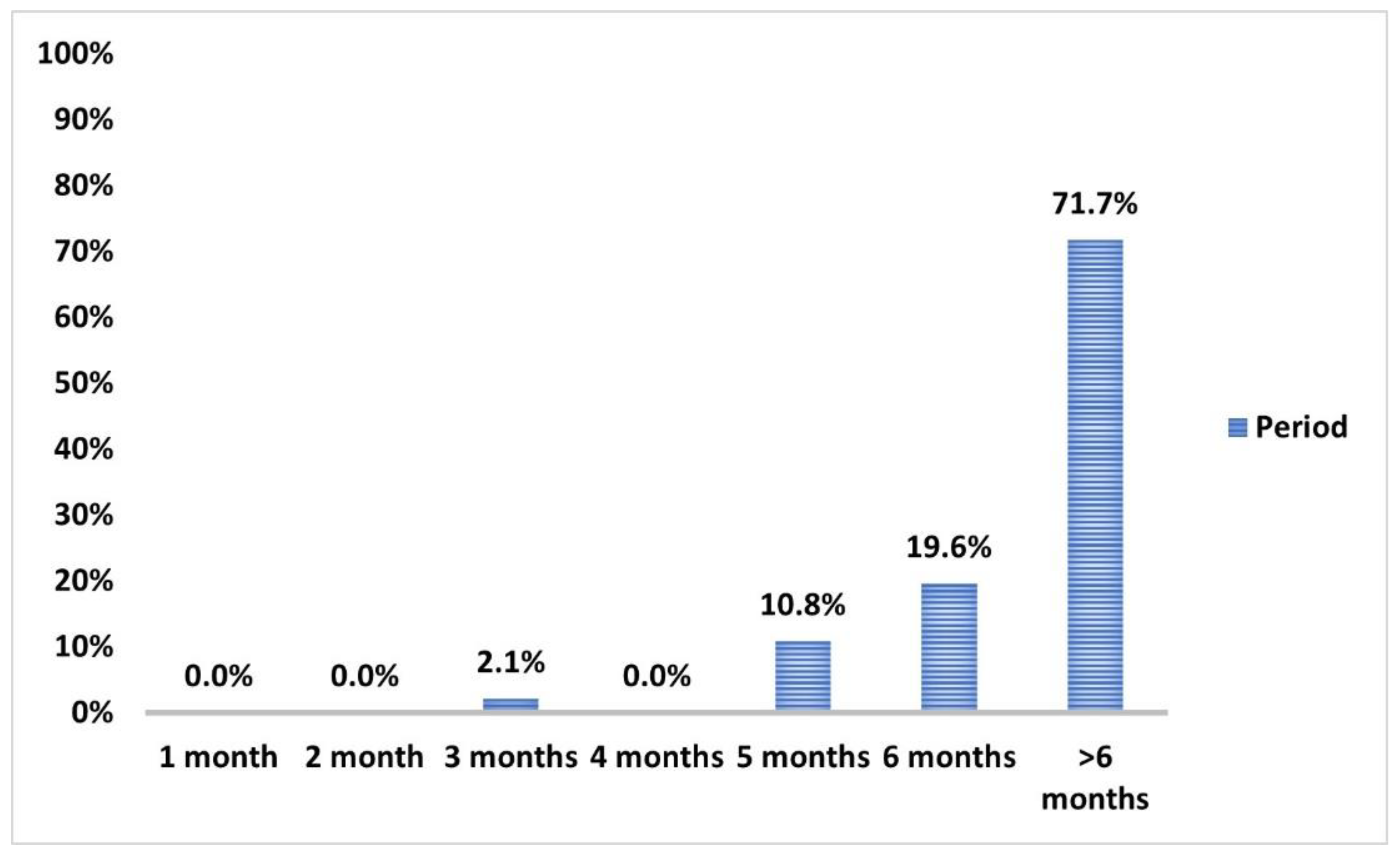
2.4. Follow-Up of Participants
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Studied HCWs
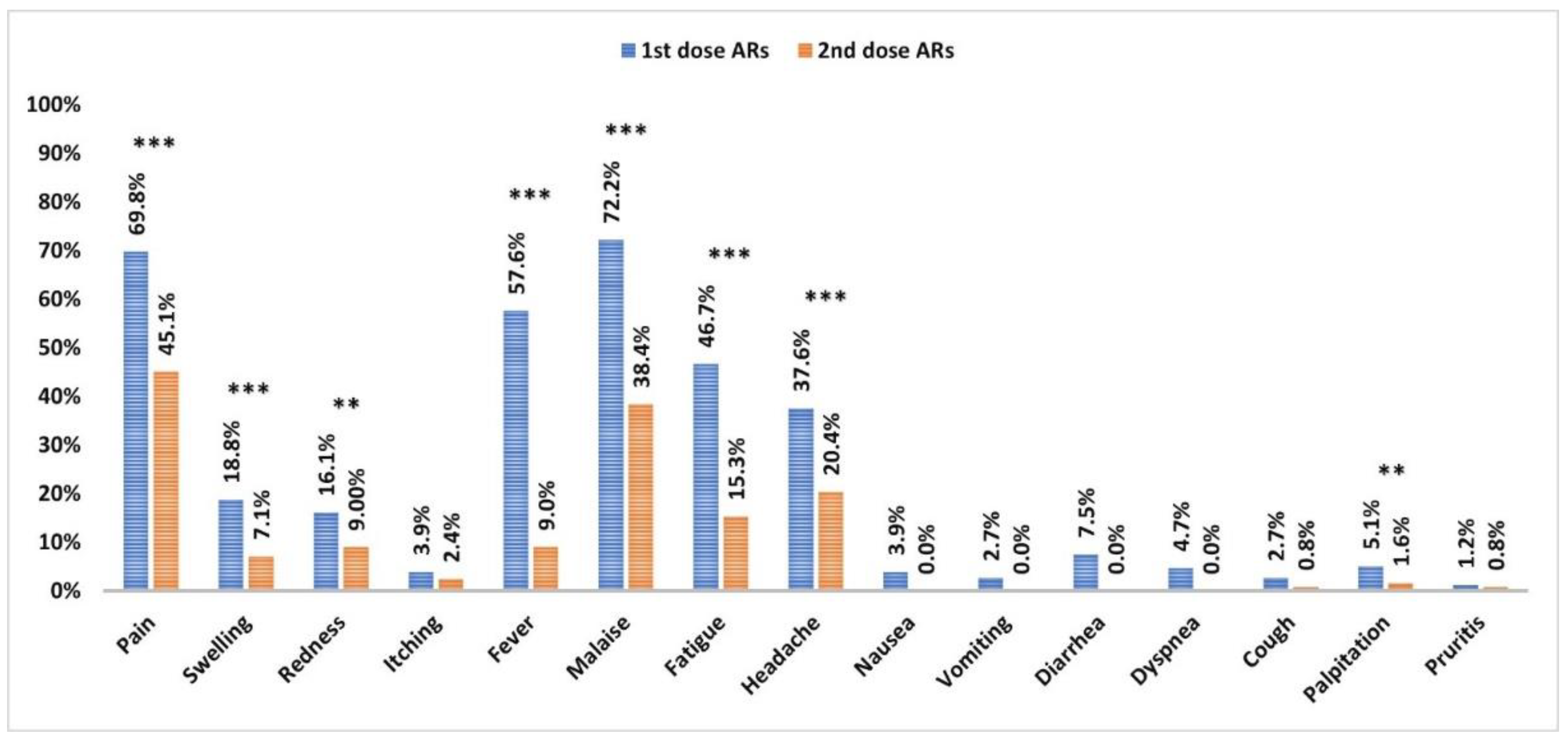
3.2. Adverse Reactions to the Vaccine
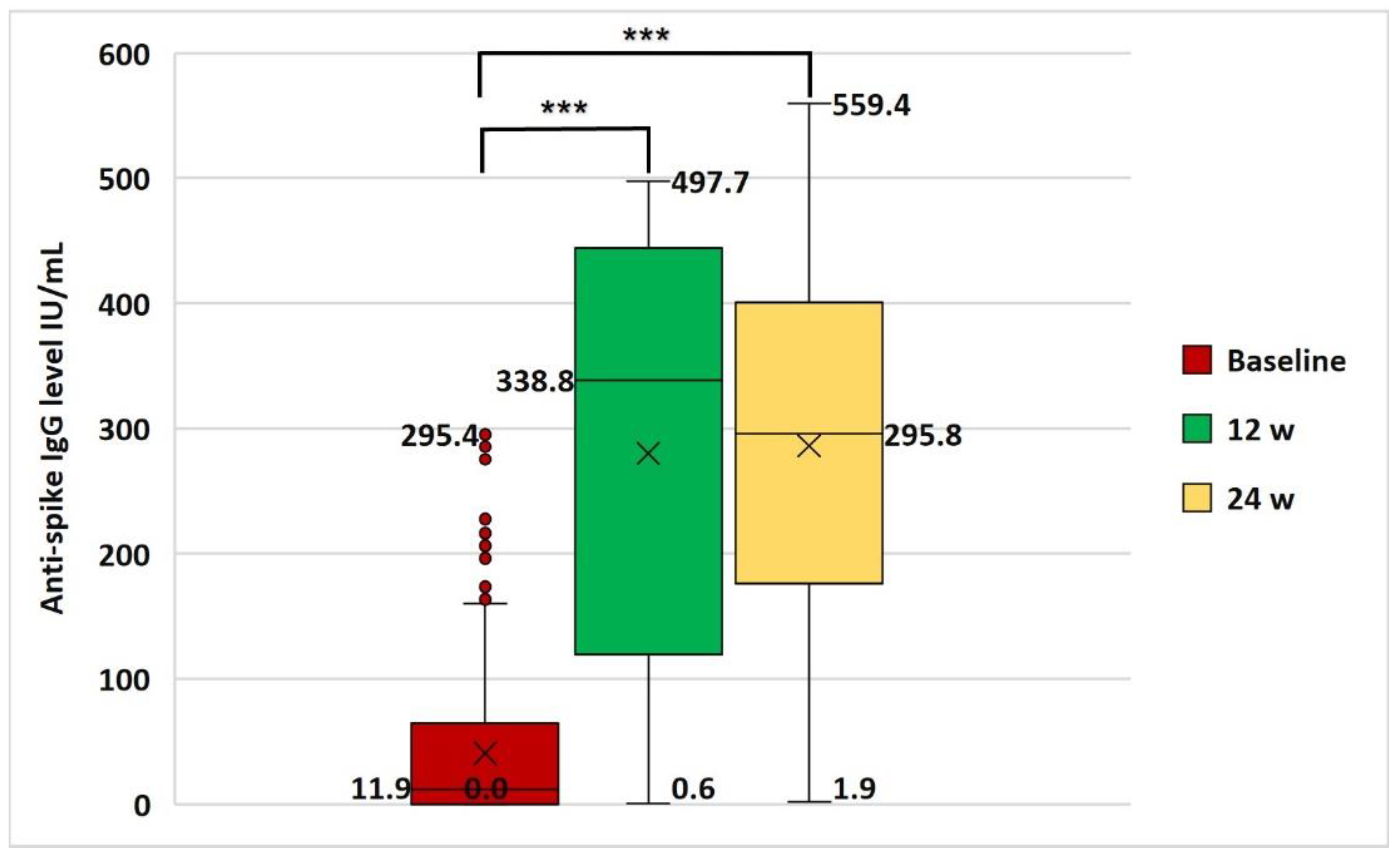
3.3. Anti-Spike IgG Serum Level and Immunoreactivity to Vaccine
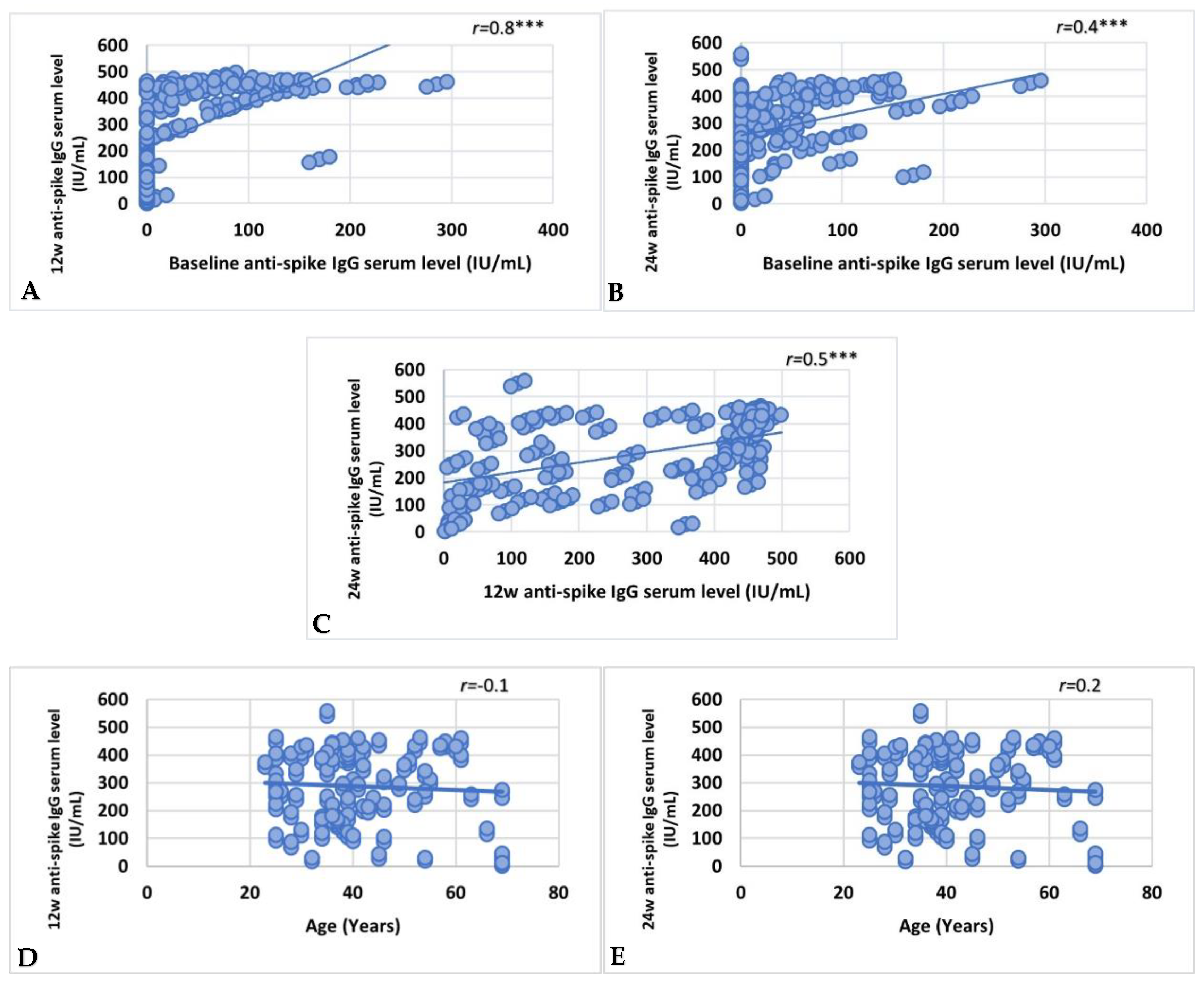
3.4. Factors Affecting Vaccine Immunoreactivity
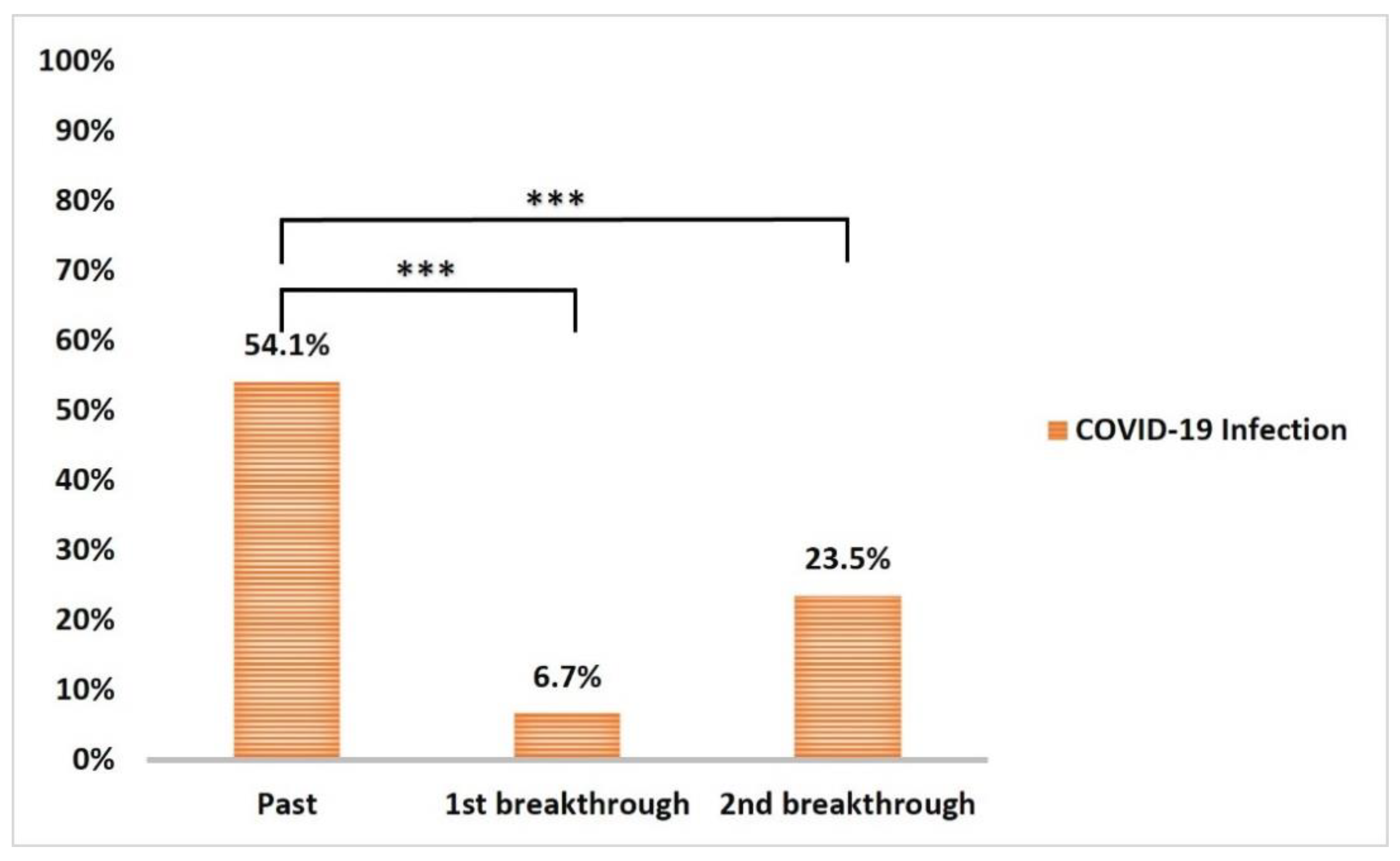
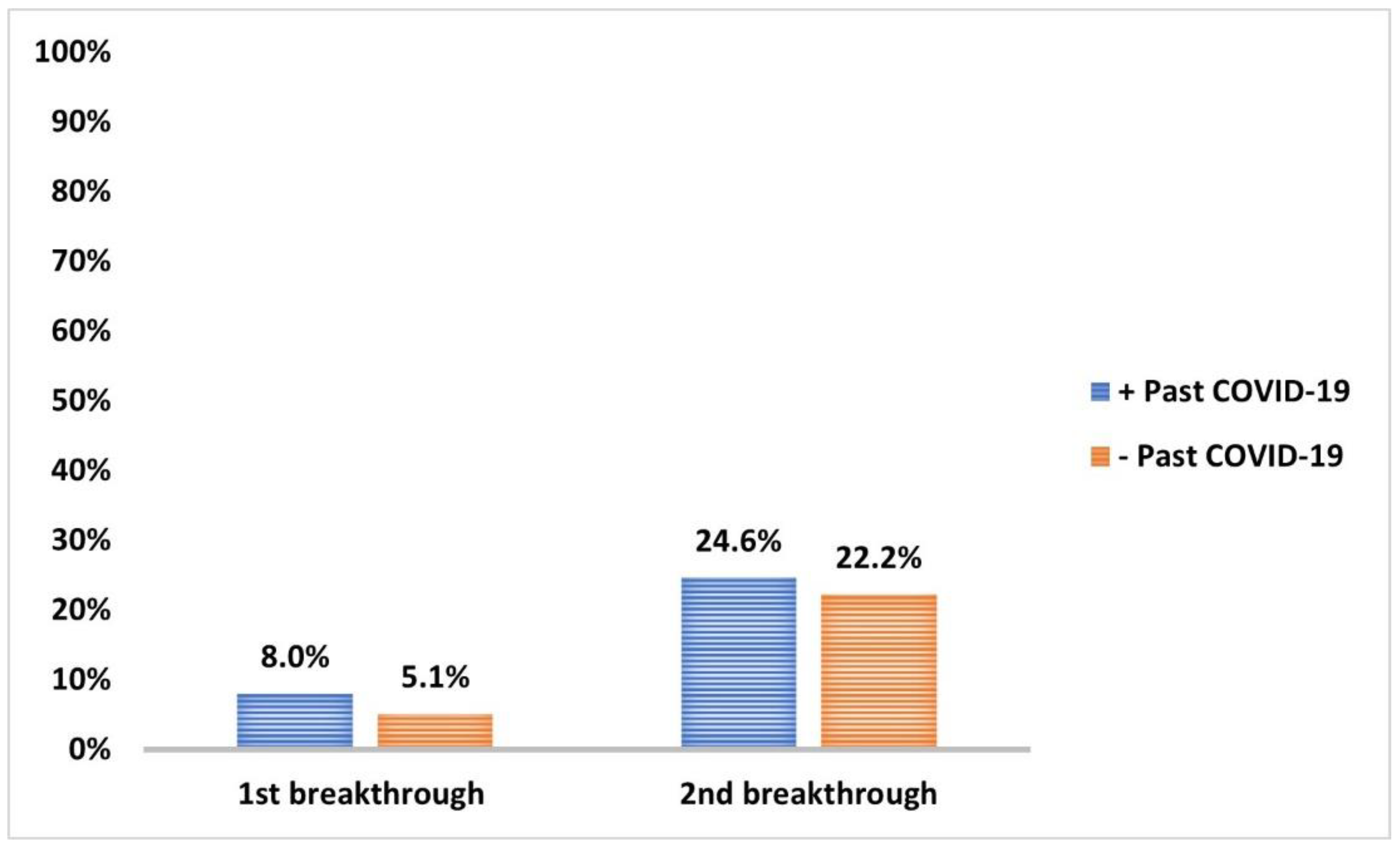
3.5. COVID-19 Infection and Impact on Anti-Spike IgG Serum Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Christie, A.; Brooks, J.T.; Hicks, L.A.; Sauber-Schatz, E.K.; Yoder, J.S.; Honein, M.A.; CDC COVID-19 Response Team. Guidance for implementing COVID-19 prevention strategies in the context of varying community transmission levels and vaccination coverage. Morb. Mortal. Wkly. Rep. 2021, 70, 1044. [Google Scholar] [CrossRef] [PubMed]
- Atnafie, S.A.; Anteneh, D.A.; Yimenu, D.K.; Kifle, Z.D. Assessment of exposure risks to COVID-19 among frontline health care workers in Amhara Region, Ethiopia: A cross-sectional survey. PLoS ONE 2021, 16, e0251000. [Google Scholar] [CrossRef]
- Khan, Z.; Karataş, Y. COVID-19 in Turkey: An urgent need for the implementation of preparedness and response strategies. Health Sci. Rep. 2020, 3. [Google Scholar] [CrossRef]
- WHO. The Impact of COVID-19 on Health and Care Workers: A Closer Look at Deaths; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- WHO. WHO SAGE Roadmap for Prioritizing Uses of COVID-19 Vaccines in the Context of Limited Supply: An Approach to Inform Planning and Subsequent Recommendations Based on Epidemiological Setting and Vaccine Supply Scenarios, First Issued, Latest Update 16 July 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- GOV.UK. Conditions of Authorisation for COVID-19 Vaccine AstraZeneca (Regulation 174). Available online: https://www.gov.uk/government/publications/regulatory-approval-of-covid-19-vaccine-astrazeneca/conditions-of-authorisation-for-covid-19-vaccine-astrazeneca (accessed on 19 April 2022).
- Mallapaty, S.; Callaway, E. What scientists do and don’t know about the Oxford-AstraZeneca COVID vaccine. Nature 2021, 592, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Wouters, O.J.; Shadlen, K.C.; Salcher-Konrad, M.; Pollard, A.J.; Larson, H.J.; Teerawattananon, Y.; Jit, M. Challenges in ensuring global access to COVID-19 vaccines: Production, affordability, allocation, and deployment. Lancet 2021, 397, 1023–1034. [Google Scholar] [CrossRef]
- Emary, K.R.; Golubchik, T.; Aley, P.K.; Ariani, C.V.; Angus, B.; Bibi, S.; Blane, B.; Bonsall, D.; Cicconi, P.; Charlton, S. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 variant of concern 202012/01 (B. 1.1. 7): An exploratory analysis of a randomised controlled trial. Lancet 2021, 397, 1351–1362. [Google Scholar] [CrossRef]
- Jeong, S.; Lee, N.; Lee, S.K.; Cho, E.J.; Hyun, J.; Park, M.J.; Song, W.; Jung, E.J.; Woo, H.; Seo, Y.B.; et al. Comparing Results of Five SARS-CoV-2 Antibody Assays Before and After the First Dose of ChAdOx1 nCoV-19 Vaccine among Health Care Workers. J. Clin. Microbiol. 2021, 59, e0110521. [Google Scholar] [CrossRef]
- Kim, N.; Minn, D.; Park, S.; Roh, E.Y.; Yoon, J.H.; Park, H.; Shin, S. Positivity of SARS-CoV-2 Antibodies among Korean Healthy Healthcare Workers 1 and 2 Weeks after Second Dose of Pfizer-BioNTech Vaccination. J. Korean Med. Sci. 2021, 36, e158. [Google Scholar] [CrossRef] [PubMed]
- Hammad, N.M.; Saeed, M.A.; Shaltout, S.W.; Nofal, H.A.; Nafae, R.M.; Arslan, K.; Tanoglu, A.; Nechifor, M.; Luca, C.; Al-kadhim, Z.H.A.; et al. RT- PCR testing of upper respiratory tract samples for diagnosis of SARS-CoV-2: Between justification and overestimation, a multi-center international study. Travel Med. Infect. Dis. 2022, 48, 102334. [Google Scholar] [CrossRef]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19-11 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Jung, J. Preparing for the coronavirus disease (COVID-19) vaccination: Evidence, plans, and implications. J. Korean Med. Sci. 2021, 36, e59. [Google Scholar] [CrossRef] [PubMed]
- Wise, J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021, 372, n699. [Google Scholar] [CrossRef] [PubMed]
- Limami, Y.; Khalki, L.; Zaid, N.; Khyatti, M.; El Turk, J.; Ammara, M.; Mtairag, E.M.; Oudghiri, M.; Naya, A.; Taberkant, M. Oxford-AstraZeneca ChAdOx1 COVID-19 vaccine does not alter platelet aggregation. Semin. Thromb. Hemost. 2022, 48, 109–111. [Google Scholar] [CrossRef]
- Willems, L.H.; Nagy, M.; Ten Cate, H.; Spronk, H.M.; Jacobs, L.M.; Kranendonk, J.; van Leeuwen, M.; Meijer, D.; Middeldorp, S.; Groh, L.A. ChAdOx1 vaccination, blood coagulation, and inflammation: No effect on coagulation but increased interleukin-6. Res. Pract. Thromb. Haemost. 2021, 5, e12630. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: WHO says rollout of AstraZeneca vaccine should continue, as Europe divides over safety. BMJ. 2021, 372, n728. [Google Scholar] [CrossRef]
- WHO. Background Document on the AZD1222 Vaccine against COVID-19 Developed by Oxford University and AstraZeneca: Background Document to the WHO Interim Recommendations for Use of the AZD1222 (ChAdOx1-S [recombinant]) Vaccine against COVID19 Developed by Oxford University and AstraZeneca, 1 March 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P.; Sudre, C.H.; Nguyen, L.H.; Drew, D.A.; Merino, J. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Kamal, D.; Thakur, V.; Nath, N.; Malhotra, T.; Gupta, A.; Batlish, R. Adverse events following ChAdOx1 nCoV-19 Vaccine (COVISHIELD) amongst health care workers: A prospective observational study. Med. J. Armed Forces India 2021, 77, S283–S288. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Alahmad, B.; Al-Shammari, A.A.; Alterki, A.; Hammad, M.; Cherian, P.; Alkhairi, I.; Sindhu, S.; Thanaraj, T.A.; Mohammad, A. Previous COVID-19 infection and antibody levels after vaccination. Front. Public Health 2021, 1964. [Google Scholar] [CrossRef]
- Tequare, M.H.; Abraha, H.E.; Adhana, M.T.; Tekle, T.H.; Belayneh, E.K.; Gebresilassie, K.B.; Wolderufael, A.L.; Ebrahim, M.M.; Tadele, B.A.; Berhe, D.F. Adverse events of Oxford/AstraZeneca’s COVID-19 vaccine among health care workers of Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia. IJID Reg. 2021, 1, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L. First month of COVID-19 vaccine safety monitoring—United States, December 14, 2020–January 13, 2021. Morb. Mortal. Wkly. Rep. 2021, 70, 283. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Gundlapalli, A.V.; Salerno, R.M.; Brooks, J.T.; Averhoff, F.; Petersen, L.R.; McDonald, L.C.; Iademarco, M.F.; CDC COVID-19 Response. SARS-CoV-2 serologic assay needs for the next phase of the US COVID-19 pandemic response. Open Forum Infect. Dis. 2020, 8, ofaa555. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gümüş, S.; Bektöre, B.; Bozkurt, H.; Gözalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2022, 94, 1060–1066. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516-e7. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Minn, D.; Chang, S.-H.; Suh, J.-S. Comparing SARS-CoV-2 Antibody Responses after Various COVID-19 Vaccinations in Healthcare Workers. Vaccines 2022, 10, 193. [Google Scholar] [CrossRef]
- Robertson, L.J.; Price, R.; Moore, J.S.; Curry, G.; Farnan, J.; Black, A.; Blighe, K.; Nesbit, M.A.; McLaughlin, J.A.; Moore, T. IgG antibody production and persistence to 6 months following SARS-CoV-2 vaccination: A Northern Ireland observational study. Vaccine 2022, 40, 2535–2539. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulla, Z.A.; Al-Bashir, S.M.; Al-Salih, N.S.; Aldamen, A.A.; Abdulazeez, M.Z. A summary of the SARS-CoV-2 vaccines and technologies available or under development. Pathogens 2021, 10, 788. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Miller, B.L. Monitoring serum spike protein with disposable photonic biosensors following SARS-CoV-2 vaccination. Sensors 2021, 21, 5857. [Google Scholar] [CrossRef]
- Ripperger, T.J.; Uhrlaub, J.L.; Watanabe, M.; Wong, R.; Castaneda, Y.; Pizzato, H.A.; Thompson, M.R.; Bradshaw, C.; Weinkauf, C.C.; Bime, C. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020, 53, 925–933.e924. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Shenhar, Y.; Green, I.; Merzon, E.; Golan-Cohen, A.; Schäffer, A.A.; Ruppin, E.; Vinker, S.; Magen, E. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Tong, P.; Gautam, A.; Windsor, I.W.; Travers, M.; Chen, Y.; Garcia, N.; Whiteman, N.B.; McKay, L.G.; Storm, N.; Malsick, L.E. Memory B cell repertoire for recognition of evolving SARS-CoV-2 spike. Cell 2021, 184, 4969–4980.e4915. [Google Scholar] [CrossRef]
- Turner, J.S.; Kim, W.; Kalaidina, E.; Goss, C.W.; Rauseo, A.M.; Schmitz, A.J.; Hansen, L.; Haile, A.; Klebert, M.K.; Pusic, I. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021, 595, 421–425. [Google Scholar] [CrossRef]
- Iwasaki, A. Exploiting mucosal immunity for antiviral vaccines. Annu. Rev. Immunol. 2016, 34, 575–608. [Google Scholar] [CrossRef]
- Dai, L.; Gao, G.F. Viral targets for vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, Á.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Whitaker, H.J.; Tsang, R.S.; Byford, R.; Andrews, N.J.; Sherlock, J.; Pillai, P.S.; Williams, J.; Button, E.; Campbell, H.; Sinnathamby, M. Pfizer-BioNTech and Oxford AstraZeneca COVID-19 vaccine effectiveness and immune response among individuals in clinical risk groups. J. Infect. 2022, 84, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2021, 24, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, G.; Tahrat, H.; Bekkat-Berkani, R. Immunogenicity, safety, and effectiveness of seasonal influenza vaccination in patients with diabetes mellitus: A systematic review. Hum. Vaccines Immunother. 2018, 14, 1853–1866. [Google Scholar] [CrossRef]
- Schillie, S.F.; Spradling, P.R.; Murphy, T.V. Immune response of hepatitis B vaccine among persons with diabetes: A systematic review of the literature. Diabetes Care 2012, 35, 2690–2697. [Google Scholar] [CrossRef] [Green Version]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- King, L.K.; March, L.; Anandacoomarasamy, A. Obesity & osteoarthritis. Indian J. Med. Res. 2013, 138, 185–193. [Google Scholar]
- Frasca, D.; Blomberg, B.B. Adipose tissue inflammation induces B cell inflammation and decreases B cell function in aging. Front. Immunol. 2017, 8, 1003. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, S.; Mizoue, T.; Tanaka, A.; Oshiro, Y.; Inamura, N.; Konishi, M.; Ozeki, M.; Miyo, K.; Sugiura, W.; Sugiyama, H. Sex–associated differences between body mass index and SARS–CoV–2 antibody titers following the BNT162b2 vaccine among 2,435 healthcare workers in Japan. medRxiv 2021. [Google Scholar] [CrossRef]
- Rifai, A.; Wahono, C.S.; Pratama, M.Z.; Handono, K.; Susianti, H.; Iskandar, A.; Diyah, N.; Santoningsih, D.; Samsu, N.; Gunawan, A. Association Between the Effectiveness and Immunogenicity of Inactivated SARS-CoV2 Vaccine (CoronaVac) with the Presence of Hypertension among Health Care Workers. Clin. Exp. Hypertens. 2022, 44, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Cheng, G.; Ma, N.; Huang, Y.; Lin, Y.; Zhou, Q.; Que, B.; Dong, J.; Zhou, Y.; Nie, S. Circulating Th1, Th2, and Th17 levels in hypertensive patients. Dis. Markers 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadikaram, G.; Ram, M.; Izadi, A.; Sheikh Fathollahi, M.; Nematollahi, M.H.; Najafipour, H.; Shahoozehi, B.; Mirhoseini, M.; Masoumi, M.; Shahrokhi, N. The study of the serum level of IL-4, TGF-β, IFN-γ, and IL-6 in overweight patients with and without diabetes mellitus and hypertension. J. Cell. Biochem. 2019, 120, 4147–4157. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, T.P.; Guzik, T.J. Adaptive immunity in hypertension. Curr. Hypertens. Rep. 2019, 21, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, J.; Nangaku, M.; Miyata, T.; Inagi, R.; Yamada, K.; Kurokawa, K.; Fujita, T. Imbalance of T-cell subsets in angiotensin II–infused hypertensive rats with kidney injury. Hypertension 2003, 42, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; de Araujo Oliveira, V.; Paixão, E.S.; Florentino, P.T.V.; Penna, G.O.; Pearce, N.; Werneck, G.L.; Barreto, M.L.; Boaventura, V.S.; Barral-Netto, M. Vaccination plus previous infection: Protection during the omicron wave in Brazil. Lancet Infect. Dis. 2022, 22, 945–946. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 19 April 2022).
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M. COVID-19 vaccine effectiveness against the omicron (B. 1.1. 529) variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef]
- Pouwels, K.B.; Pritchard, E.; Matthews, P.C.; Stoesser, N.; Eyre, D.W.; Vihta, K.-D.; House, T.; Hay, J.; Bell, J.I.; Newton, J.N. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat. Med. 2021, 27, 2127–2135. [Google Scholar] [CrossRef]
- Du, X.; Tang, H.; Gao, L.; Wu, Z.; Meng, F.; Yan, R.; Qiao, S.; An, J.; Wang, C.; Qin, F. Omicron adopts a different strategy from Delta and other variants to adapt to host. Signal Transduct. Target. Ther. 2022, 7, 45. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]



| Variable | (N = 255) | % |
|---|---|---|
| Age (Years) | ||
| Mean ± SD | 40.7 ± 11.4 | |
| Median (Min–Max) | 38 (23–69) | |
| 20–39 | 147 | 57.6 |
| 40–59 | 84 | 32.9 |
| 60–80 | 24 | 9.4 |
| Gender | ||
| Female | 144 | 56.5 |
| Male | 111 | 43.5 |
| HCWs | ||
| Medical | 183 | 71.8 |
| Paramedical | 72 | 28.2 |
| Associated comorbidity 1 | 132 | 51.8 |
| Regular drug use 2 | 57 | 22.4 |
| Ivermectin Administration | 138 | 54.1 |
| Prophylactic | 69 | 27.1 |
| Therapeutic | 15 | 5.8 |
| Both | 54 | 21.2 |
| Past COVID-19 infection | 138 | 54.1 |
| 1st dose ARs | 2nd dose AR | |||||
|---|---|---|---|---|---|---|
| Variable | Local N (%) 178 (69) | Systemic N (%) 212 (83.1) | p Value, OR (CI) | Local N (%) 120 (47.1) | Systemic N = 130 130 (51.0) | p Value, OR (CI) |
| Age | ||||||
| 20–39 n = 147 | 112 (62.9) | 128 (60.4) | 0.01 *, NA 1 | 84 (70.0) | 72 (55.4) | 0.001 *, NA 1 |
| 40–59 n = 84 | 54 (30.3) | 69 (32.5) | 0.01 *, NA 2 | 27 (22.5) | 49 (37.7) | 0.2, NA 2 |
| 60–80 n = 24 | 12 (6.7) | 15 (7.1) | 9 (7.5) | 9 (6.9) | ||
| Sex | ||||||
| Female n = 144 | 116 (65.2) | 128 (60.4) | <0.001 *, 5.2 (3.0–9.2) 1 | 84 (70.0) | 75 (57.7) | <0.001 *, 2.9 (1.7–4.9) 1 |
| Male n = 111 | 62 (34.8) | 84 (39.6) | 0.005 *, 2.6 (1.3–5.1) 2 | 36 (30.0) | 55 (42.3) | 0.7, 1.6 (0.7–1.8) 2 |
| Comorbidity3 n = 132 | 90 (50.6) | 112 (52.8) | 0.6, 0.9 (0.5–1.5) 1 0.5, 1.3 (0.7–2.5) 2 | 54 (45.0) | 67 (51.5) | 0.04 *, 0.6 (0.4–1.0) 1 0.9, 1.0 (0.6–1.6) 2 |
| Variable | (N = 255) | % |
|---|---|---|
| SARS-CoV-2 IgG Immunoreactivity | ||
| Baseline | 90 | 35.3 |
| After 1st dose of vaccination | 225 | 88.2 |
| After 2nd dose of vaccination | 244 | 95.7 |
| Seroconversion rate (n = 165) | ||
| After 1st dose of vaccination | 135 | 81.8 |
| After 2nd dose of vaccination | 154 | 93.3 |
| Variable | Immunoreactive N = 244 N (%) | Non-Immunoreactive N = 11 N (%) | p-Value | OR | CI |
|---|---|---|---|---|---|
| Age | <0.009 * | NA | NA | ||
| 20–39 n = 147 | 144 (59.0) | 3 (27.3) | |||
| 40–59 n = 84 | 80 (32.8) | 4 (36.4) | |||
| 60–80 n = 24 | 20 (8.2) | 4 (36.4) | |||
| Sex | 0.8 | 1.4 | 0.4–4.8 | ||
| Female n = 144 | 137 (56.1) | 7 (63.6) | |||
| Male n = 111 | 107 (43.9) | 4 (36.4) | |||
| HCWs | 0.5 | 0.7 | 0.2–2.4 | ||
| Medical n = 183 | 176 (72.1) | 7 (63.6) | |||
| Paramedical n = 72 | 68 (27.9) | 4 (36.4) | |||
| Ivermectin administration | |||||
| Prophylaxis n = 123 | 120 (49.2) | 3 (27.3) | 0.2 | 0.4 | 0.1–1.5 |
| Therapeutic n = 69 | 66 (27.0) | 3 (27.3) | 1.0 | 1.0 | 0.3–3.9 |
| Diabetes n = 28 | 22 (9.0) | 6 (54.5) | <0.001 * | 12.1 | 3.4–42.9 |
| Hypertension n = 35 | 31 (12.7) | 4 (36.4) | 0.049 * | 3.9 | 1.1–14.2 |
| Anemia n = 18 | 18 (7.4) | 0 (0.0) | 1.0 | NA | NA |
| Liver disease n = 7 | 7 (2.9) | 0 (0.0) | 1.0 | NA | NA |
| Neurologic disorders n = 6 | 6 (2.5) | 0 (0.0) | 1.0 | NA | NA |
| Thyroid disorders n = 13 | 13 (5.3) | 0 (0.0) | 1.0 | NA | NA |
| Osteoarthritis n = 35 | 28 (11.5) | 7 (63.6) | <0.001 * | 14.8 | 4.1–53.8 |
| Chest disease n = 12 | 12 (4.9) | 0 (0.0) | 1.0 | NA | NA |
| Allergic rhinitis n = 46 | 45 (18.4) | 1 (9.1) | 0.7 | 0.4 | 0.1–3.5 |
| Urticaria n = 19 | 19 (7.8) | 0 (0.0) | 1.0 | NA | NA |
| Drug allergy n = 11 | 11 (4.5) | 0 (0.0) | 1.0 | NA | NA |
| Food allergy n = 6 | 6 (2.5) | 0 (0.0) | 1.0 | NA | NA |
| Immunoreactivity to 1st Dose | Immunoreactivity to 2nd Dose | |||||
|---|---|---|---|---|---|---|
| Variable | Immuno-Reactive N = 225 N (%) | Non-Immunoreactive N = 30 N (%) | p Value, OR (CI) | Immuno-Reactive N = 244 N (%) | Non-Immunoreactive N = 11 N (%) | p Value, OR (CI) |
| Local | 163 (72.4) | 15 (50) | 0.01 *, 2.6 (1.2–5.7) | 114 (46.7) | 6 (54.5) | 0.6, 0.7 (0.2–2.5) |
| Pain | 163 (72.4) | 15 (50) | 0.01 *, 2.6 (1.2–5.7) | 122 (45.9) | 3 (27.3) | 0.4, 2.3 (0.6–8.7) |
| Swelling | 42 (18.7) | 6 (20.0) | 0.9, 0.9 (0.4–2.4) | 15 (6.1) | 3 (27.3) | 0.03 *, 0.2 (0.0–0.7) |
| Redness | 38 (16.9) | 3 (10.0) | 0.4, 1.8 (0.5–6.3) | 20 (8.2) | 3 (27.3) | 0.07, 0.2 (0.1–1.0) |
| Itching | 10 (4.4) | 0 (0.0) | 0.6, NA | 6 (2.5) | 0 (0.0) | 1.0, NA |
| Systemic | 196 (87.1) | 16 (53.3) | <0.001 *, 5.9 (2.6–13.4) | 127 (52.0) | 3 (27.3) | 0.1, 2.9 (0.8–11.2) |
| Fever | 138 (61.3) | 9 (30.0) | 0.001 *, 3.7 (1.6–8.5) | 23 (9.4) | 0 (0.0) | 0.6, NA |
| Malaise | 168 (61.3) | 16 (53.3) | 0.01 *, 2.6 (1.2–5.6) | 95 (38.9) | 3 (27.3) | 0.5, 1.7 (4.4–6.6) |
| Fatigue | 113 (50.2) | 6 (20.0) | 0.002 *, 4.0 (1.6–10.3) | 52 (21.3) | 0 (0.0) | 0.1, NA |
| Headache | 89 (39.6) | 7 (23.3) | 0.09, 2.2 (0.9–5.2) | 36 (14.8) | 3 (27.3) | 0.3, 0.4 (0.1–1.8) |
| Nausea | 10 (4.4) | 0 (0.0) | 0.6, NA | 0 (0.0) | 0 (0.0) | NA |
| Vomiting | 7 (3.1) | 0 (0.0) | 1.0, NA | 0 (0.0) | 0 (0.0) | NA |
| Diarrhea | 19 (8.4) | 0 (0.0) | 0.1, NA | 0 (0.0) | 0 (0.0) | NA |
| Dyspnea | 12 (5.3) | 0 (0.0) | 0.4, NA | 0 (0.0) | 0 (0.0) | NA |
| Cough | 7 (3.1) | 0 (0.0) | 1.0, NA | 2 (0.8) | 0 (0.0) | 1.0, NA |
| Palpitation | 10 (4.4) | 3 (10.0) | 0.2, 0.4 (0.1–1.6) | 4 (1.6) | 0 (0.0) | 1.0, NA |
| Pruritis | 3 (1.3) | 0 (0.0) | 1.0, NA | 2 (0.8) | 0 (0.0) | 1.0, NA |
| Baseline Immunoreactivity | Past COVID-19 Infection | p | OR | CI | |
|---|---|---|---|---|---|
| Yes N = 138 N (%) | No N = 117 N (%) | ||||
| Immunoreactive | 73 (52.9) | 18 (15.4) | <0.001 * | 6.2 | 3.4–11.3 |
| Non-Immunoreactive | 65 (39.1) | 99 (84.6) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammad, N.M.; Kadry, H.M.; Malek, M.M.; Bahgat, S.M.; Abdelsalam, N.M.; Afifi, A.H.M.; Abo-alella, D.A. Maintenance of Antibody Response in Egyptian Healthcare Workers Vaccinated with ChAdOx1 nCoV-19 Vaccine during Delta and Omicron Variants Pandemic: A Prospective Study. Vaccines 2022, 10, 1706. https://doi.org/10.3390/vaccines10101706
Hammad NM, Kadry HM, Malek MM, Bahgat SM, Abdelsalam NM, Afifi AHM, Abo-alella DA. Maintenance of Antibody Response in Egyptian Healthcare Workers Vaccinated with ChAdOx1 nCoV-19 Vaccine during Delta and Omicron Variants Pandemic: A Prospective Study. Vaccines. 2022; 10(10):1706. https://doi.org/10.3390/vaccines10101706
Chicago/Turabian StyleHammad, Noha M., Heba M. Kadry, Mai M. Malek, Shereen Mohamed Bahgat, Noha M. Abdelsalam, Amira Hamed Mohamed Afifi, and Doaa Alhussein Abo-alella. 2022. "Maintenance of Antibody Response in Egyptian Healthcare Workers Vaccinated with ChAdOx1 nCoV-19 Vaccine during Delta and Omicron Variants Pandemic: A Prospective Study" Vaccines 10, no. 10: 1706. https://doi.org/10.3390/vaccines10101706
APA StyleHammad, N. M., Kadry, H. M., Malek, M. M., Bahgat, S. M., Abdelsalam, N. M., Afifi, A. H. M., & Abo-alella, D. A. (2022). Maintenance of Antibody Response in Egyptian Healthcare Workers Vaccinated with ChAdOx1 nCoV-19 Vaccine during Delta and Omicron Variants Pandemic: A Prospective Study. Vaccines, 10(10), 1706. https://doi.org/10.3390/vaccines10101706






