AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses
Abstract
:1. Introduction
2. Methods and Materials
2.1. Cell Lines and Strains
2.2. Plasmid Construction and Vaccine Candidate Virus Rescue
2.3. Vaccine Preparation
2.4. Vaccine Immunization
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Hemagglutination Inhibition (HI) Assay
2.7. Microneutralization Assay (MN)
2.8. Neuraminidase Inhibition (NI) Assay
2.9. Statistics
3. Results
3.1. Generation of Vaccine Candidate Virus and Preparation of H5N8 Inactivated Vaccine
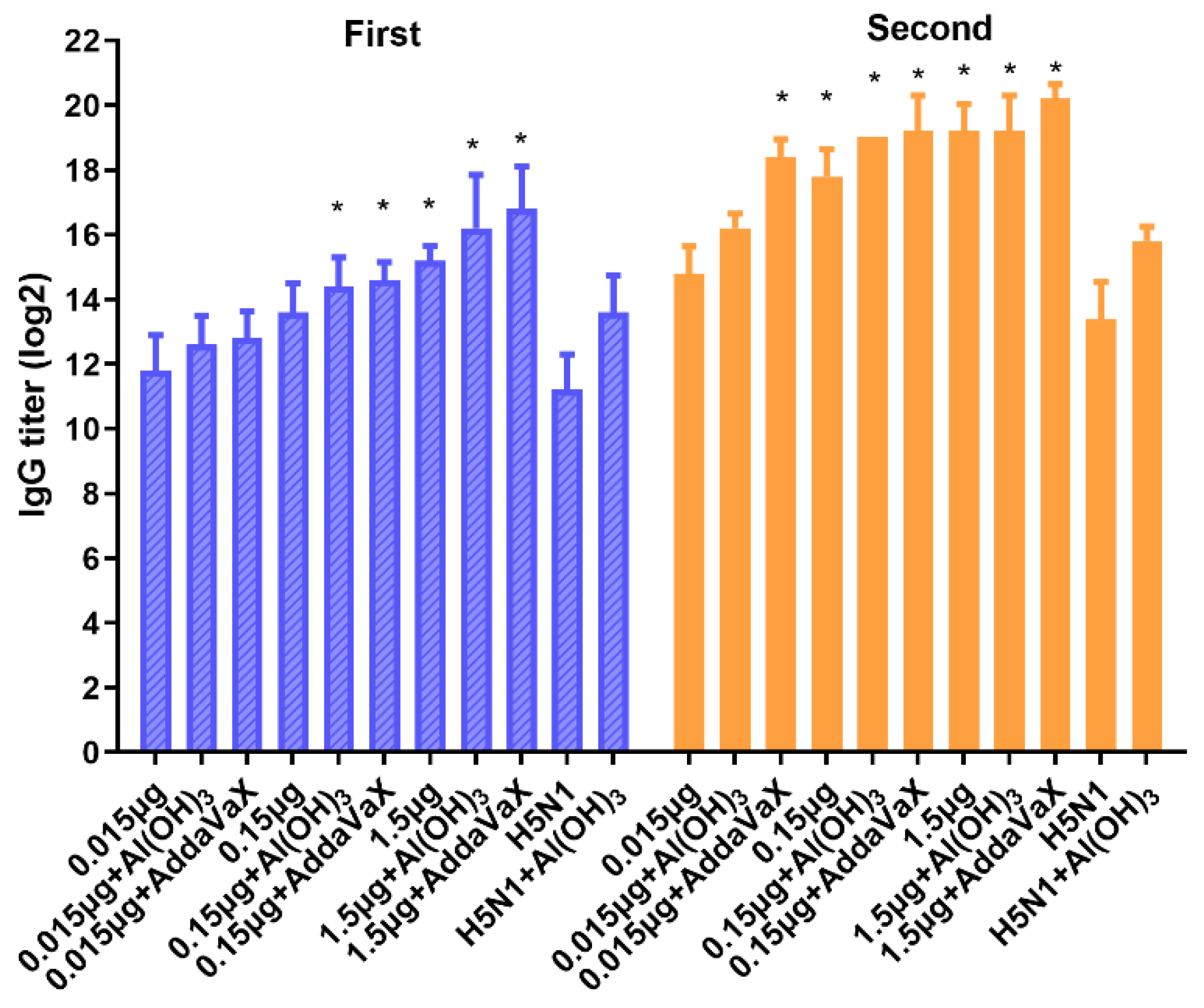
3.2. Immune Response Induced by H5N8 Inactivated Vaccine in Mouse
3.3. Cross-Reactive Antibody Response against Different Clades of H5 Viruses
3.4. Correlation Analysis between Functional Antibodies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Huang, C.-W.; Chen, L.-H.; Lee, D.-H.; Liu, Y.-P.; Li, W.-C.; Lee, M.-S.; Chen, Y.-P.; Lee, F.; Chiou, C.-J.; Lin, Y.-J. Evolutionary history of H5 highly pathogenic avian influenza viruses (clade 2.3.4.4c) circulating in Taiwan during 2015–2018. Infect. Genet. Evol. 2021, 92, 104885. [Google Scholar] [CrossRef] [PubMed]
- Evolution Working Group. Toward a unified nomenclature system for highly pathogenic avian influenza virus (H5N1). Emerg. Infect. Dis. 2008, 14, e1. [Google Scholar] [CrossRef] [PubMed]
- Nunez, I.; Ross, T.M. A review of H5Nx avian influenza viruses. Ther. Adv. Vaccines Immunother. 2019, 7, 2515135518821625. [Google Scholar] [PubMed] [Green Version]
- WHO. Cumulative Number of Confirmed Human Cases for Avian Influenza A(H5N1) Reported to WHO, 2003–2021; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Shi, W.; Gao, G.F. Emerging H5N8 avian influenza viruses. Science 2021, 372, 784–786. [Google Scholar] [CrossRef]
- Jeong, J.H.; Kim, E.-H.; Lloren, K.K.S.; Kwon, J.J.; Kwon, H.-I.; Ahn, S.J.; Kim, Y.-I.; Choi, W.-S.; Si, Y.-J.; Lee, O.-J.; et al. Preclinical evaluation of the efficacy of an H5N8 vaccine candidate (IDCDC-RG43A) in mouse and ferret models for pandemic preparedness. Vaccine 2018, 37, 484–493. [Google Scholar] [CrossRef]
- Jang, Y.; Seo, S.H. H5 cleavage-site peptide vaccine protects chickens from lethal infection by highly pathogenic H5 avian influenza viruses. Arch. Virol. 2022, 167, 67–75. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.; Dang, A.; Sun, S.; Zhang, D.; Wang, M.; Chen, F.; Li, Y.; Xue, R.; Chen, J.; et al. Pathological analysis and genetic characterization of the first outbreak H5N8 subtype avian influenza virus isolated from wild swan in Shandong, China. Transbound. Emerg. Dis. 2021, 68, 3200–3206. [Google Scholar] [CrossRef]
- Mostafa, A.; Abdelwhab, E.M.; Mettenleiter, T.C.; Pleschka, S. Zoonotic Potential of Influenza A Viruses: A Comprehensive Overview. Viruses 2018, 10, 497. [Google Scholar] [CrossRef] [Green Version]
- Moatasim, Y.; Kandeil, A.; Mostafa, A.; Kutkat, O.; Sayes, M.; El Taweel, A.; AlKhazindar, M.; AbdElSalam, E.; El-Shesheny, R.; Kayali, G.; et al. Impact of Individual Viral Gene Segments from Influenza A/H5N8 Virus on the Protective Efficacy of Inactivated Subtype-Specific Influenza Vaccine. Pathogens 2021, 10, 368. [Google Scholar] [CrossRef]
- Reed, S.G.; Orr, M.T.; Fox, C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013, 19, 1597–1608. [Google Scholar] [CrossRef]
- Giuseppe, D.G.; Rino, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar]
- De Jonge, J.; van Dijken, H.; de Heij, F.; Spijkers, S.; Mouthaan, J.; de Jong, R.; Roholl, P.; Adami, E.A.; Akamatsu, M.A.; Ho, P.L.; et al. H7N9 influenza split vaccine with SWE oil-in-water adjuvant greatly enhances cross-reactive humoral immunity and protection against severe pneumonia in ferrets. npj Vaccines 2020, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Chearwae, W.; Castellino, F.; Manischewitz, J.; King, L.R.; Honorkiewicz, A.; Rock, M.T.; Edwards, K.M.; Del Giudice, G.; Rappuoli, R.; et al. Vaccines with MF59 Adjuvant Expand the Antibody Repertoire to Target Protective Sites of Pandemic Avian H5N1 Influenza Virus. Sci. Transl. Med. 2010, 2, 15ra5. [Google Scholar] [CrossRef] [PubMed]
- Hawksworth, D. Advancing Freund’s and AddaVax Adjuvant Regimens Using CpG Oligodeoxynucleotides. Monoclon. Antib. Immunodiagn. Immunother. 2018, 37, 195–199. [Google Scholar] [CrossRef]
- Ramanunninair, M.; Le, J.; Onodera, S.; Fulvini, A.A.; Pokorny, B.A.; Silverman, J.; Devis, R.; Arroyo, J.M.; He, Y.; Boyne, A.; et al. Molecular Signature of High Yield (Growth) Influenza A Virus Reassortants Prepared as Candidate Vaccine Seeds. PLoS ONE 2013, 8, e65955. [Google Scholar]
- Hoffmann, E.; Neumann, G.; Kawaoka, Y.; Hobom, G.; Webster, R.G. A DNA transfection system for generation of influenza A virus from eight plasmids. Proc. Natl. Acad. Sci. USA 2000, 97, 6108–6113. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.Q.; Li, Z.; Jiao, M.; Lu, J.; Zhou, J.F.; Li, X.Y.; Liu, J.; Guo, J.F.; Xiao, N.; Zhao, X.; et al. Development and Assessment of Two Highly Pathogenic Avian Influenza (HPAI) H5N6 Candidate Vaccine Viruses for Pandemic Preparedness. Biomed. Environ. Sci. 2020, 33, 670–679. [Google Scholar]
- Shao, M.; Li, J.; Song, Y.L.; Cui, X.Y.; Yuan, L.Y.; Fang, H.H. Development and Validation of Alternative Method for Determination of Haemagglutinin Content in Influenza Vaccine. Chin. J. Biol. 2010, 23, 770–773. [Google Scholar]
- Gao, F.; Yang, T.; Liu, X.; Xiong, F.; Luo, J.; Yi, Y.; Fan, J.; Chen, Z.; Tan, W.-S. MiRNA Targeted NP Genome of Live Attenuated Influenza Vaccines Provide Cross-Protection against a Lethal Influenza Virus Infection. Vaccines 2020, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, T.-T.; Chen, P.-L.; Weng, T.-C.; Tsai, S.-Y.; Lai, C.-C.; Chou, H.-I.; Chen, P.-W.; Lu, C.-C.; Liu, M.-T.; Sung, W.-C.; et al. Development of high-growth influenza H7N9 prepandemic candidate vaccine viruses in suspension MDCK cells. J. Biomed. Sci. 2020, 27, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Xiong, F.-F.; Liu, X.-Y.; Gao, F.-X.; Luo, J.; Duan, P.; Tan, W.-S.; Chen, Z. Protective efficacy of anti-neuraminidase monoclonal antibodies against H7N9 influenza virus infection. Emerg. Microbes Infect. 2020, 9, 78–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heeringa, M.L.B.S. Comparability of Titers of Antibodies against Seasonal Influenza Virus Strains as Determined by Hemagglutination Inhibition and Microneutralization Assays. J. Clin. Microbiol. 2020, 58, e00750-20. [Google Scholar] [CrossRef] [PubMed]
- Memoli, M.J.; Shaw, P.A.; Han, A.; Czajkowski, L.; Reed, S.; Athota, R.; Bristol, T.; Fargis, S.; Risos, K.; Powers, J.H.; et al. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. mBio 2016, 7, e00417. [Google Scholar] [CrossRef] [PubMed]
- Couzens, L.; Gao, J.; Westgeest, K.; Sandbulte, M.; Lugovtsev, V.; Fouchier, R.; Eichelberger, M. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. J. Virol. Methods 2014, 210, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Belshe, R.B.; Frey, S.E.; Graham, I.; Mulligan, M.J.; Edupuganti, S.; Jackson, L.A.; Wald, A.; Poland, G.; Jacobson, R.; Keyserling, H.L.; et al. Safety and Immunogenicity of Influenza A H5 Subunit Vaccines: Effect of Vaccine Schedule and Antigenic Variant. J. Infect. Dis. 2011, 203, 666–673. [Google Scholar] [CrossRef]
- Czajka, H.; Unal, S.; Ulusoy, S.; Usluer, G.; Strus, A.; Sennaroglu, E.; Guzik, J.; Iskit, A.T.; Dargiewicz, A.; Musial, D.; et al. A phase II, randomised clinical trial to demonstrate the non-inferiority of low-dose MF59®-adjuvanted pre-pandemic A/H5N1 influenza vaccine in adult and elderly subjects. J. Prev. Med. Hyg. 2012, 53, 136–142. [Google Scholar]
- He, P.; Zou, Y.; Hu, Z. Advances in aluminum hydroxide-based adjuvant research and its mechanism. Hum. Vaccines Immunother. 2015, 11, 477–488. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef]
- O’Hagan, D.; Ott, G.; De Gregorio, E.; Seubert, A. The mechanism of action of MF59—An innately attractive adjuvant formulation. Vaccine 2012, 30, 4341–4348. [Google Scholar] [CrossRef]
- Nyon, M.P.; Du, L.; Tseng, C.-T.K.; Seid, C.A.; Pollet, J.; Naceanceno, K.S.; Agrawal, A.; Algaissi, A.; Peng, B.-H.; Tai, W.; et al. Engineering a stable CHO cell line for the expression of a MERS-coronavirus vaccine antigen. Vaccine 2018, 36, 1853–1862. [Google Scholar] [CrossRef] [PubMed]
- Nian, X.; Zhang, J.; Deng, T.; Liu, J.; Gong, Z.; Lv, C.; Yao, L.; Li, J.; Huang, S.; Yang, X. AddaVax Formulated with PolyI:C as a Potential Adjuvant of MDCK-based Influenza Vaccine Enhances Local, Cellular, and Antibody Protective Immune Response in Mice. AAPS PharmSciTech 2021, 22, 270. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Gordy, J.T.; Zavala, F.; Markham, R.B. A chemokine-fusion vaccine targeting immature dendritic cells elicits elevated antibody responses to malaria sporozoites in infant macaques. Sci. Rep. 2021, 11, 1220. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, C.M.; Montomoli, E. Influenza immunology evaluation and correlates of protection: A focus on vaccines. Expert Rev. Vaccines 2016, 15, 967–976. [Google Scholar] [CrossRef]
- Versage, E.; van Twuijver, E.; Jansen, W.; Theeuwes, A.; Sawlwin, D.; Hohenboken, M. Analyses of Safety Profile and Homologous Antibody Responses to a Mammalian Cell-Based, MF59-Adjuvanted, A/H5N1, Pandemic Influenza Vaccine across Four Phase II/III Clinical Trials in Healthy Children, Adults, and Older Adults. Vaccines 2021, 9, 1468. [Google Scholar] [CrossRef]
- Chanthavanich, P.; Versage, E.; Van Twuijver, E.; Hohenboken, M. Antibody responses against heterologous A/H5N1 strains for an MF59-adjuvanted cell culture–derived A/H5N1 (aH5N1c) influenza vaccine in healthy pediatric subjects. Vaccine 2021, 39, 6930–6935. [Google Scholar] [CrossRef]
- Antigua, K.J.C.; Choi, W.-S.; Baek, Y.H.; Song, M.-S. The Emergence and Decennary Distribution of Clade 2.3.4.4 HPAI H5Nx. Microorganisms 2019, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-S.; Jang, E.Y.; Cho, J.; Kim, K.; Lee, C.H.; Yi, H. Development and comparison of two H5N8 influenza A vaccine candidate strains. Arch. Virol. 2019, 164, 127–136. [Google Scholar] [CrossRef]
- Rockman, S.; Brown, L.E.; Barr, I.G.; Gilbertson, B.; Lowther, S.; Kachurin, A.; Kachurina, O.; Klippel, J.; Bodle, J.; Pearse, M.; et al. Neuraminidase-Inhibiting Antibody Is a Correlate of Cross-Protection against Lethal H5N1 Influenza Virus in Ferrets Immunized with Seasonal Influenza Vaccine. J. Virol. 2013, 87, 3053–3061. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Hansen, L.; Pedersen, G.; Grødeland, G.; Cox, R. Matrix M Adjuvanted H5N1 Vaccine Elicits Broadly Neutralizing Antibodies and Neuraminidase Inhibiting Antibodies in Humans That Correlate with In Vivo Protection. Front. Immunol. 2021, 12, 747774. [Google Scholar] [CrossRef]



| A/Astrakhan/3212/2020 H5N8 | Modification of Hemagglutinin Cleavage Site |
|---|---|
| Wild virus HA | L R E K R R K R ↓ G L F |
| CTA AGA GAA AAG AGA AGA AAA AGA GGC CTG TTT | |
| Modified HA | CTA AGA GAA ACG AGA GGC CTG TTT |
| L R E T R G L F |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Liu, X.; Dang, Y.; Duan, P.; Xu, W.; Zhang, X.; Wang, S.; Luo, J.; Li, X. AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines 2022, 10, 1683. https://doi.org/10.3390/vaccines10101683
Gao F, Liu X, Dang Y, Duan P, Xu W, Zhang X, Wang S, Luo J, Li X. AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines. 2022; 10(10):1683. https://doi.org/10.3390/vaccines10101683
Chicago/Turabian StyleGao, Feixia, Xueying Liu, Yudong Dang, Peng Duan, Wenting Xu, Xin Zhang, Shilei Wang, Jian Luo, and Xiuling Li. 2022. "AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses" Vaccines 10, no. 10: 1683. https://doi.org/10.3390/vaccines10101683
APA StyleGao, F., Liu, X., Dang, Y., Duan, P., Xu, W., Zhang, X., Wang, S., Luo, J., & Li, X. (2022). AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines, 10(10), 1683. https://doi.org/10.3390/vaccines10101683





