1. Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic has devastated economies and caused unprecedented challenges to healthcare and food systems around the world. In March 2021, vaccination using the ChAdOx1 nCov-19 (AZD1222) vaccine (Oxford-AstraZeneca) was paused in several European countries because of unusual thrombotic events observed after vaccine administration, sharing distinct clinical features (thrombosis in uncommon locations, thrombocytopenia, predominantly observed in young adults and women) [
1,
2]. Subsequently, vaccine-induced immune thrombotic thrombocytopenia (VITT), a variant of heparin-induced thrombocytopenia (HIT), was identified as a cause of thrombosis in these patients [
3]. HIT is a progressive thrombotic condition that can cause both venous and arterial thrombosis, typically 5 to 14 days after exposure to heparin. It is more common in female patients, particularly those who receive unfractionated heparin, and the diagnosis is confirmed by the presence of platelet factor 4-polyanion complexes (PF4) antibodies [
3]. Moreover, other severe side effects thromboses combined with immunothrombocytopenia (ITP) were reported after the administration of the ChAdOx1 nCov-19 vaccine [
4].
Proteolytic inactivation of factors Va (FVa) andVIIIa (FVIIIa) by activated protein C (APC) and its cofactors protein S and factor V (FV) is a key process in the physiological down-regulation of blood coagulation [
5]. A poor response of plasma to exogenous APC, better known as APC resistance, was first discovered in selected thrombophilic families [
6] but was soon recognized as the most frequent cause of inherited thrombophilia [
5,
7]. In most cases, APC resistance is caused by a single point mutation in the factor V gene (FV
Leiden, Leiden mutation, G1691A). The FV
Leiden mutation is present in about 5% of the general population and predisposes them to venous thromboembolism (VTE
) [
5,
8]. The risk of VTE is increased approximately 7-fold and 80-fold in heterozygous and homozygous carriers, respectively [
9]. Most of the FV
Leiden carriers never experience VTE, however, additional risk factors (e.g., protein C or protein S deficiency, oral contraceptive use, trauma, surgery, pregnancy) may be identified in those patients who develop VTE [
5,
10].
Here, we report on the radiological and laboratory diagnostic workup of an otherwise healthy 58-year-old man with extensive pulmonary embolism (PE) 18 days following the first dose of the ChAdOx1 nCov-19 vaccine.
2. Case
A 58-year-old man presented to his practitioner with right-sided pleuritic chest pain, dyspnea, and fatigue 18 days following the first dose of the ChAdOx1 nCov-19 vaccine. At the time of anamnesis, we could not identify risk factors for VTE (e.g., malignant, autoimmune, or infectious disease), however, chest auscultation indicated a reduced air entry over the right lower quadrant.
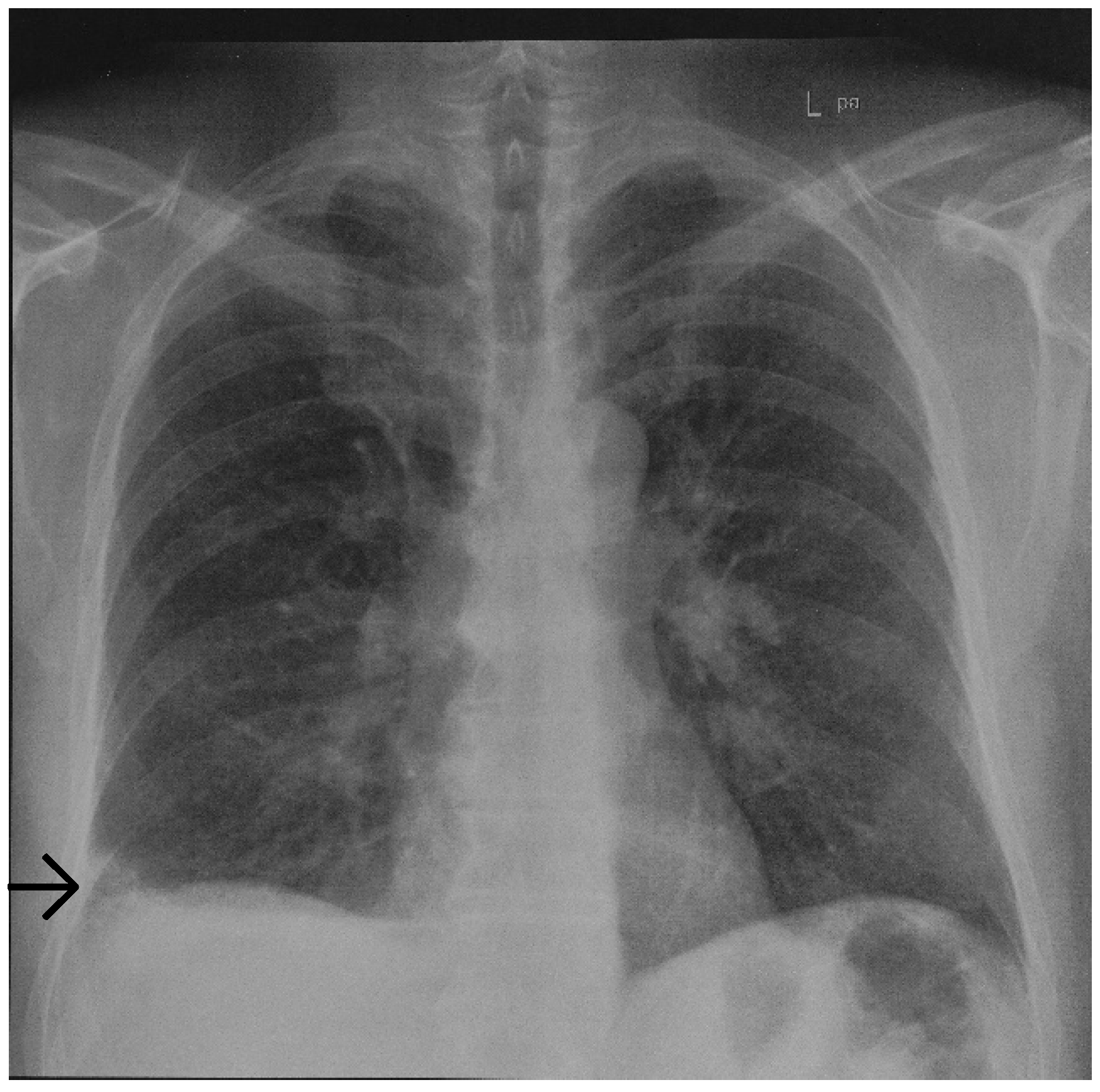
Initial chest radiography revealed a basal, wedged-shaped consolidation in the right lower lobe (
Figure 1) which was concerning for a Hampton’s hump. This radiological sign was first described in 1940 by Hampton and Castleman, who performed an autopsy series to demonstrate the site of opacities seen on chest radiography in patients with PE compared with pulmonary infarction (PI) seen at autopsy [
11,
12]. PE is a common cause of PI; however, the true incidence of subsequent PI is variable. In patients diagnosed with PE, PI has been reported in 15% to 31% of patients on follow-up autopsy and in 9% to 36% of patients on computed tomography [
13,
14,
15]. Hampton’s hump was demonstrated to be modestly specific for the diagnosis of PE but lacks sensitivity [
16]. In a study evaluating radiographs of patients in the multicenter Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) trial, Hampton’s hump showed a sensitivity of 22% and a specificity of 82% [
17]. Therefore, computed tomography pulmonary angiography (CTPA) was performed showing an area of subpleural PI in the right lower lobe (
Figure 2).
The only abnormal blood tests reported were a mild leukocytosis of 11.62 G/L (normal range 4.00–10.00 G/L), a mild thrombocytopenia of 143 G/L (normal range 150–350 G/L), a CRP and fibrinogen level of 62.8 mg/L (normal range < 5 mg/L) and 542 mg/dL (normal range 180–350 mg/dL), respectively, a markedly elevated D-dimer level of 7.53 µg/mL (normal range < 0.5 µg/mL), and a previously unknown APC resistance of 1.3 (normal range ≥ 1.8). Follow-up measurements six weeks after the initial examination revealed normalized blood cell counts with a negative CRP and a reduced D-dimer level of 1.73 µg/mL (normal range < 0.5 µg/mL).
Of note, antibodies against PF4 could not be observed, thereby ruling out VITT as a cause of PE. Moreover, antiphospholipid syndrome (APS) seems unlikely because the activated partial thromboplastin time (aPTT) was not found prolonged (24 s; normal range 23–32 s). Additionally, we observed normal levels of protein C, protein S, and antithrombin, and failed to demonstrate Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection as indicated by repeatedly negative nasopharyngeal swab testing. Subsequent genetic analysis identified the patient to be heterozygous for the FVLeiden mutation. The patient fully recovered with no sequelae but is being continued on long-term anticoagulation given his risk of recurrent VTE.
3. Discussion
Recently, a novel pathomechanism for the development of thrombosis after ChAdOx1 nCov-19 vaccination has been proposed [
4]. Accordingly, splice variants of the SARS-CoV-19 Spike protein lacking their transmembrane anchor are shed into the bloodstream. Upon dissemination throughout the vascular system, secreted Spike variants could have the potential to induce severe side effects when binding to cells via the ACE2 receptor. These side effects may be explained by the formation of anti-Spike antibodies directed against Spike variants bound to the ACE2 receptor with the potential to result in antibody-dependent cell-mediated cytotoxicity (ADCC) and/or complement-dependent cytotoxicity (CDC)-mediated inflammatory reaction [
4]. Moreover, other pathological reactions such as the neutrophil extracellular traps (NETs) host damage [
18] or the capillary leak syndrome could potentially explain the side effects observed after ChAdOx1 nCov-19 vaccination [
4,
19].
In their population-based cohort study, Pottegård and colleagues [
1] assessed the rates of cardiovascular and hemostatic events in the first 28 days after vaccination with the Oxford-AstraZeneca vaccine ChAdOx1-S in Denmark and Norway in order to compare them with rates observed in the general populations. Among 281,264 individuals, 59 venous thromboembolic events, including cerebral venous thrombosis, were observed in the vaccinated cohort compared with 30 expected based on the incidence rates in the general population. The authors, however, concluded, that the absolute risks of venous thromboembolic events were small, and their findings should be interpreted in the light of the proven beneficial effects of the vaccine, the context of the given country, and the limitations to the generalizability of the study findings.
While VITT-associated VTE has already been observed in a patient carrying the FV
Leiden mutation [
20], this is, to the best of our knowledge, the first report on a patient heterozygous for FV
Leiden with PE and without the concomitant presence of anti-PF4 antibodies.
4. Conclusions
Here we report on the temporal association between the first dose of the ChAdOx1 nCov-19 vaccine and extensive PE in an otherwise healthy patient with APC resistance, however, further investigation is needed to prove causality. Nevertheless, it seems tempting to speculate that a secreted Spike variant or even anti-Spike antibodies synergized with FVLeiden in our patient. Therefore, preemptive testing for APC resistance in individuals receiving ChAdOx1 nCov-19 vaccination could become a moot point.
Author Contributions
Study design: G.K.; data collection, analysis, and interpretation: A.B. and G.K.; manuscript preparation: G.K.; critically reviewed by A.B. and G.K. All authors have read and agreed to the published version of the manuscript.
Funding
No specific funding was received from any bodies in the public, commercial or not-for-profit sectors to carry out the work described in this article.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patient.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.; et al. Arterial events, venous thromboembolism, thrombocytopenia, and bleeding after vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population based cohort study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef] [PubMed]
- Schultz, N.H.; Sorvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aarnodt, A.H.; SkattØr, T.H.; TjØnnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Kowarz, E.; Krutzke, L.; Külp, M.; Streb, P.; Larghero, P.; Reis, J.; Bracharz, S.; Engler, T.; Kochanek, S.; Marschalek, R. Vaccine-induced COVID-19 mimicry syndrome. Elife 2022, 11, e74974. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, E.; Rosing, J. APC resistance: Biological basis and aquired influences. J. Thromb. Haemost. 2010, 8, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Dahlbäck, B.; Carlsson, M.; Svensson, P.J. Familial thrombophilia due to a previously unrecognized mechanism characterized by poor anticoagulant response to activated protein C: Prediction of a cofactor to activated protein C. Proc. Natl. Acad. Sci. USA 1993, 90, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, J.H.; Evatt, B.; Wideman, C.; Fernandez, J.A. Anticoagulant protein C pathway defective in majority of thrombophilic patients. Blood 1993, 82, 1989–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desmarais, S.; de Moerloose, P.; Reber, G.; Minazio, P.; Perrier, A.; Bounameaux, H. Resistance to activated protein C in an unselected population of patients with pulmonary embolism. Lancet 1996, 347, 1374–1375. [Google Scholar] [CrossRef]
- Rosendaal, F.R.; Koster, T.; Vandenbroucke, J.P.; Reitsma, P.H. High risk of thrombosis in patients homozygous for factor V Leiden (activated protein C resistance). Blood 1995, 85, 1504–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corral, J.; Zuaza-Jausoro, I.; Rivera, J.; González-Conejero, R.; Ferrer, F.; Vicente, V. Clinical and analytical relevance of the combination of prothrombin 20210A/A and factor V Leiden: Results from a large family. Br. J. Hematol. 1999, 105, 560–563. [Google Scholar] [CrossRef]
- Hampton, A.; Castleman, B. Correlation of postmortem chest tele-roentgenograms with autopsy findings with special reference to pulmonary embolism and infarction. AJR Am. J. Roentgenol. 1940, 43, 305–326. [Google Scholar]
- Mc Grath, B.M.; Groom, A.G. Images in clinical medicine. Hampton’s hump. N. Engl. J. Med. 2013, 368, 2219. [Google Scholar]
- Kaptein, F.H.J.; Kroft, L.M.; Hammerschlag, G.; Ninaber, M.K.; Bauer, M.P.; Huisman, M.V.; Klok, F.A. Pulmonary infarction in acute pulmonary embolism. Thromb. Res. 2021, 202, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Freiman, D.G.; Suyemoto, J.; Wessler, S. Frequency of pulmonary thromboembolism in man. N. Engl. J. Med. 1965, 272, 1278–1280. [Google Scholar] [CrossRef] [PubMed]
- Tsao, W.S.; Schraufnagel, D.; Wang, N.S. Pathogenesis of pulmonary infarction. Am. J. Med. 1982, 72, 599–606. [Google Scholar] [CrossRef]
- De Sirkar, S.; Newman, J.; Allen, S.; Elkaryoni, A.; Marginean, A.; Darki, A. Hampton hump in acute pulmonary embolism. Clevel. Clin. J. Med. 2002, 89, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Worsley, D.E.; Alavi, A.; Aronchick, J.W.; Chen, J.T.; Greenspan, R.H.; Ravin, C.E. Chest radiographic findings in patients with acute pulmonary embolism: Observations from the PIOPED study. Radiology 1993, 189, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef] [PubMed]
- Kawabe, S.; Saeki, T.; Yamazaki, H.; Nagai, M.; Miyamura, S. Systemic capillary leak syndrome. Intern. Med. 2002, 41, 211–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChaAdOx1 nCov-19 Vaccination. N. Eng. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).