Vaccination Coverage among Immunocompromised Patients in a Large Health Maintenance Organization: Findings from a Novel Computerized Registry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting
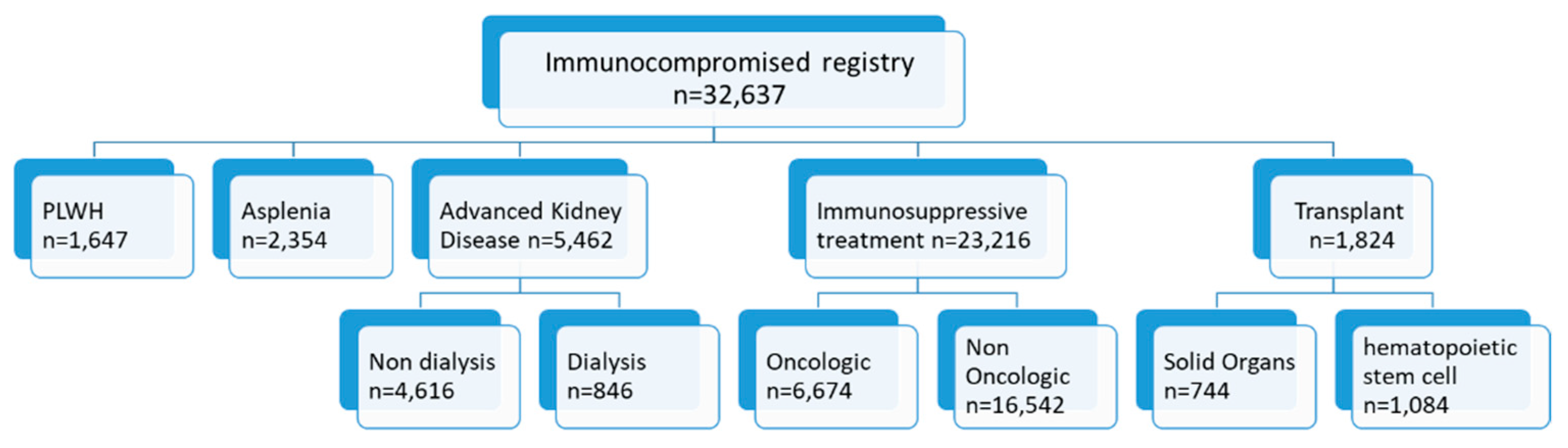
2.2. Registry Population
- (1)
- Receiving immunosuppressive treatment in the previous six months, including chemotherapy and radiation therapy for oncologic patients;
- (2)
- Patients living with HIV (PLWH)—HIV diagnosis (according to the International Classification of Diseases version 9 with clinical modifications (ICD-9-CM) codes: 044.9, 043.9, 795.71, 043.2) with a record of central MHS preauthorization for HIV-specific combination antiretroviral treatment (patients who purchase antiretroviral treatment for pre- or post- HIV exposure prophylaxis were excluded);
- (3)
- Patients with advanced chronic kidney disease (CKD) identified based on two consecutive serum creatinine levels corresponding to an estimated glomerular filtration rate < 30 mL/min, or receiving renal replacement therapy for at least three consecutive months during the previous seven months;
- (4)
- Transplant recipients (TR)—patients who had received solid organ (kidney, liver, intestines, heart, lung, pancreas) and/or hematopoietic stem cell transplant (HSCT);
- (5)
- Asplenia—record of a splenectomy procedure or a physician diagnosis of asplenia (ICD-9-CM codes: 759.0, 41.5).
2.3. Recommended Vaccines
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harpaz, R.; Dahl, R.M.; Dooling, K.L. Prevalence of Immunosuppression Among US Adults, 2013. JAMA 2016, 23, 2547–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dropulic, L.K.; Lederman, H.M. Overview of infections in the immunocompromised host. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M.; Janoff, E.N. Influenza in immunosuppressed populations: A review of infection frequency, morbidity, mortality, and vaccine responses. Lancet. Infect. Dis. 2009, 9, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Backhaus, E.; Berg, S.; Andersson, R.; Ockborn, G.; Malmström, P.; Dahl, M.; Nasic, S.; Trollfors, B. Epidemiology of invasive pneumococcal infections: Manifestations, incidence and case fatality rate correlated to age, gender and risk factors. BMC Infect. Dis. 2016, 16, 367. [Google Scholar] [CrossRef] [Green Version]
- van Aalst, M.; Lötsch, F.; Spijker, R.; van der Meer, J.T.M.; Langendam, M.W.; Goorhuis, A.; Grobusch, M.P.; de Bree, G.J. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2018, 24, 89–100. [Google Scholar] [CrossRef]
- Israeli Ministry of Health National Vaccine Guideline. Hebrew. Available online: https://www.health.gov.il/Subjects/vaccines/Pages/tadrich_Chisunim.aspx (accessed on 10 September 2019).
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, e44–e100. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.-J.; Hung, M.-C.; Srivastav, A.; Grohskopf, L.A.; Kobayashi, M.; Harris, A.M.; Dooling, K.L.; Markowitz, L.E.; Rodriguez-Lainz, A.; Williams, W.W. Surveillance of Vaccination Coverage Among Adult Populations—United States, 2018. MMWR Surveill. Summ. 2021, 70, 1–26. [Google Scholar] [CrossRef]
- Pittet, L.F.; Verolet, C.M.; Michetti, P.; Gaillard, E.; Girardin, M.; Juillerat, P.; Mottet, C.; Maillard, M.H.; Siegrist, C.A.; Posfay-Barbe, K.M.; et al. Risk of Vaccine-Preventable Infections in Swiss Adults with Inflammatory Bowel Disease. Digestion 2021, 102, 956–964. [Google Scholar] [CrossRef]
- Teich, N.; Klugmann, T.; Tiedemann, A.; Holler, B.; Mössner, J.; Liebetrau, A.; Schiefke, I. Vaccination coverage in immunosuppressed patients: Results of a regional health services research study. Dtsch. Arztebl. Int. 2011, 108, 105–111. [Google Scholar]
- Doornekamp, L.; van Leeuwen, L.; van Gorp, E.; Voeten, H.; Goeijenbier, M. Determinants of vaccination uptake in risk populations: A comprehensive literature review. Vaccines 2020, 8, 480. [Google Scholar] [CrossRef]
- Poeppl, W.; Lagler, H.; Raderer, M.; Sperr, W.R.; Zielinski, C.; Herkner, H.; Burgmann, H. Influenza vaccination perception and coverage among patients with malignant disease. Vaccine 2015, 33, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Larsen, L.; Bistrup, C.; Sørensen, S.S.; Boesby, L.; Nguyen, M.T.T.; Johansen, I.S. The coverage of influenza and pneumococcal vaccination among kidney transplant recipients and waiting list patients: A cross-sectional survey in Denmark. Transpl. Infect. Dis. 2021, 23, e13537. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Schmidt-Ott, R.; Santos, J.I.; Stanberry, L.R.; Hofstetter, A.M.; Rosenthal, S.L.; Cunningham, A.L. Vaccination of special populations: Protecting the vulnerable. Vaccine 2016, 34, 6681–6690. [Google Scholar] [CrossRef] [Green Version]
- Pop, B.; Fetica, B.; Blaga, M.L.; Trifa, A.P.; Achimas-Cadariu, P.; Vlad, C.I.; Achimas-Cadariu, A. The role of medical registries, potential applications and limitations. Med. Pharm. Rep. 2019, 92, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Hoque, D.M.E.; Kumari, V.; Hoque, M.; Ruseckaite, R.; Romero, L.; Evans, S.M. Impact of clinical registries on quality of patient care and clinical outcomes: A systematic review. PLoS ONE 2017, 12, e0183667. [Google Scholar] [CrossRef] [Green Version]
- Israeli Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population 2015. Available online: https://din-online.info/pdf/lst92e.pdf (accessed on 15 November 2021).
- Harrison, N.; Poeppl, W.; Herkner, H.; Tillhof, K.D.; Grabmeier-Pfistershammer, K.; Rieger, A.; Forstner, C.; Burgmann, H.; Lagler, H. Predictors for and coverage of influenza vaccination among HIV-positive patients: A cross-sectional survey. HIV Med. 2017, 18, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Schulte, K.; Schierke, H.; Tamayo, M.; Hager, L.; Engehausen, R.; Raspe, M.; Hübner, R.-H.; Schlieper, G.; Borzikowsky, C.; Urbschat, A.; et al. Strategies for Improving Influenza Vaccination Rates in Patients with Chronic Renal Disease. Dtsch. Arztebl. Int. 2019, 116, 413–419. [Google Scholar] [CrossRef]
- Battistella, C.; Quattrin, R.; Celotto, D.; d’Angelo, M.; Fabbro, E.; Brusaferro, S.; Agodi, A.; Astengo, M.; Baldo, V.; Baldovin, T.; et al. Factors predicting influenza vaccination adherence among patients in dialysis: An Italian survey. Hum Vaccin. Immunother. 2019, 15, 2434–2439. [Google Scholar] [CrossRef]
- Restivo, V.; Vizzini, G.; Mularoni, A.; Di Benedetto, C.; Gioè, S.M.; Vitale, F. Determinants of influenza vaccination among solid organ transplant recipients attending Sicilian reference center. Hum Vaccin. Immunother. 2017, 13, 346–350. [Google Scholar] [CrossRef] [Green Version]
- Ghaswalla, P.K.; Bengtson, L.G.S.; Marshall, G.S.; Buikema, A.R.; Bancroft, T.; Schladweiler, K.M.; Koep, E.; Novy, P.; Hogea, C.S. Meningococcal vaccination in patients with newly diagnosed asplenia in the United States. Vaccine 2021, 39, 272–281. [Google Scholar] [CrossRef]
- Sadlier, M.; Sadlier, C.; Alani, A.; Ahmad, K.; Bergin, C.; Ramsay, B. Poor adherence to vaccination guidelines in dermatology patients on immunosuppressive therapies: An issue that needs addressing. Br. J. Dermatol. 2015, 173, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Sowden, E.; Mitchell, W.S. An audit of influenza and pneumococcal vaccination in rheumatology outpatients. BMC Musculoskelet. Disord. 2007, 8, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monier, A.; Puyade, M.; Hernanz, M.P.G.; Bouchaert, P.; Leleu, X.; Tourani, J.M.; Roblot, F.; Rammaert, B. Observational study of vaccination in cancer patients: How can vaccine coverage be improved? Med. Mal. Infect. 2020, 50, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Pierron, A.; Bozon, F.; Berceanu, A.; Fontan, J.; Brion, A.; Deconinck, E.; Chirouze, C.; Brunel, A.-S. Vaccination coverage in hematological patients undergoing chemotherapy: Should we move towards personalized vaccination? Vaccine 2021, 39, 7036–7043. [Google Scholar] [CrossRef]
- Loubet, P.; Kernéis, S.; Groh, M.; Loulergue, P.; Blanche, P.; Verger, P.; Launay, O. Attitude, knowledge and factors associated with influenza and pneumococcal vaccine uptake in a large cohort of patients with secondary immune deficiency. Vaccine 2015, 33, 3703–3708. [Google Scholar] [CrossRef]
- Costello, R.; Winthrop, K.L.; Pye, S.R.; Brown, B.; Dixon, W.G. Influenza and Pneumococcal Vaccination Uptake in Patients with Rheumatoid Arthritis Treated with Immunosuppressive Therapy in the UK: A Retrospective Cohort Study Using Data from the Clinical Practice Research Datalink. PLoS ONE 2016, 11, e0153848. [Google Scholar] [CrossRef] [Green Version]
- National Program for Quality Indicators in Community Healthcare. Elderly adults. Available online: https://en.israelhealthindicators.org/elderly-adults (accessed on 12 August 2021).
- Harris, M.F.; Priddin, D.; Ruscoe, W.; Infante, F.A.; O’Toole, B.I. Quality of care provided by general practitioners using or not using Division-based diabetes registers. Med. J. Aust. 2002, 177, 250–252. [Google Scholar] [CrossRef]
- Gliklich, R.; Dreyer, N. Registries for Evaluating Patient Outcomes; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2010. [Google Scholar]
- Pollard, C.; Bailey, K.A.; Petitte, T.; Baus, A.; Swim, M.; Hendryx, M. Electronic patient registries improve diabetes care and clinical outcomes in rural community health centers. J. Rural Health 2009, 25, 77–84. [Google Scholar] [CrossRef]
| Variable | Total n = 32,367 | Transplant n = 1824 | IT n = 22,851 | Advance CKD * n = 5462 | Asplenia n = 2354 | PLWH n = 1647 |
|---|---|---|---|---|---|---|
| Age (years)—mean (SD) | 57 (17.4) | 55.86 (13.3) | 54.2 (16.6) | 73.4 (13.9) | 56 (15.1) | 46.1 (10.6) |
| 18–64 years no. (%) | 20,502 (62.8) | 1244 (68.3) | 15,835 (69.3) | 1180 (21.6) | 1602 (68) | 1560 (95) |
| ≥ 65 years no. (%) | 12,135 (37.2) | 580 (31.7) | 7016 (30.7) | 4282 (78.4) | 752 (32) | 87 (5) |
| Gender, Female—no. (%) | 17,087 (52.4) | 751 (27.3) | 13,113 (56.5) | 2483 (45.5) | 1122 (47.7) | 421 (25.6) |
| Socioeconomic status ‡—no. (%) | ||||||
| low | 6146 (18.8) | 332 (18.2) | 3934 (16.9) | 1335 (23.9) | 455 (19.3) | 462 (28.1) |
| med | 15,938 (48.8) | 846 (46.2) | 11,124 (47.9) | 2779 (50.9) | 1136 (48.3) | 857 (52) |
| high | 10,552 (32.3) | 650 (35.6) | 8158 (35.1) | 1250 (22.9) | 773 (32.8) | 328 (19.9) |
| Current Smoker—no. (%) | 4226 (12.9) | 192 (10.5) | 2765 (12.1) | 496 (9.1) | 373 (15.8) | 542 (32.9) |
| BMI—mean, SD | 27.1 (5.8) | 27 (5.2) | 26.9 (5.7) | 28.8 (6.2) | 26.8 (5.9) | 24.9 (5.1) |
| Comorbidities—no. (%) | ||||||
| Obesity ^ | 8811 (27) | 457 (25) | 5841 (25.6) | 2073 (38) | 594 (25.2) | 218 (13.2) |
| Underweight ^^ | 2264 (6.9) | 110 (6) | 193 (0.8) | 27 (0.5) | 153 (6.5) | 172 (10.4) |
| Hypertension | 13,036 (39.9) | 917 (50.1) | 7633 (33.4) | 4436 (81.2) | 800 (34) | 211 (12.8) |
| Diabetes | 6805 (20.9) | 459 (25.1) | 3624 (81.2) | 2657 (48.6) | 478 (20.3) | 83 (5) |
| Cardiovascular disease | 4532 (13.9) | 290 (15.9) | 2113 (9.2) | 2117 (38.3) | 280 (11.9) | 64 (3.9) |
| COPD | 1609 (4.9) | 93 (4.6) | 962 (4.2) | 510 (9.3) | 99 (4.2) | 27 (1.6) |
| Cognitive impairment | 722 (2.2) | 19 (1) | 292 (1.3) | 383 (7) | 42 (1.8) | 5 (0.3) |
| Osteoporosis | 6656 (20.4) | 434 (23.7) | 4609 (20.2) | 1515 (27.7) | 399 (16.9) | 96 (5.8) |
| IBD | 4384 (13.4) | 34 (1.8) | 4269 (18.7) | 73 (1.3) | 36 (1.5) | 20 (1.2) |
| Time from entry to registry, (Yr)—mean (SD) | 5.6 (5.4) | 7.3 (5.4) | 5.3 (5.4) | 4.5 (4.6) | 9.5 (5.7) | 7.2 (4.4) |
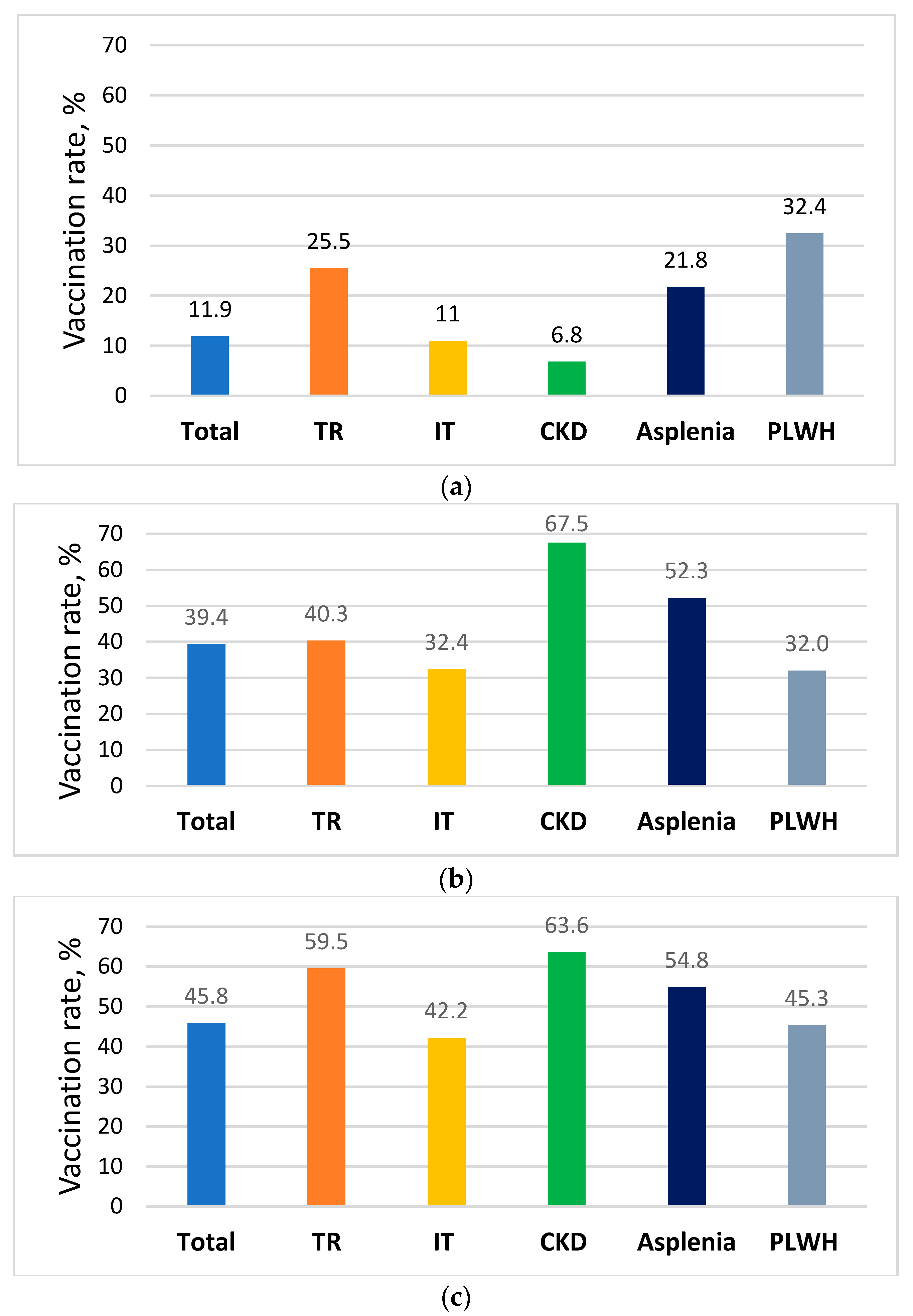
| PCV13 | PPV23 | Influenza 2018–2019 | Pneumo. + Annual Influenza | MenACWY * | Hepatitis B ** | |
|---|---|---|---|---|---|---|
| Total registry N = 32,637 | 3882 (11.9) | 12,857 (39.4) | 14,963 (45.8) | 1728 (5.3) | ||
| PLWH n = 1647 | 534 (32.4) | 527 (32) | 746 (45.3) | 161 (9.8) | 447 (27.1) | |
| Asplenia n = 2354 | 513 (21.8) | 1230 (52.3) | 1291 (54.8) | 314 (13.3) | ||
| CKD n = 4616 | 201 (4.4) | 3260 (70.6) | 2916 (63.2) | |||
| Dialysis n = 846 | 173 (20.4) | 429 (50.7) | 611 (70.7) | 342 (40.4) | ||
| Non Oncologic IT n = 16,542 | 2113 (12.8) | 4794 (29) | 8648 (52.3) | |||
| Oncologic IT n = 6674 | 498 (7.5) | 2613 (39.2) | 3641 (54.6) | |||
| HSC TR n = 1084 | 224 (20.7) | 422 (38.9) | 612 (56.5) | |||
| Solid organ TR n = 744 | 242 (32.5) | 313 (42) | 476 (48.3) | |||
| 18–64 years n = 20,502 | 2846 (13.9) | 3275 (16) | 7023 (34.3) | |||
| 65 years and Above n = 12,135 | 1036 (8.5) | 9582 (79) | 7940 (65.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shapiro Ben David, S.; Goren, I.; Mourad, V.; Cahan, A. Vaccination Coverage among Immunocompromised Patients in a Large Health Maintenance Organization: Findings from a Novel Computerized Registry. Vaccines 2022, 10, 1654. https://doi.org/10.3390/vaccines10101654
Shapiro Ben David S, Goren I, Mourad V, Cahan A. Vaccination Coverage among Immunocompromised Patients in a Large Health Maintenance Organization: Findings from a Novel Computerized Registry. Vaccines. 2022; 10(10):1654. https://doi.org/10.3390/vaccines10101654
Chicago/Turabian StyleShapiro Ben David, Shirley, Iris Goren, Vered Mourad, and Amos Cahan. 2022. "Vaccination Coverage among Immunocompromised Patients in a Large Health Maintenance Organization: Findings from a Novel Computerized Registry" Vaccines 10, no. 10: 1654. https://doi.org/10.3390/vaccines10101654
APA StyleShapiro Ben David, S., Goren, I., Mourad, V., & Cahan, A. (2022). Vaccination Coverage among Immunocompromised Patients in a Large Health Maintenance Organization: Findings from a Novel Computerized Registry. Vaccines, 10(10), 1654. https://doi.org/10.3390/vaccines10101654






