Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Study Procedure
2.3. Immunogenicity Outcomes
2.3.1. Quantitative IgG against Spike Protein Receptor Binding Domain of Ancestral Strain (Anti-S-RBD IgG) ELISA
2.3.2. Surrogate Virus Neutralization Test (sVNT)
2.3.3. Pseudovirus Neutralization Test (pVNT)
2.3.4. Enzyme-Linked Immunospot (ELISpot) Assay to Evaluate T Cell and Memory B Cell Responses
2.4. Statistical Analysis
3. Results
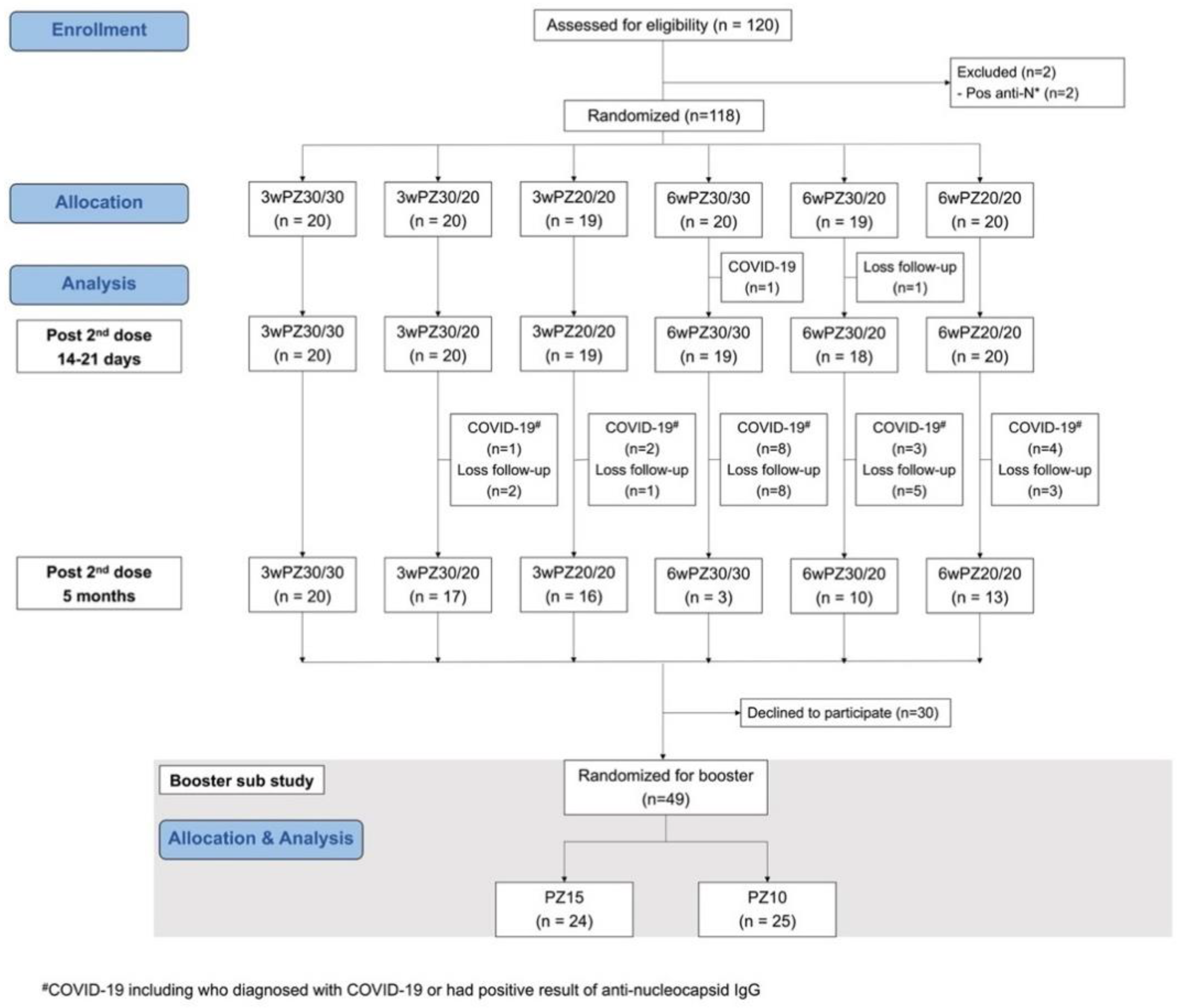
3.1. Study Populations
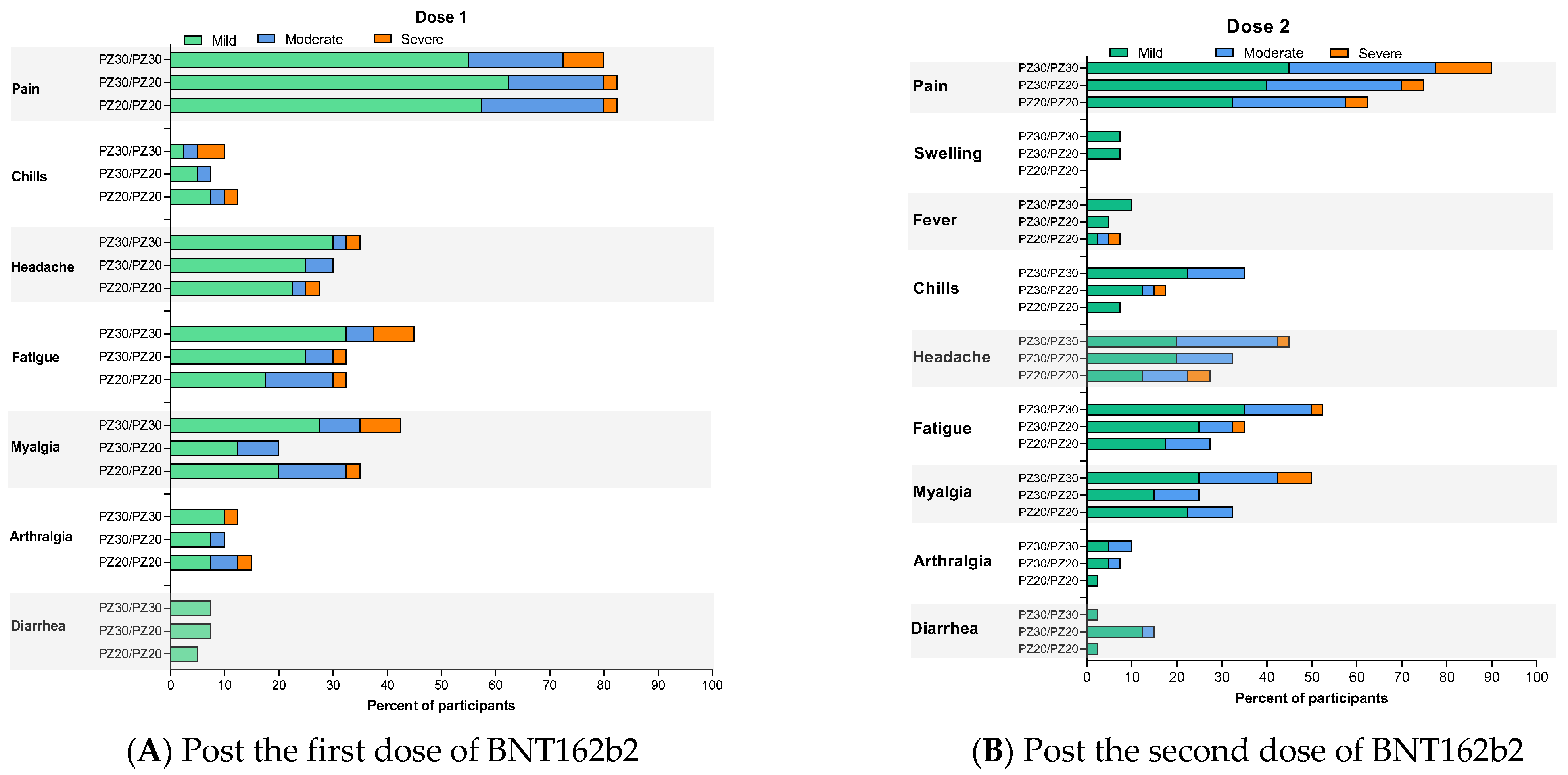
3.2. Reactogenicity
3.3. Immunogenicity
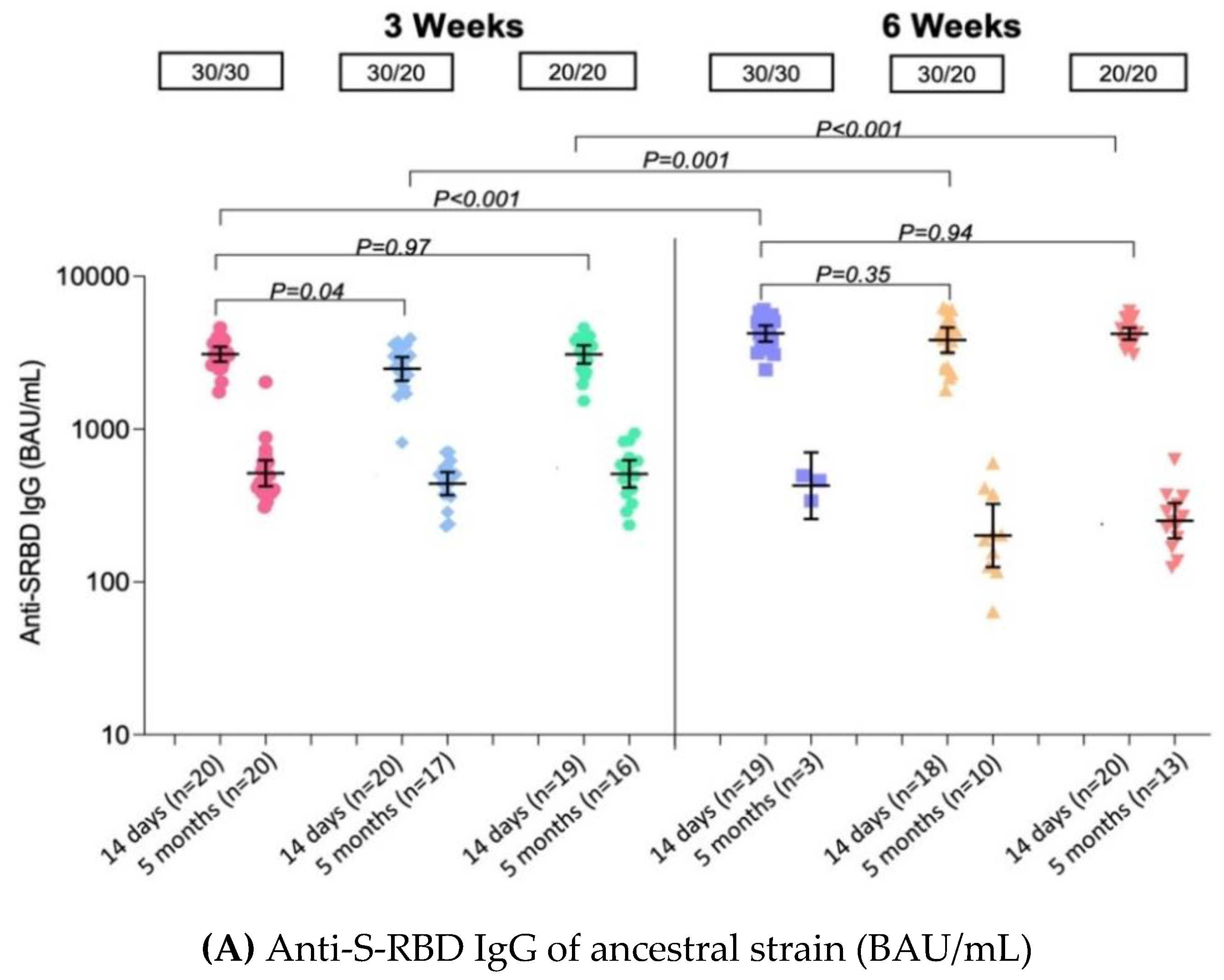
3.3.1. Quantitative IgG against Spike Protein Receptor Binding Domain of Ancestral Strain (Anti-S-RBD IgG) ELISA
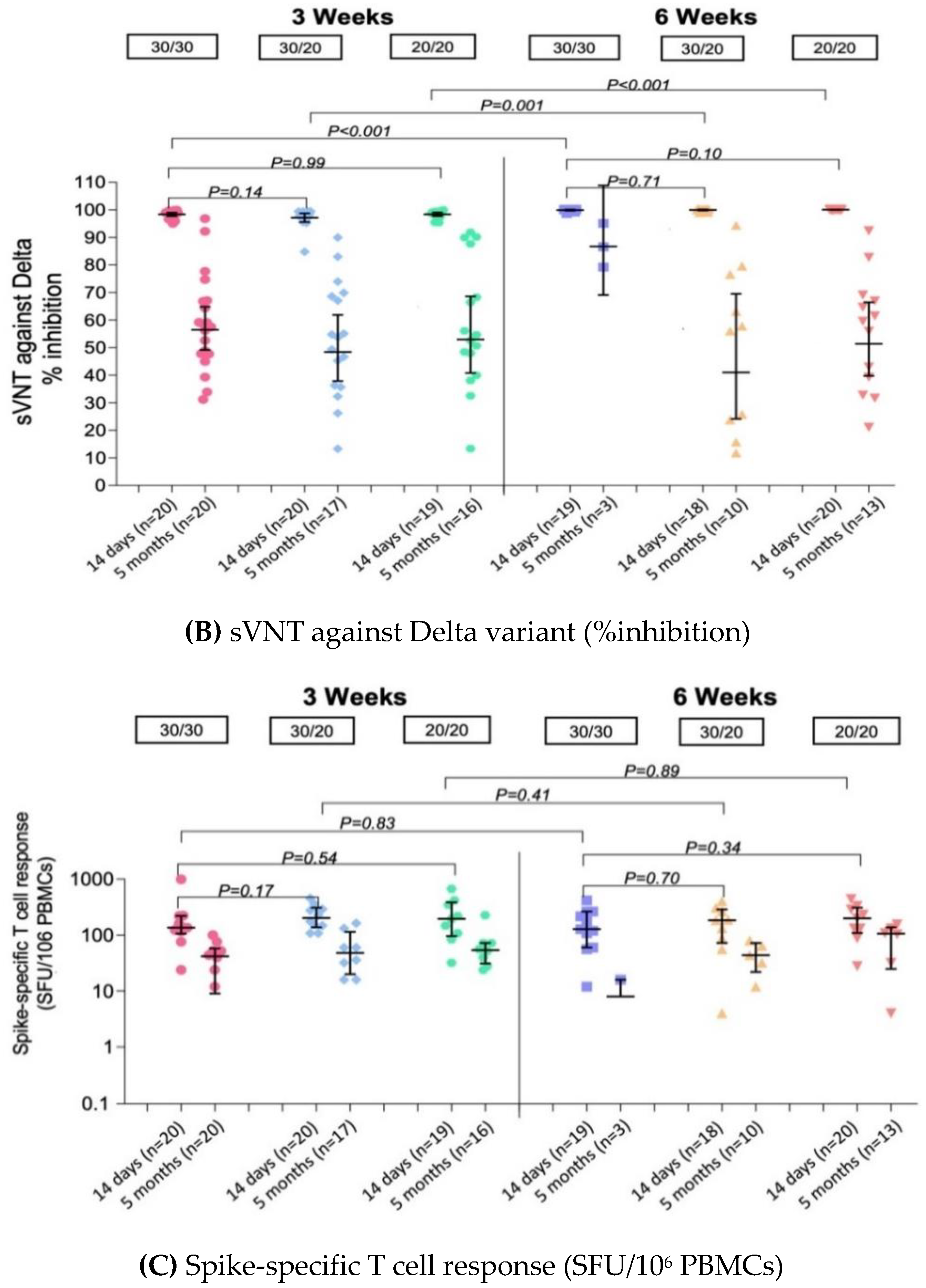
3.3.2. Surrogate Virus Neutralization Test (sVNT)
3.3.3. Pseudovirus Neutralization Test (pVNT)
3.3.4. Enzyme-Linked Immunospot (ELISpot) Assay to Evaluate T Cell and Memory B Cell Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. 2022. Available online: https://covid19.who.int/ (accessed on 28 August 2022).
- Department of Disease Control, Ministry of Public Health (Thailand). COVID-19 Dash Board. 2022. Available online: https://ddc.moph.go.th/covid19-dashboard/?dashboard=select-trend-line (accessed on 28 August 2022).
- World Health Organization. COVID-19 Weekly Epidemiological Update Edition 42. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-june-2021 (accessed on 14 August 2022).
- World Health Organization. COVID-19 Weekly Epidemiological Update Edition 74. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---11-january-2022 (accessed on 14 August 2022).
- Kao, C.M.; Orenstein, W.A.; Anderson, E.J. The Importance of Advancing Severe Acute Respiratory Syndrome Coronavirus 2 Vaccines in Children. Clin. Infect. Dis. 2021, 72, 515–518. [Google Scholar] [CrossRef]
- Wallace, M.; Woodworth, K.R.; Gargano, J.W.; Scobie, H.M.; Blain, A.E.; Moulia, D.; Chamberland, M.; Reisman, N.; Hadler, S.C.; MacNeil, J.R.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12–15 Years—United States, May 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA News Release: FDA Approves First COVID-19 Vaccine 23 August 2021. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine (accessed on 14 August 2022).
- Fowlkes, A.L.; Yoon, S.K.; Lutrick, K.; Gwynn, L.; Burns, J.; Grant, L.; Phillips, A.L.; Ellingson, K.; Ferraris, M.V.; LeClair, L.B.; et al. Effectiveness of 2-Dose BNT162b2 (Pfizer BioNTech) mRNA Vaccine in Preventing SARS-CoV-2 Infection Among Children Aged 5–11 Years and Adolescents Aged 12–15 Years—PROTECT Cohort, July 2021–February 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Munro, A.; Janani, L.; Cornelius, V.; Aley, P.K.; Babbage, G.; Baxter, D.; Bula, M.; Cathie, K.; Chatterjee, K.; Dodd, K.; et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): A blinded, multicentre, randomised, controlled, phase 2 trial. Lancet 2021, 398, 2258–2276. [Google Scholar] [CrossRef]
- Mbaeyi, S.; Oliver, S.E.; Collins, J.P.; Godfrey, M.; Goswami, N.D.; Hadler, S.C.; Jones, J.; Moline, H.; Moulia, D.; Reddy, S.; et al. The Advisory Committee on Immunization Practices’ Interim Recommendations for Additional Primary and Booster Doses of COVID-19 Vaccines—United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1545–1552. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration Center for Biologics Evaluation and Research. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials, 2007. Available online: https://www.fda.gov/media/73679/download (accessed on 10 August 2022).
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef]
- Nanthapisal, S.; Puthanakit, T.; Jaru-Ampornpan, P.; Nantanee, R.; Sodsai, P.; Himananto, O.; Sophonphan, J.; Suchartlikitwong, P.; Hiransuthikul, N.; Angkasekwinai, P.; et al. A randomized clinical trial of a booster dose with low versus standard dose of AZD1222 in adult after 2 doses of inactivated vaccines. Vaccine 2022, 40, 2551–2560. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Wanitchang, A.; Koonpaew, S.; Srisutthisamphan, K.; Saenboonrueng, J.; Im-Erbsin, R.; Inthawong, M.; Sunyakumthorn, P.; Thaweerattanasinp, T.; Tanwattana, N.; et al. Chimeric Virus-like Particle-Based COVID-19 Vaccine Confers Strong Protection against SARS-CoV-2 Viremia in K18-hACE2 Mice. Vaccines 2022, 10, 786. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- Kitro, A.; Sirikul, W.; Dilokkhamaruk, E.; Sumitmoh, G.; Pasirayut, S.; Wongcharoen, A.; Panumasvivat, J.; Ongprasert, K.; Sapbamrer, R. COVID-19 vaccine hesitancy and influential factors among Thai parents and guardians to vaccinate their children. Vaccine X 2022, 11, 100182. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; He, Y.; Shi, Y. Parents’ and Guardians’ Willingness to Vaccinate Their Children against COVID-19: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 179. [Google Scholar]
- Więcek, W.; Ahuja, A.; Chaudhuri, E.; Kremer, M.; Simoes Gomes, A.; Snyder, C.M.; Tabarrok, A.; Tan, B.J. Testing fractional doses of COVID-19 vaccines. Proc. Natl. Acad. Sci. USA 2022, 119, e2116932119. [Google Scholar] [CrossRef]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Amirthalingam, G.; Bernal, J.L.; Andrews, N.J.; Whitaker, H.; Gower, C.; Stowe, J.; Tessier, E.; Subbarao, S.; Ireland, G.; Baawuah, F.; et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat. Commun. 2021, 12, 7217. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Febriani, Y.; Ouakki, M.; Setayeshgar, S.; El Adam, S.; Zou, M.; Talbot, D.; Prystajecky, N.; Tyson, J.R.; Gilca, R.; et al. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: Test-negative design studies from British Columbia and Quebec, Canada. Clin. Infect. Dis. 2022, ciac290. [Google Scholar] [CrossRef]
- Buchan, S.A.; Seo, C.Y.; Johnson, C.; Alley, S.; Kwong, J.C.; Nasreen, S.; Calzavara, A.; Lu, D.; Harris, T.M.; Yu, K. Epidemiology of Myocarditis and Pericarditis following mRNA Vaccination by Vaccine Product, Schedule, and Interdose Interval among Adolescents and Adults in Ontario, Canada. JAMA Netw. Open 2022, 5, e2218505. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Cai, C.; Grifoni, A.; Müller, T.R.; Niessl, J.; Olofsson, A.; Humbert, M.; Hansson, L.; Österborg, A.; Bergman, P.; et al. Ancestral SARS-CoV-2-specific T cells cross-recognize the Omicron variant. Nat. Med. 2022, 28, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Jeong, S.D.; Noh, J.Y.; Kim, D.U.; Jung, S.; Song, J.Y.; Jeong, H.W.; Park, S.H.; Shin, E.C. BNT162b2-induced memory T cells respond to the Omicron variant with preserved polyfunctionality. Nat. Microbiol. 2022, 7, 909–917. [Google Scholar] [CrossRef] [PubMed]
- Agrati, C.; Castilletti, C.; Goletti, D.; Sacchi, A.; Bordoni, V.; Mariotti, D.; Notari, S.; Matusali, G.; Meschi, S.; Petrone, L.; et al. Persistent Spike-specific T cell immunity despite antibody reduction after 3 months from SARS-CoV-2 BNT162b2-mRNA vaccine. Sci. Rep. 2022, 12, 6687. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, G.; Picozza, M.; D’Orso, S.; Placido, R.; Pirronello, M.; Verdiani, A.; Termine, A.; Fabrizio, C.; Giannessi, F.; Sambucci, M.; et al. BNT162b2 vaccination induces durable SARS-CoV-2-specific T cells with a stem cell memory phenotype. Sci. Immunol. 2021, 6, eabl5344. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Britton, A.; Shang, N.; Derado, G.; Link-Gelles, R.; Accorsi, E.K.; Smith, Z.R.; Miller, J.; Verani, J.R.; Schrag, S.J. Association of Prior BNT162b2 COVID-19 Vaccination with Symptomatic SARS-CoV-2 Infection in Children and Adolescents During Omicron Predominance. JAMA 2022, 327, 2210–2219. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Pfizer-BioNTech COVID-19 Vaccine Letter of Authorization (Reissued) on 17 June 2022. Available online: https://www.fda.gov/media/150386/download (accessed on 14 August 2022).
- Assavavongwaikit, P.; Chantasrisawad, N.; Himananto, O.; Phasomsap, C.; Klawaja, P.; Cartledge, S.; Nadsasarn, R.; Jupimai, T.; Kawichai, S.; Anugulruengkitt, S.; et al. Immunogenicity of BNT162b2 Vaccination against SARS-CoV-2 Omicron Variant and Attitudes toward a COVID-19 Booster Dose among Healthy Thai Adolescents. Vaccines 2022, 10, 1098. [Google Scholar] [CrossRef]
- Link-Gelles, R. Updates on COVID-19 Vaccine Effectiveness during Omicron. Presented at: ACIP Meeting, 1 September 2022. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-09-01/04-COVID-Link-Gelles-508.pdf (accessed on 25 September 2022).
- BioNTech SE. To Evaluate the Safety, Tolerability, Efficacy and Immunogenicity of BNT162b2 Boosting Strategies against COVID-19 in Participants ≥12 Years of Age. Available online: https://clinicaltrials.gov/ct2/show/NCT04955626 (accessed on 28 August 2022).
- ModernaTX Inc. A Study to Evaluate the Immunogenicity and Safety of mRNA-1273.529 Vaccine for the COVID-19 Omicron Variant B.1.1.529. Available online: https://clinicaltrials.gov/ct2/show/NCT05249829 (accessed on 28 August 2022).
- Center of Disease Control and Prevention. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html (accessed on 25 September 2022).
| 3-Week Interval | 6-Week Interval | |||||
|---|---|---|---|---|---|---|
| Groups | 3wPZ30/30 | 3wPZ30/20 | 3wPZ20/20 | 6wPZ30/30 | 6wPZ30/20 | 6wPZ20/20 |
| Anti-S-RBD IgG of Ancestral Strain (BAU/mL) | ||||||
| Pre 2nd dose, GMs (95% CI) | n = 20 | n = 20 | n = 19 | n = 19 | n = 19 | n = 20 |
| 490 | 503 | 557 | 303 | 357 | 239 | |
| (399–601) | (409–618) | (445–695) | (227–406) | (241–527) | (183–313) | |
| Post 2nd dose: 14–21 days, GMs (95% CI) | n = 20 | n = 20 | n = 19 | n = 19 | n = 18 | n = 20 |
| 3090 | 2480 | 3080 | 4226 | 3823 | 4205 | |
| (2761–3460) | (2078–2961) | (2685–3535) | (3745–4769) | (3162–4622) | (3852–4590) | |
| GMR | ref | 0.80 | 1.00 | 1.37 | 1.24 | 1.36 |
| (0.67–0.97) | (0.83–1.20) | (1.13–1.65) | (1.02–1.50) | (1.13–1.64) | ||
| Post 2nd dose: 5 months, GMs (95% CI) | n = 20 | n = 17 | n = 16 | n = 3 | n = 10 | n = 13 |
| 514 | 440 | 509 | 426 | 201 | 251 | |
| (423–626) | (370–523) | (414–627) | (259–702) | (125–324) | (193–328) | |
| GMR | ref | 0.86 | 0.99 | 0.83 | 0.39 | 0.49 |
| (0.64–1.14) | (0.74–1.32) | (0.48–1.42) | (0.28–0.55) | (0.36–0.67) | ||
| sVNT against Delta variant (%inhibition) | ||||||
| Pre 2nd dose, GMs (95% CI) | n = 20 | n = 20 | n = 19 | n = 19 | n = 19 | n = 20 |
| 60.9 | 62.6 | 64.8 | 48.5 | 56.8 | 51.7 | |
| (54.8–67.8) | (56.1–69.8) | (55.8–75.2) | (40.7–57.9) | (47.9–67.3) | (46.3–57.6) | |
| Post 2nd dose: 14–21 days, GMs (95% CI) | n = 20 | n = 20 | n = 19 | n = 19 | n = 18 | n = 20 |
| 98.4 | 97.2 | 98.4 | 99.9 | 100.0 | 100.1 | |
| (97.7–99.0) | (95.6–98.7) | (97.7–99.0) | (99.7–100.1) | (99.7–100.2) | (100.0–100.1) | |
| GMR | ref | 0.99 | 1.00 | 1.02 | 1.02 | 1.02 |
| (0.98–1.00) | (0.99–1.01) | (1.00–1.03) | (1.01–1.03) | (1.01–1.03) | ||
| Post 2nd dose: 5 months, GMs (95% CI) | n = 20 | n = 17 | n = 16 | n = 3 | n = 10 | n = 13 |
| 56.5 | 48.4 | 52.9 | 86.7 | 41.0 | 51.4 | |
| (49.2–64.9) | (37.9–61.9) | (40.8–68.6) | (69.1–108.9) | (24.2–69.5) | (39.7–66.5) | |
| GMR | ref | 0.86 | 0.94 | 1.54 | 0.73 | 0.91 |
| (0.63–1.17) | (0.69–1.28) | (0.86–2.74) | (0.51–1.04) | (0.65–1.27) | ||
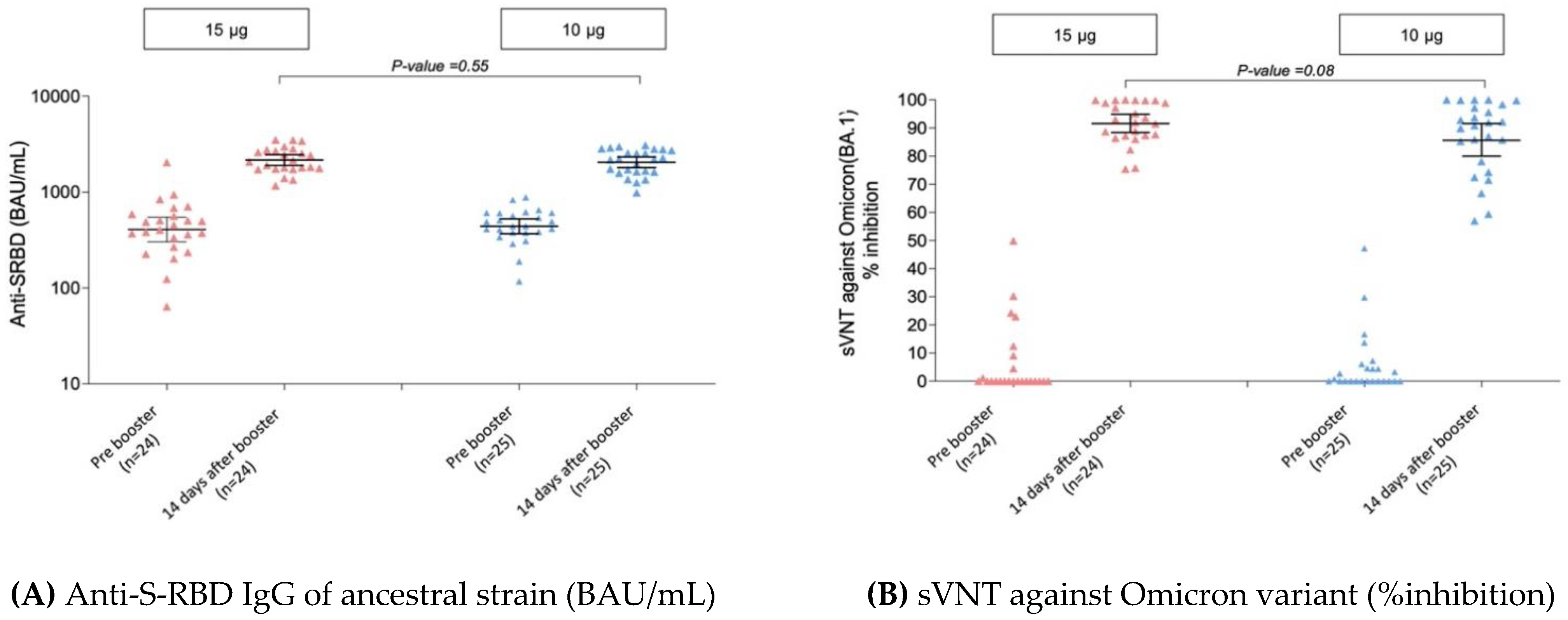
| Booster 15 μg (n = 24) | Booster 10 μg (n = 25) | p-Value | |
|---|---|---|---|
| sVNT against Omicron (BA.1) variant (%inhibition) | |||
| Pre booster dose, GMs (95% CI) | 12.2 | 6.7 | 0.28 |
| (4.4–33.9) | (3.2–13.8) | ||
| Post booster dose: 14 days, GMs (95% CI) | 91.6 | 85.6 | 0.08 |
| (88.4–94.9) | (80.0–91.6) | ||
| GMR | ref | 0.93 (0.87–1.01) | |
| pVNT against Omicron (BA.2) variant (ID50), GMs (95% CI) | |||
| Post booster dose: 14 days, GMs (95% CI) | 330.7 | 396.6 | 0.51 |
| (221.1–494.9) | (266.4–590.4) | ||
| GMR | ref | 1.20 (0.69–2.08) | |
| Anti-S-RBD IgG of ancestral strain (BAU/mL) | |||
| Pre booster dose, GMs (95% CI) | 407 | 440 | 0.64 |
| (304–546) | (368–526) | ||
| Post booster dose: 14 days, GMs (95% CI) | 2158 | 2047 | 0.55 |
| (1897–2455) | (1798–2330) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puthanakit, T.; Chantasrisawad, N.; Yoohat, K.; Nantanee, R.; Sophonphan, J.; Meepuksom, T.; Sodsai, P.; Phanthanawiboon, S.; Jantarabenjakul, W.; Hirankarn, N.; et al. Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents. Vaccines 2022, 10, 1646. https://doi.org/10.3390/vaccines10101646
Puthanakit T, Chantasrisawad N, Yoohat K, Nantanee R, Sophonphan J, Meepuksom T, Sodsai P, Phanthanawiboon S, Jantarabenjakul W, Hirankarn N, et al. Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents. Vaccines. 2022; 10(10):1646. https://doi.org/10.3390/vaccines10101646
Chicago/Turabian StylePuthanakit, Thanyawee, Napaporn Chantasrisawad, Kirana Yoohat, Rapisa Nantanee, Jiratchaya Sophonphan, Thutsanun Meepuksom, Pimpayao Sodsai, Supranee Phanthanawiboon, Watsamon Jantarabenjakul, Nattiya Hirankarn, and et al. 2022. "Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents" Vaccines 10, no. 10: 1646. https://doi.org/10.3390/vaccines10101646






