Development of Therapeutic Vaccine for Chronic Hepatitis B: Concept, Cellular and Molecular Events, Design, Limitation, and Future Projection
Abstract
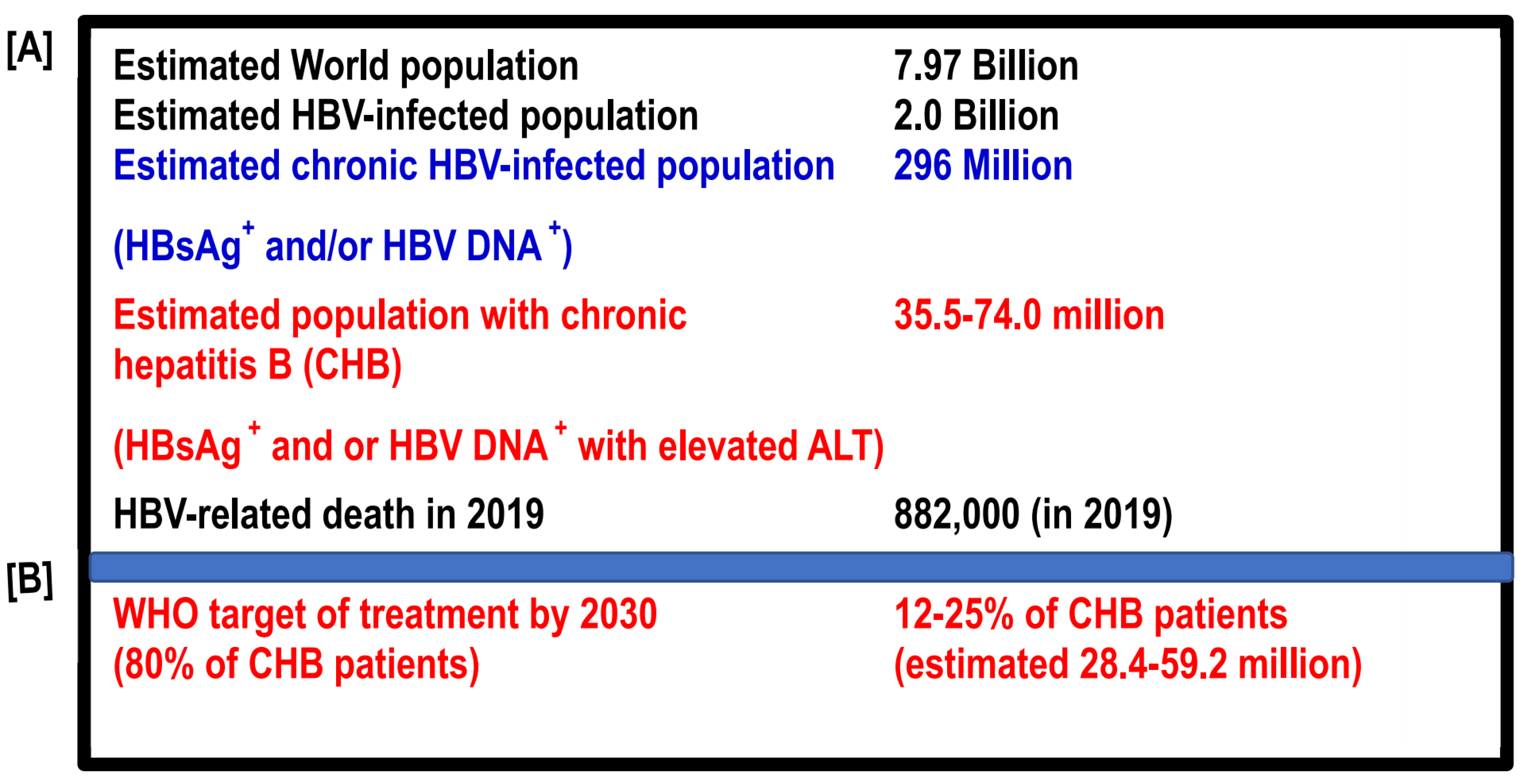
:1. Introduction
2. Limitation of Commercially Available Antiviral Drugs
3. Why Immune Therapy Became an Option for Treatment of CHB
4. The Advent of Vaccine Therapy for Treating CHB Patients
5. Different Modes of Vaccine Therapies for CHB Patients (from 1994 to 2022)
5.1. HBsAg-Based Vaccine Therapy
5.2. Different Forms of HBsAg-Based Vaccine Therapy for Treating CHB Patients and Their Limitations
5.3. Other Forms of Vaccine Therapy in Addition to HBsAg-Based Vaccine
5.4. A Bird’s Eye View of the “Vaccine Therapy” in CHB Patients
6. Limitations of “Vaccine Therapy” in CHB Patients
- HBsAg-based vaccine therapy is not an evidence-based immune therapy for CHB patients. Immunity to HBsAg, such as anti-HBs, is an essential factor for controlling circulating HBV. Thus, the HBsAg-based vaccine is critical in the context of preventing HBV infection. However, treatment of CHB patients is related to the control of intracellular HBV DNA, especially cccDNA. This cannot be accomplished by the anti-HB response initiated by the HBsAg-based vaccine.
- Almost all investigators have used their own protocol for accomplishing vaccine therapy. Thus, the dose and duration of immunization have varied among protocols. This makes it difficult to assess the real implication of the optimum dose or duration of HBsAg that should be used in vaccine therapy.
- Almost all studies about vaccine therapy in CHB patients have been accomplished as pilot studies or observational studies, or as clinical trials of a limited spectrum. Thus, phase I/II/III clinical trials along with a follow-up study of several years with vaccine therapy remain to be accomplished.
- In almost all cases of vaccine therapy for CHB, there has been no publication regarding the short- or long-term follow-up information of this therapy.
- In most cases of vaccine therapy in CHB patients, the mechanism of action has been elucidated. The query remains regarding the presence of HBsAg in all CHB patients. Further, what specific functions can be achieved by administering HBsAg? Thus, there is a need to show differences in the immune modulation of circulating HBsAg and administered HBsAg-based vaccines. This has not been properly dissected.
7. Role of HBcAg as a Component of Vaccine Therapy
8. Positive Sides of Vaccine Therapy for the Treatment of CHB Patients
- Almost all protocols of vaccine therapy against CHB have shown their excellent safety profiles.
- Most of the projects of vaccine therapy have been endowed with antiviral potentials in CHB patients.
- Some protocols of vaccine therapy have shown the capacity of therapy to induce HBeAg negativity or anti-HBeAg seroconversion.
- Normalization of ALT has been reported by most of the designs of vaccine therapy.
9. Future Projections of Vaccine Therapy for CHB Patients
- Nature of the antigen: various pieces of evidence indicate that only HBsAg-based vaccine therapy may not be an optimum type of vaccine therapy as HBsAg-based immunity is unlikely to have an impact on intracellular HBV DNA and cccDNA.
- In spite of using HBsAg-based vaccine therapy, more attention should be directed to the use of HBcAg or other HBV-related antigens as candidate antigens for vaccine therapy.
- Studies about vaccine therapy should be properly designed regarding the dose and duration of therapy.
- Studies should be formulated to provide safety and efficacy information for the end of treatment (EOT) and after the EOT for a prolonged duration (data from over one to five years).
- When antiviral and liver protection is recorded by vaccine therapy, the underlying mechanisms should be explored.
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, J.J.; Mohtashemi, N.; Bhattacharya, D. Significance and management of isolated hepatitis B core antibody (Anti-HBc) in HIV and HCV: Strategies in the DAA Era. Curr. HIV AIDS Rep. 2018, 15, 172–181. [Google Scholar] [CrossRef]
- WHO. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf (accessed on 28 August 2022).
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 1, a021410. [Google Scholar] [CrossRef]
- Ni, Y.-H. Natural history of hepatitis B virus infection: Pediatric perspective. J. Gastroenterol. 2010, 46, 1–8. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Hu, K.Q.; Duan, Z. New perspective on the natural course of chronic HBV infection. Front. Med. 2014, 8, 129–134. [Google Scholar] [CrossRef]
- Chang, M.L.; Liaw, Y.F. Hepatitis B flares in chronic hepatitis B: Pathogenesis, natural course, and management. J. Hepatol. 2014, 61, 1407–1417. [Google Scholar] [CrossRef]
- Wong, G.L.-H.; Wong, V.W.-S. Eliminating hepatitis B virus as a global health threat. Lancet Infect. Dis. 2016, 16, 1313–1314. [Google Scholar] [CrossRef]
- Sandhu, H.S.; Roesel, S.; Sharifuzzaman, M.; Chunsuttiwat, S.; Tohme, R.A. Progress toward hepatitis B control—South-East Asia region, 2016–2019. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 988–992. [Google Scholar] [CrossRef]
- World Health Organization. Combating Hepatitis B and C to Reach Elimination by 2030. May 2016. Available online: https://apps.who.int/iris/bitstream/handle/10665/206453/WHO_HIV_2016.04_eng.pdf (accessed on 20 August 2022).
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Zhang, M.; Liu, F.; Zhang, L.; Yang, B.; Wang, L. Nucleoside/nucleotide analog consolidation therapy in hepatitis B e-antigen-postive chronic hepatitis B patients: Three years should be preferred. Hepatol. Res. 2021, 51, 633–640. [Google Scholar] [CrossRef]
- Charlton, M.R.; Alam, A.; Shukla, A.; Dashtseren, B.; Lesmana, C.R.A.; Duger, D.; Hashmi, Z.Y. An expert review on the use of tenofovir alafenamide for the treatment of chronic hepatitis B virus infection in Asia. J. Gastroenterol. 2020, 55, 811–823. [Google Scholar] [CrossRef]
- Ozaras, R.; Khodor, H.; Yetim, N.; Unal, U.K.; Demirhan, Y.E.; Gultekin, G.; Isal, B. Monotherapy for hepatitis B infection: A review of treatment options. Expert Rev. Anti-Infect. Ther. 2015, 13, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, Y.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar] [PubMed]
- Liem, K.S.; Fung, S.; Wong, D.K.; Yim, C.; Noureldin, S.; Chen, J.; Feld, J.J.; Hansen, B.E.; Janseen, H.L. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: Results from a randomised controlled trial (Toronto STOP study). Gut 2019, 68, 2206–2213. [Google Scholar] [CrossRef]
- Wahhed, Y.; Siddiq, M.; Jamil, Z.; Najmi, M.H. Hepatitis elimination by 2030: Progress and challenges. World J. Gastroenterol. 2018, 24, 4959–4961. [Google Scholar] [CrossRef] [PubMed]
- Dienstag, J.L.; Stevens, C.E.; Bhan, A.K.; Szmuness, W. Hepatitis B vaccine administered to chronic carriers of hepatitis b surface antigen. Ann. Intern. Med. 1982, 96, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Pol, S.; Driss, F.; Michel, M.L.; Nalpas, B.; Berthelot, P.; Brechot, C. Specific vaccine therapy in chronic hepatitis B infection. Lancet 1994, 344, 342. [Google Scholar] [CrossRef]
- Viganò, M.; Grossi, G.; Loglio, A.; Lampertico, P. Treatment of hepatitis B: Is there still a role for interferon? Liver Int. 2018, 38, 79–83. [Google Scholar] [CrossRef]
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813. [Google Scholar] [CrossRef]
- Asselah, T.; Lada, O.; Moucari, R.; Martinot, M.; Boyer, N.; Marcellin, P. Interferon therapy for chronic hepatitis B. Clin. Liver Dis. 2007, 11, 839–849. [Google Scholar] [CrossRef]
- Grossi, G.; Viganò, M.; Loglio, A.; Lampertico, P. Hepatitis B virus long-term impact of antiviral therapy nucleot(s)ide analogues (NUCs). Liver Int. 2017, 37, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Zoulim, F.; Lebossé, F.; Levrero, M. Current treatments for chronic hepatitis B virus infections. Curr. Opin. Virol. 2016, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.; Wong, V.W.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Zhang, X.; Li, G.L.; Lui, G.C.Y.; Chan, H.L.Y.; Wong, G.L.H. Similarly low risk of hepatocellular carcinoma after either spontaneous or nucleos(t)ide analogue-induced hepatitis B surface antigen loss. Aliment. Pharmacol. Ther. 2020, 53, 321–331. [Google Scholar]
- De Fraga, R.S.; Van Vaisberg, V.; Mendes, L.C.A.; Carrilho, F.J.; Ono, S.K. Adverse events of nucleos(t)ide analogues for chronic hepatitis B: A systematic review. J. Gastroenterol. 2020, 55, 496–514. [Google Scholar] [CrossRef] [PubMed]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef]
- Wu, J.; Han, M.; Li, J.; Yang, X.; Yang, D. Immunopathogenesis of HBV Infection. Adv. Exp. Med. Biol. 2020, 1179, 71–107. [Google Scholar]
- Balmasova, I.P.; Yushchuk, N.D.; Mynbaev, O.; Alla, N.R.; Malova, E.; Shi, Z.; Gao, C.-L. Immunopathogenesis of chronic hepatitis B. World J. Gastroenterol. 2014, 20, 14156–14171. [Google Scholar] [CrossRef]
- Levy, G.A.; Chisari, F.V. The Immunopathogenesis of Chronic HBV Induced Liver Disease. Springer Semin. Immunopathol. 1981, 3, 439–459. [Google Scholar] [CrossRef]
- Nassal, M. HBV cccDNA: Viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut 2015, 64, 1972–1984. [Google Scholar] [CrossRef]
- Akbar, S.F.; Kajino, K.; Tanimoto, K.; Kurose, K.; Masumoto, T.; Michitaka, K.; Horiike, N.; Onji, M. Placebo-controlled trial of vaccination with hepatitis B virus surface antigen in hepatitis B virus transgenic mice. J. Hepatol. 1997, 26, 131–137. [Google Scholar] [CrossRef]
- Wirth, S.; Guidotti, L.G.; Ando, K.; Schlicht, H.J.; Chisari, F.V. Breaking tolerance leads to autoantibody production but not au-toimmune liver disease in hepatitis B virus envelope transgenic mice. J. Immunol. 1995, 154, 2504–2515. [Google Scholar] [PubMed]
- Shen, Z.Y.; Zheng, W.P.; Liu, T.; Yang, Y.; Song, H.L. Effects of dendritic cells from hepatitis B virus transgenic mice-stimulated au-tologous lymphocytes on hepatitis B virus replication: A study on the impact of specific sensitized effector cells on in vitro virus replication. Viral Immunol. 2015, 29, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maini, M.K.; Boni, C.; Lee, C.K.; Larrubia, J.R.; Reignat, S.; Ogg, G.S.; King, A.S.; Herberg, J.; Gilson, R.; Alisa, A.; et al. The role of virus-specific CD8(+) cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 2000, 191, 1269–1280. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Le Bert, N. Immunotherapy for Chronic Hepatitis B Virus Infection. Gut Liver 2018, 12, 497–507. [Google Scholar] [CrossRef]
- Gehring, A.J.; Xue, S.A.; Ho, Z.Z.; Teoh, D.; Ruedl, C.; Chia, A.; Koh, S.; Lim, S.G.; Maini, M.K.; Stauss, H.; et al. Engineering vi-rus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 2011, 55, 103–110. [Google Scholar] [CrossRef]
- Tilg, H.; Vogel, W.; Tratkiewicz, J.; Aulizky, W.E.; Herold, M.; Gruber, M.; Geissler, D.; Umalauft, F.; Judmaier, G.; Schwuelra, U. Pilot study of natural human interleukin-2 in patients with chronic hepatitis B. Immunomodulatory and antiviral effects. J. Hepatol. 1993, 19, 259–267. [Google Scholar] [CrossRef]
- Artillo, S.; Pastore, G.; Alberti, A.; Milella, M.; Santantonio, T.; Fattovitch, G.; Guistina, G.; Ryff, J.C.; Chaneac, M.; Bartolome, J.; et al. Double-blind, randomized controlled trial of interleukin-2 treatment of chronic hepatitis B. J. Med. Virol. 1998, 54, 167–172. [Google Scholar] [CrossRef]
- Carreño, V.; Zeuzem, S.; Hopf, U.; Marcellin, P.; Cooksley, W.G.; Fevery, J.; Diuago, M.; Reddy, R.; Peters, M.; Rittweger, K.; et al. A phase I/II study of recombinant human interleukin-12 in patients with chronic hepatitis B. J. Hepatol. 2000, 32, 317–324. [Google Scholar] [CrossRef]
- Martín, J.; Quiroga, J.A.; Bosch, O.; Carreño, V. Changes in cytokine production during therapy with granulocyte-macrophage colony-stimulating factor in patients with chronic hepatitis B. Hepatology 1994, 20, 1156–1161. [Google Scholar] [CrossRef]
- Ruiz-Moreno, M.; García, R.; Rua, M.J.; Serrano, B.; Moraleda, G.; Feijoo, E.; Bartolome, J.; Ortiz, F.; Castillo, I.; Carreno, V. Levamisole and interferon in children with chronic hepatitis B. Hepatology 1993, 18, 264–269. [Google Scholar] [CrossRef]
- Keating, G.M.; Noble, S. Recombinant hepatitis B vaccine (Engerix-B): A review of its immunogenicity and protective efficacy against hepatitis B. Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-H.; You, S.-L.; Chen, C.-J.; Liu, C.-J.; Lai, M.-W.; Wu, T.-C.; Wu, S.-F.; Lee, C.-M.; Yang, S.-S.; Chu, H.-C.; et al. Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology 2016, 151, 472–480.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikipedia. Expanded Program on Immunization. Available online: https://en.wikipedia.org/wiki/Expanded_Program_on_Immunization (accessed on 4 April 2021).
- Senturk, H.; Tabak, F.; Akdogan, M.; Erdem, L.; Mert, A.; Ozaras, R.; Sander, E.; Ozbay, G.; Badur, S. Therapeutic vaccination in chronic hepatitis B. J. Gastroenterol. Hepatol. 2002, 17, 72–76. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, X.X.; Yao, X.; Jiang, J.-H.; Xie, Y.-H.; Yuan, Z.-H.; Wen, Y.-M. Serum HbeAg sero-conversion correlated with decrease of HbsAg and HBV DNA in chronic hepatitis B patients treated with a therapeutic vaccine. Vaccine 2010, 28, 8169–8174. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, K.; Acar, M.; Degertekin, H. Specific hepatitis B vaccine therapy in inactive HbsAg carriers: A Randomized Controlled Trial. Infection 2003, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.-M.; Wu, X.-H.; Hu, D.-C.; Zhang, Q.-P.; Guo, S.-Q. Hepatitis B vaccine and anti-HBs complex as approach for vaccine therapy. Lancet 1995, 345, 1575–1576. [Google Scholar] [CrossRef]
- Xu, D.Z.; Zhao, K.; Guo, L.M.; Li, L.J.; Xie, Q.; Ren, H.; Zhang, J.M.; Xu, M.; Wang, H.F.; Huang, W.X.; et al. A randomized con-trolled phase Iib trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE 2008, 3, e2565. [Google Scholar] [CrossRef]
- Xu, D.Z.; Wang, X.Y.; Shen, X.L.; Gong, G.Z.; Ren, H.; Guo, L.M.; Sun, A.M.; Xu, M.; Li, L.J.; Guo, X.H.; et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: Experiences and findings. J. Hepatol. 2013, 59, 450–453. [Google Scholar] [CrossRef]
- Fontaine, H.; Kahi, S.; Chazallon, C.; Bourgine, M.; Varaut, A.; Buffet, C.; Godon, O.; Meritet, J.F.; Saidi, Y.; Michel, M.L.; et al. Anti-HBV DNA vaccination does not prevent relapse after discontinuation of analogues in the treatment of chronic hepatitis B: A randomised trial-ANRS HB02 VAC-ADN. Gut 2015, 64, 139–147. [Google Scholar] [CrossRef]
- Mancini-Bourgine, M.; Fontaine, H.; Bréchot, C.; Pol, S.; Michel, M.L. Immunogenicity of a hepatitis B DNA vaccine administered to chronic HBV carriers. Vaccine 2006, 24, 4482–4489. [Google Scholar] [CrossRef]
- Cova. L. Present and future DNA vaccines for chronic hepatitis B treatment. Expert Opin. Biol. Ther. 2017, 17, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.G.; Zhang, D.Z.; Wang, Z.Y.; Zeng, W.Q.; Shi, X.F.; Ren, H. Therapeutic effect of autologous dendritic cell vaccine on patients with chronic hepatitis B: A clinical study. World J. Gastroenterol. 2005, 11, 1806–1808. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.M.; Furukawa, S.; Horiike, N.; Abe, M.; Hiasa, Y.; Onji, M. Safety and immunogenicity of hepatitis B surface antigen-pulsed dendritic cells in patients with chronic hepatitis B. J. Viral Hepat. 2011, 18, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, J.; Chen, R.L.; Nie, L.; Huang, J.; Liu, Z.W.; Luo, L.; Yan, X.J. Autologus dendritic cell vaccine for chronic hepatitis B carriers: A pilot, open label, clinical trial in human volunteers. Vaccine 2010, 28, 2497–2504. [Google Scholar] [CrossRef] [PubMed]
- Horiike, N.; Fazle Akbar, S.M.; Michitaka, K.; Joukou, K.; Yamamoto, K.; Kojima, N.; Hiasa, Y.; Abe, M.; Onji, M. In vivo immun-ization by vaccine therapy following virus suppression by lamivudine: A novel approach for treating patients with chronic hepa-titis B. J. Clin. Virol. 2005, 32, 156–161. [Google Scholar] [CrossRef]
- Le Hoa, P.T.; Huy, N.T.; Thu, L.T.; Nga, C.N.; Nakao, K.; Eguchi, K.; Chi, N.H.; Hoang, B.H.; Hirayama, K. Randomized controlled study investigating viral suppression and serological response following pre-S1/pre-S2/S vaccine therapy combined with lamivudine treatment in HBeAg-positive patients with chronic hepatitis B. Antimicrob. Agents Chemother. 2009, 53, 5134–5140. [Google Scholar] [CrossRef]
- Vandepapelière, P.; Lau, G.K.; Leroux-Roels, G.; Horsmans, Y.; Gane, E.; Tawandee, T.; bin Merican, M.S.; Win, K.M.; Treop, C.; Cooksley, G.; et al. Therapeutic vaccination of chronic hepatitis B patients with virus suppression by antiviral therapy: A ran-domized, controlled study of co-administration of HBsAg/AS02 candidate vaccine and lamivudine. Vaccine 2007, 25, 8585–8597. [Google Scholar] [CrossRef]
- Heathcote, J.; McHutchison, J.; Lee, S.; Tong, M.; Benner, K.; Minuk, G.; Wright, T.; Fikes, J.; Livingston, B.; Sette, A.; et al. A pilot study of the CY-1899 T-cell vaccine in subjects chronically infected with hepatitis B virus. The CY1899 T Cell Vaccine Study Group. Hepatology 1999, 30, 531–536. [Google Scholar] [CrossRef]
- Vitiello, A.; Ishioka, G.; Grey, H.M.; Rose, R.; Farness, P.; LaFond, R.; Yuan, L.; Chisari, F.V.; Furze, J.; Bartholomeuz, R.; et al. Development of a lipopeptide-based therapeutic vaccine to treat chronic HBV infection. I. Induction of a primary cytotoxic T lymphocyte response in humans. J. Clin. Investig. 1995, 95, 341–349. [Google Scholar] [CrossRef]
- Tsai, E. Review of Current and Potential Treatments for Chronic Hepatitis B Virus Infection. Gastroenterol. Hepatol. 2021, 17, 367–376. [Google Scholar]
- Duraisamy, G.S.; Bhosale, D.; Lipenská, I.; Huvarova, I.; Růžek, D.; Windisch, M.P.; Miller, A.D. Advanced Therapeutics, Vaccinations, and Precision Medicine in the Treatment and Management of Chronic Hepatitis B Viral Infections; Where Are We and Where Are We Going? Viruses 2020, 12, 998. [Google Scholar] [CrossRef] [PubMed]
- Spyrou, E.; Smith, C.I.; Ghany, M.G. Hepatitis B: Current Status of Therapy and Future Therapies. Gastroenterol. Clin. N. Am. 2020, 49, 215–238. [Google Scholar] [CrossRef] [PubMed]
- Ghozy, S.; Nam, N.H.; Radwan, I.; Karimzadeh, S.; Tieu, T.M.; Hashan, M.R.; Abbas, A.S.; Eid, P.S.; Vuong, N.L.; Khang, N.V.; et al. Therapeutic efficacy of hepatitis B virus vaccine in treatment of chronic HBV infections: A systematic review and meta-analysis. Rev. Med. Virol. 2020, 30, e2089. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Uddin, H.; Khan, S.I.; Rahman, S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol. Int. 2013, 7, 981–989. [Google Scholar] [CrossRef]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treat-ment-controlled phase III clinical trial). PLoS ONE 2018, 13, e0201236. [Google Scholar] [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Penton, E.; Guillen, G.; Hiasa, Y. Sustained antiviral and liver protection by a nasal therapeutic vaccine (NASVAC), containing both HBsAg and HBcAg) in patients with chronic hepatitis B; 2-year fol-low-up of phase III clinical trial. Pathogens 2021, 10, 1440. [Google Scholar] [CrossRef]
- Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Khan, S.; Penton, E.; Gerardo, G.N.; Hiasa, Y. The Safety and Efficacy of a Therapeutic Vaccine for Chronic Hepatitis B: A Follow-Up Study of Phase III Clinical Trial. Vaccines 2021, 10, 45. [Google Scholar] [CrossRef]
- Yoshida, O.; Imai, Y.; Shiraishi, K.; Tokumoto, Y.; Sanada, T.; Tsukiyama-Kohara, K.; Miyazaki, T.; Kamishita, T.; Aguilar, J.C.; Guillen, G.E. HBsAg Reduction by Nasal Administration of a Therapeutic Vaccine Containing HBsAg and HBcAg (NASVAC) in Patients with Chronic HBV Infection: The Results of 18 Months Follow-Up. In The Liver Meeting Digital ExperienceTM; American Association for the Study of Liver Diseases: Boston, MA, USA, 2020. [Google Scholar]
- Burdette, D.L.; Lazerwith, S.; Yang, J.; Chan, H.L.Y.; Delaney Iv, W.E.; Fletcher, S.P.; Cihlar, T.; Feierbach, B. Ongoing viral replication and production of infectious virus in patients with chronic hepatitis B virus suppressed below the limit of quantitation on long-term nucleos(t)ide therapy. PLoS ONE 2022, 17, e0262516. [Google Scholar] [CrossRef]
- Dusheiko, G. Will we need novel combinations to cure HBV infection? Liver Int. 2020, 40, 35–42. [Google Scholar] [CrossRef] [Green Version]
| Limitation of IFNs and NUCs | Possible Causes |
|---|---|
| IFNs and NUCs are unable to eradicate or substantially control cccDNA that acts as a template for HBV replication. | Interferons and nucleoside analogs are repurposed antiviral drugs developed for other viral infections. Thus, these drugs are not evidence-based drugs for CHB. |
| The liver-protecting capacities and potency to block progression to LC and HCC are nominal for IFNs and NUCs. | The immune modulatory capacity and the nature of immune modulation of IFNs and NUCs are not purpose-oriented. |
| NUCs is an infinite mode of therapy, and there is a need to continue NUC intake for years or even for life. | The half-life of NUCs is, at best, 60 h. Thus, the effect of NUCs would be of limited duration. Cessation of NUCs will favor a milieu of HBV replication. |
| NUCs are not patient-friendly for developing and resource-constrained countries. | The infinite usage of NUCs along with periodic check-ups is unfriendly to developing countries that harbor 80% of CHB patients. |
| Use of NUCs confuses patients in developing countries. | NUCs induce HBV DNA negativity after usage for a short duration. Many CHB patients consider this as a remedy from the disease and give up taking medication. However, the drug must be taken for a prolonged duration. |
| Parameters | Explanations |
|---|---|
| Availability | The HBsAg-based vaccine has been used as a preventive vaccine for HBV infection since the 1980s and is available commercially around the world. |
| Safety | The safety of the HBsAg-based vaccine has been validated in millions of individuals. |
| Scientific rationality | Loss of HBsAg and development of antibodies to HBsAg (anti-HBs) have been regarded as a complete cure for HBV infection. It was assumed that the HBsAg-based vaccine will be able to accomplish immune modulation in favor of anti-HBs. |
| Different Candidates of HBsAg-Based Vaccine Therapy | References |
|---|---|
| 1. HBsAg-based protein vaccine | [46,47,48] |
| 2. HBsAg/anti-HB-based antigen/antibody complex vaccine | [49,50,51] |
| 3. HBsAg-based DNA vaccine | [53,54] |
| 4. HBsAg-based cellular vaccine | [55,56,57] |
| 5. HBsAg-based vaccine as part of combination therapy with antiviral drugs | [58,59,60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, S.M.F.; Mahtab, M.A.; Khan, S.; Yoshida, O.; Hiasa, Y. Development of Therapeutic Vaccine for Chronic Hepatitis B: Concept, Cellular and Molecular Events, Design, Limitation, and Future Projection. Vaccines 2022, 10, 1644. https://doi.org/10.3390/vaccines10101644
Akbar SMF, Mahtab MA, Khan S, Yoshida O, Hiasa Y. Development of Therapeutic Vaccine for Chronic Hepatitis B: Concept, Cellular and Molecular Events, Design, Limitation, and Future Projection. Vaccines. 2022; 10(10):1644. https://doi.org/10.3390/vaccines10101644
Chicago/Turabian StyleAkbar, Sheikh Mohammad Fazle, Mamun Al Mahtab, Sakirul Khan, Osamu Yoshida, and Yoichi Hiasa. 2022. "Development of Therapeutic Vaccine for Chronic Hepatitis B: Concept, Cellular and Molecular Events, Design, Limitation, and Future Projection" Vaccines 10, no. 10: 1644. https://doi.org/10.3390/vaccines10101644
APA StyleAkbar, S. M. F., Mahtab, M. A., Khan, S., Yoshida, O., & Hiasa, Y. (2022). Development of Therapeutic Vaccine for Chronic Hepatitis B: Concept, Cellular and Molecular Events, Design, Limitation, and Future Projection. Vaccines, 10(10), 1644. https://doi.org/10.3390/vaccines10101644






