Vaccination Offer during the Occupational Health Surveillance Program for Healthcare Workers and Suitability to Work: An Italian Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
- determinants of vaccination refusal
- determinants of susceptibility to serological evaluation.
3. Results
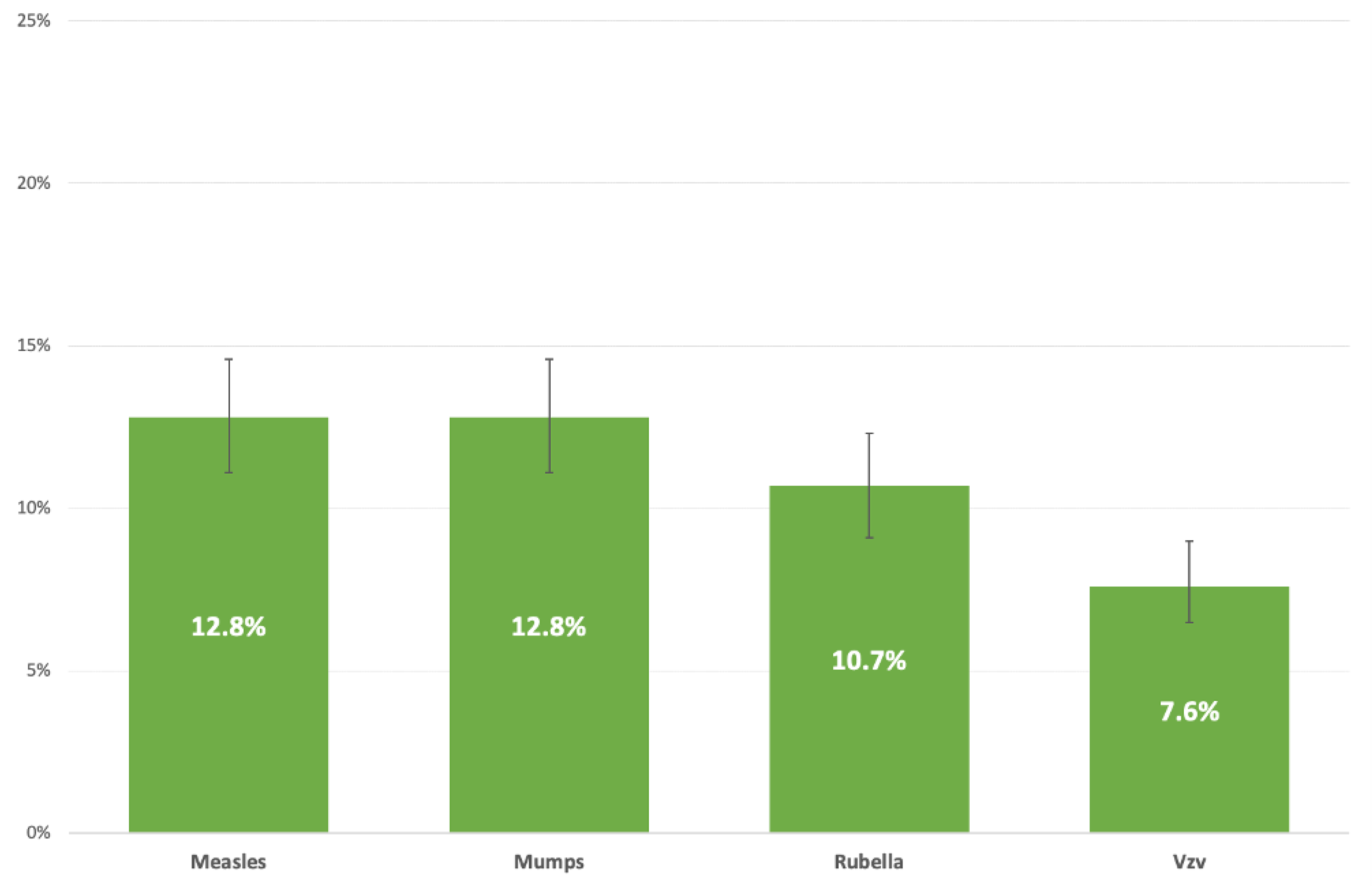
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Occupational Health and Safety Risks in the Healthcare Sector. Occupational Health and Safety Risks in the Healthcare Sector—Guide to Prevention and Good Practice. Available online: https://publications.europa.eu/en/publication-detail/-/publication/b29abb0a-f41e-4cb4-b787-4538ac5f0238 (accessed on 22 December 2021).
- CDC. Immunization of Health-Care Personnel. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report. Recommendations and Reports. Volume 60, No. 7. 25 November 2011. Available online: https://www.cdc.gov/mmwr/pdf/rr/rr6007.pdf (accessed on 21 December 2021).
- Fortunato, F.; Tafuri, S.; Cozza, V.; Martinelli, D.; Prato, R. Low vaccination coverage among italian healthcare workers in 2013. Hum. Vaccines Immunother. 2015, 11, 133–139. [Google Scholar] [CrossRef]
- Squeri, R.; Genovese, C.; Trimarchi, G.; Palamara, M.A.R.; La Fauci, V. An evaluation of attitude toward vaccines among healthcare workers of a University Hospital in Southern Italy. Ann. Ig. 2017, 29, 595–606. [Google Scholar] [PubMed]
- Italian Ministry of Health. National Plan of Vaccinal Prevention (PNPV) 2017–2019. Available online: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf (accessed on 12 December 2021).
- Rémy, V.; Largeron, N.; Quilici, S.; Carroll, S. The economic value of vaccination: Why prevention is wealth. J. Mark. Access Health Policy 2015, 3, 29284. [Google Scholar] [CrossRef]
- Squeri, R.; Di Pietro, A.; La Fauci, V.; Genovese, C. Healthcare workers’ vaccination at European and Italian level: A narrative review. Acta Biomed. 2019, 90, 45–53. [Google Scholar] [PubMed]
- Cologni, L.; Belotti, L.; Bacis, M.; Moioli, F.; Goglio, A.; Mosconi, G. Measles, mumps, rubella and varicella: Antibody titration and vaccinations in a large hospital. G. Ital. Med. Lav. Ergon. 2012, 34 (Suppl. 3), 272–274. [Google Scholar] [PubMed]
- Maltezou, H.C.; Wicker, S.; Borg, M.; Heininger, U.; Puro, V.; Theodoridou, M.; Poland, G.A. Vaccination policies for health-care workers in acute health-care facilities in Europe. Vaccine 2011, 29, 9557–9562. [Google Scholar] [CrossRef]
- Porretta, A.; Quattrone, F.; Aquino, F.; Pieve, G.; Bruni, B.; Gemignani, G.; Vatteroni, M.L.; Pistello, M.; Privitera, G.P.; Lopalco, P.L. A nosocomial measles outbreak in Italy, February–April 2017. Eur. Surveill. 2017, 22, 30597. [Google Scholar] [CrossRef]
- Botelho-Nevers, E.; Gautret, P.; Biellik, R.; Brouqui, P. Nosocomial transmission of measles: An updated review. Vaccine 2012, 30, 3996–4001. [Google Scholar] [CrossRef]
- Filia, A.; Bella, A.; Cadeddu, G.; Milia, M.; Del Manso, M.; Rota, M.; Magurano, F.; Nicoletti, L.; Declich, S. Extensive Nosocomial Transmission of Measles Originating in Cruise Ship Passenger, Sardinia, Italy, 2014. Emerg. Infect. Dis. 2015, 21, 1444–1446. [Google Scholar] [CrossRef]
- Baxi, R.; Mytton, O.T.; Abid, M.; Maduma-Butshe, A.; Iyer, S.; Ephraim, A.; Brown, K.E.; O’Moore, V.A. Outbreak report: Nosocomial transmission of measles through an unvaccinated healthcare worker-implications for public health. J. Public Health 2014, 36, 375–381. [Google Scholar] [CrossRef]
- Sá Machado, R.; Perez Duque, M.; Almeida, S.; Cruz, I.; Sottomayor, A.; Almeida, I.; Roliveira, J.; Antunes, D. Measles outbreak in a tertiary level hospital, Porto, Portugal, 2018: Challenges in the post-elimination era. Eurosurveillance 2018, 23, 18–00224. [Google Scholar] [CrossRef] [PubMed]
- Tafuri, S.; Germinario, C.; Rollo, M.; Prato, R. Occupational risk from measles in healthcare personnel: A case report. J. Occup. Health 2009, 51, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, U.L.R.; Scala, C.; Toletone, A.; Debarbieri, N.; Perria, M.; D’Amico, B.; Montecucco, A.; Martini, M.; Dini, G.; Durando, P. Susceptibility to vaccine-preventable diseases and vaccination adherence among healthcare workers in Italy: A cross-sectional survey at a regional acute-care university hospital and a systematic review. Hum. Vaccines Immunother. 2017, 13, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.; Picerno, I.A.M.; Trimarchi, G.; Cannavo, G.; Egitto, G.; Cosenza, B.; Merlina, V.; Icardi, G.; Panatto, D.; Amicizia, D.; et al. Vaccination coverage in healthcare workers: A multicenter cross-sectional study in Italy. J. Prev. Med. Hyg. 2019, 60, E12–E17. [Google Scholar] [PubMed]
- Durando, P.; Alicino, C.; Dini, G.; Barberis, I.; Bagnasco, A.M.; Iudici, R.; Zanini, M.; Martini, M.; Toletone, A.; Paganino, C.; et al. Determinants of adherence to seasonal influenza vaccination among healthcare workers from an Italian region: Results from a cross-sectional study. BMJ Open 2016, 6, e010779. [Google Scholar] [CrossRef]
- Di Martino, G.; Di Giovanni, P.; Di Girolamo, A.; Scampoli, P.; Cedrone, F.; D’Addezio, M.; Meo, F.; Romano, F.; Di Sciascio, M.B.; Staniscia, T. Knowledge and Attitude towards Vaccination among Healthcare Workers: A Multicenter Cross-Sectional Study in a Southern Italian Region. Vaccines 2020, 8, 248. [Google Scholar] [CrossRef]
- National Vaccine Advisory Committee. Strategies to Achieve the Healthy People 2020 Annual Influenza Vaccine Coverage Goal for Health-Care Personnel: Recommendations from the National Vaccine Advisory Committee. Public Health Rep. 2013, 128, 7–25. [Google Scholar] [CrossRef]
- Italian Government. Legislative Decree No. 81/2008. Unique Text on Health and Safety at Work. Available online: https://www.ispettorato.gov.it/it-it/Documenti-Norme/Documents/Testo-Unico-Dlgs-81-08-edizione-di-luglio-2018.pdf (accessed on 12 December 2021).
- Carrico, R.M.; Wiemken, T.; Westhusing, K.; Christensen, D.; McKinney, W.P. Health care personnel immunization programs: An assessment of knowledge and practice among infection preventionists in US health care facilities. Am. J. Infect. Control 2013, 41, 581–584. [Google Scholar] [CrossRef]
- Vimercati, L.; Bianchi, F.P.; Mansi, F.; Ranieri, B.; Stefanizzi, P.; de Nitto, S.; Tafuri, S. Influenza vaccination in health-care workers: An evaluation of an on-site vaccination strategy to increase vaccination uptake in HCWs of a South Italy Hospital. Hum. Vaccines Immunother. 2019, 15, 2927–2932. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Vimercati, L.; Mansi, F.; de Nitto, S.; Stefanizzi, P.; Rizzo, L.A.; Fragnelli, G.R.; Cannone, E.S.S.; de Maria, L.; Larocca, A.M.V.; et al. Compliance with immunization and a biological risk assessment of health care workers as part of an occupational health surveillance program: The experience of a university hospital in southern Italy. Am. J. Infect. Control 2020, 48, 368–374. [Google Scholar] [CrossRef]
- Pedote, P.D.; Termite, S.; Gigliobianco, A.; Lopalco, P.L.; Bianchi, F.P. Influenza Vaccination and Health Outcomes in COVID-19 Patients: A Retrospective Cohort Study. Vaccines 2021, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.G.; Klein, M. Survival Analysis: A Self-Learning Text, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-1441966452. [Google Scholar]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; de Nitto, S.; Germinario, C.A.; Lopalco, P.; Tafuri, S. Prevalence and management of measles susceptibility in healthcare workers in Italy: A systematic review and meta-analysis. Expert Rev. Vaccines 2020, 19, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; Stefanizzi, P.; de Nitto, S.; Larocca, A.M.V.; Germinario, C.; Tafuri, S. Long-term Immunogenicity of Measles Vaccine: An Italian Retrospective Cohort Study. J. Infect. Dis. 2020, 221, 721–728. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Mascipinto, S.; Stefanizzi, P.; de Nitto, S.; Germinario, C.; Tafuri, S. Long-term immunogenicity after measles vaccine vs. wild infection: An Italian retrospective cohort study. Hum. Vaccines Immunother. 2021, 17, 2078–2084. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Kim, S.K.; Kwak, S.H.; Hong, M.J.; Kim, S.H. Seroprevalence of Measles in Healthcare Workers in South Korea. Infect. Chemother. 2019, 51, 58–61. [Google Scholar] [CrossRef]
- Campins, M.; Urbiztondo, L.; Costa, J.; Broner, S.; Esteve, M.; Bayas, J.M.; Borras, E.; Dominguez, A.; Working Group for the Study of the Immune Status in Healthcare Workers of Catalonia. Serological survey of mumps immunity among health care workers in the Catalonia region of Spain. Am. J. Infect. Control 2013, 41, 378–380. [Google Scholar] [CrossRef]
- Kumakura, S.; Shibata, H.; Onoda, K.; Nishimura, N.; Matsuda, C.; Hirose, M. Seroprevalence survey on measles, mumps, rubella and varicella antibodies in healthcare workers in Japan: Sex, age, occupational-related differences and vaccine efficacy. Epidemiol. Infect. 2014, 142, 12–19. [Google Scholar] [CrossRef]
- Bianchi, F.P.; de Nitto, S.; Stefanizzi, P.; Larocca, A.M.V.; Germinario, C.A.; Tafuri, S. Long time persistence of antibodies against Mumps in fully MMR immunized young adults: An Italian retrospective cohort study. Hum. Vaccines Immunother. 2020, 16, 2649–2655. [Google Scholar] [CrossRef]
- Bianchi, F.P.; de Nitto, S.; Stefanizzi, P.; Larocca, A.M.V.; Germinario, C.A.; Tafuri, S. Immunity to rubella: An Italian retrospective cohort study. BMC Public Health 2019, 19, 1490. [Google Scholar] [CrossRef]
- Haviari, S.; Bénet, T.; Saadatian-Elahi, M.; Andric, P.; Loulergue, P.; Vanhems, P. Vaccination of healthcare workers: A review. Hum. Vaccines Immunother. 2015, 11, 2522–2537. [Google Scholar] [CrossRef]
- Bianchi, F.P.; Tafuri, S.; Larocca, A.M.V.; Germinario, C.A.; Stefanizzi, P. Long -term persistence of antibodies against varicella in fully immunized healthcare workers: An Italian retrospective cohort study. BMC Infect. Dis. 2021, 25, 475. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, A.; Nicolli, A.; de Nuzzo, D.; Lago, L.; Artuso, E.; Maso, S. Varicella seroepidemiology and immunization in a cohort of future healthcare workers in the pre-vaccination era. Int. J. Infect. Dis. 2020, 96, 228–232. [Google Scholar] [CrossRef]
- Griffin, D.E. Measles Vaccine. Viral Immunol. 2018, 31, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.P. Understanding sex-related differences in immune responses. Sci. Transl. Med. 2020, 12, eabd3631. [Google Scholar] [CrossRef]
- Bellia, C.; Setbon, M.; Zylberman, P.; Flahault, A. Healthcare worker compliance with seasonal and pandemic influenza vaccination. Influenza Other Respir. Viruses 2013, 7 (Suppl. 2), 97–104. [Google Scholar] [CrossRef]
- Dini, G.; Toletone, A.; Sticchi, L.; Orsi, A.; Bragazzi, N.L.; Durando, P. Influenza vaccination in healthcare workers: A comprehensive critical appraisal of the literature. Hum. Vaccines Immunother. 2018, 14, 772–789. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.P.; Tafuri, S.; Spinelli, G.; Carlucci, M.; Migliore, G.; Calabrese, G.; Daleno, A.; Melpignano, L.; Vimercati, L.; Stefanizzi, P. Two years of on-site influenza vaccination strategy in an Italian university hospital: Main results and lessons learned. Hum. Vaccines Immunother. 2021, 4, 1–6. [Google Scholar] [CrossRef]
- Scatigna, M.; Fabiani, L.; Micolucci, G.; Santilli, F.; Mormile, P.; Giuliani, A.R. Attitudinal variables and a possible mediating mechanism for vaccination practice in health care workers of a local hospital in L’Aquila (Italy). Hum. Vaccines Immunother. 2017, 13, 198–205. [Google Scholar] [CrossRef]
- Stefanizzi, P.; de Nitto, S.; Patano, F.; Bianchi, F.P.; Ferorelli, D.; Stella, P.; Ancona, D.; Bavaro, V.; Tafuri, S. Post-marketing surveillance of adverse events following measles, mumps, rubella and varicella (MMRV) vaccine: Retrospecive study in Apulia region (ITALY), 2009–2017. Hum. Vaccines Immunother. 2020, 16, 1875–1883. [Google Scholar] [CrossRef]
- Fiebelkorn, A.P.; Seward, J.F.; Orenstein, W.A. A global perspective of vaccination of healthcare personnel against measles: Systematic review. Vaccine 2014, 32, 4823–4839. [Google Scholar] [CrossRef]
- Lu, P.J.; Graitcer, S.B.; O’Halloran, A.; Liang, J.L. Tetanus, diphtheria and acellular pertussis (Tdap) vaccination among healthcare personnel-United States, 2011. Vaccine 2014, 32, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Apulia Region. Regional Law No. 27 of 19 June 2018. Provisions for the Execution of the Vaccination Obligations of Healthcare Professionals. Official Bulletin of the Apulia Region No. 82, Suppl. of 21 June 2018. Available online: https://www.quotidianosanita.it/allegati/allegato3125404.pdf (accessed on 29 December 2021).
- Maltezou, H.C.; Theodoridou, K.; Ledda, C.; Rapisarda, V.; Theodoridou, M. Vaccination of healthcare workers: Is mandatory vaccination needed? Expert. Rev. Vaccines 2019, 18, 5–13. [Google Scholar] [CrossRef] [PubMed]
| Variable | Value |
|---|---|
| Age (mean ± SD; range) | 35.7 ± 10.6 (20.0–68.0) |
| Females; n (%) | 936 (63.4%) |
| Chronic disease; n (%) | 287 (19.4%) |
| Allergies; n (%) | 348 (23.6%) |
| Profession; n (%) | |
| 485 (32.8%) |
| 471 (31.9%) |
| 217 (14.7%) |
| 187 (12.7%) |
| 117 (7.9%) |
| Operative Unit; n (%) | |
| 661 (44.8%) |
| 258 (17.5%) |
| 306 (20.7%) |
| Vaccine | n | % |
|---|---|---|
| Measles | ||
| 994 | 67.3 |
| 121 | 8.2 |
| 362 | 24.5 |
| Mumps | ||
| 1031 | 69.8 |
| 106 | 7.2 |
| 340 | 23.0 |
| Rubella | ||
| 1007 | 68.2 |
| 125 | 8.5 |
| 345 | 23.4 |
| Varicella | ||
| 1425 | 96.5 |
| 17 | 1.2 |
| 35 | 2.3 |
| Determinants | Measles | Mumps | Rubella | Vzv | ||||
|---|---|---|---|---|---|---|---|---|
| aOR (95%CI) | p-Value | aOR (95%CI) | p-Value | aOR (95%CI) | p-Value | aOR (95%CI) | p-Value | |
| Sex (male vs. female) | 1.27 (0.89–1.83) | 0.193 | 1.43 (1.01–2.03) | 0.049 | 1.18 (0.80–1.74) | 0.394 | 1.10 (0.69–1.75) | 0.697 |
| Age (yrs) | 0.91 (0.88–0.94) | <0.0001 | 0.95 (0.92–0.97) | <0.0001 | 0.94 (0.92–0.97) | <0.0001 | 0.98 (0.96–1.01) | 0.146 |
| Immunization status | ||||||||
| 1.02 (0.57–1.83) | 0.955 | 1.23 (0.69–2.19) | 0.476 | 0.29 (0.13–0.66) | 0.003 | 1.82 (0.39–8.53) | 0.449 |
| 1.11 (0.72–1.71) | 0.629 | 0.69 (0.43–1.10) | 0.119 | 0.95 (0.06–0.25) | <0.0001 | 7.48 (3.22–17.36) | <0.0001 |
| Professional category | ||||||||
| 0.91 (0.57–1.46) | 0.704 | 1.07 (0.68–1.67) | 0.775 | 1.36 (0.85–2.28) | 0.200 | 1.20 (0.66–2.18) | 0.558 |
| 0.63 (0.42–0.95) | 0.027 | 0.88 (0.58–1.32) | 0.523 | 0.95 (0.60–1.53) | 0.843 | 1.27 (0.76–2.14) | 0.360 |
| Specialty | ||||||||
| 1.18 (0.71–1.97) | 0.529 | 1.09 (0.67–1.78) | 0.719 | 1.17 (0.68–2.00) | 0.571 | 0.60 (0.28–1.29) | 0.192 |
| 1.29 (0.85–1.97) | 0.236 | 0.93 (0.61–1.42) | 0.747 | 1.12 (0.69–1.82) | 0.634 | 1.40 (0.81–2.42) | 0.227 |
| Chronic disease (yes/no) | 1.35 (0.89–2.06) | 0.161 | 1.16 (0.75–1.78) | 0.505 | 1.41 (0.89–2.25) | 0.144 | 0.93 (0.53–1.63) | 0.795 |
| Allergies (yes/no) | 1.34 (0.91–1.96) | 0.133 | 1.21 (0.82–1.78) | 0.344 | 0.75 (0.47–1.19) | 0.224 | 1.21 (0.73–2.00) | 0.457 |
| Chi-square = 6.2; p = 0.627 | Chi-square = 852.3; p = 0.183 | Chi-square = 11.3; p = 0.184 | Chi-square = 5.4; p = 0.717 | |||||
| Infection | Seronegative HCWs (n) | HCWs Offered the Vaccine | HCWs Who Accepted Vaccine Prophylaxis | Re-Titered HCWs | Seroconverted | GMT after Booster(s) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | Mean (95%CI) | ||
| Measles | 188 | 153 | 81.3 | 150 | 98.0 | 61 | 40.7 | 54 | 88.5 | 73.6 (62.8–118.5) |
| Mumps | 188 | 124 | 66.0 | 120 | 97.6 | 52 | 43.3 | 43 | 82.7 | 38.3 (20.5–71.5) |
| Rubella | 157 | 97 | 61.8 | 95 | 97.9 | 38 | 40.0 | 36 | 94.7 | 59.7 (37.3–97.5) |
| Varicella | 111 | 82 | 73.8 | 77 | 93.9 | 29 | 37.7 | 25 | 86.2 | 636.2 (322.8–1297.2) |
| Determinant | aOR | 95%CI | p-Value |
|---|---|---|---|
| Age (years) | 1.16 | 1.09–1.25 | <0.0001 |
| Sex (male vs. female) | 0.54 | 0.12–2.33 | 0.411 |
| Professional category | |||
| 1.21 | 0.24–6.03 | 0.820 |
| 2.31 | 0.47–11.35 | 0.303 |
| Specialty | |||
| 0.43 | 0.07–2.68 | 0.362 |
| 0.39 | 0.09–1.75 | 0.220 |
| Chronic disease (yes/no) | 1.24 | 0.35–4.43 | 0.737 |
| Allergies (yes/no) | 1.26 | 0.37–4.29 | 0.711 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bianchi, F.P.; Stefanizzi, P.; De Maria, L.; Martinelli, A.; Diella, G.; Larocca, A.M.V.; Vimercati, L.; Tafuri, S. Vaccination Offer during the Occupational Health Surveillance Program for Healthcare Workers and Suitability to Work: An Italian Retrospective Cohort Study. Vaccines 2022, 10, 1633. https://doi.org/10.3390/vaccines10101633
Bianchi FP, Stefanizzi P, De Maria L, Martinelli A, Diella G, Larocca AMV, Vimercati L, Tafuri S. Vaccination Offer during the Occupational Health Surveillance Program for Healthcare Workers and Suitability to Work: An Italian Retrospective Cohort Study. Vaccines. 2022; 10(10):1633. https://doi.org/10.3390/vaccines10101633
Chicago/Turabian StyleBianchi, Francesco Paolo, Pasquale Stefanizzi, Luigi De Maria, Andrea Martinelli, Giusy Diella, Angela Maria Vittoria Larocca, Luigi Vimercati, and Silvio Tafuri. 2022. "Vaccination Offer during the Occupational Health Surveillance Program for Healthcare Workers and Suitability to Work: An Italian Retrospective Cohort Study" Vaccines 10, no. 10: 1633. https://doi.org/10.3390/vaccines10101633
APA StyleBianchi, F. P., Stefanizzi, P., De Maria, L., Martinelli, A., Diella, G., Larocca, A. M. V., Vimercati, L., & Tafuri, S. (2022). Vaccination Offer during the Occupational Health Surveillance Program for Healthcare Workers and Suitability to Work: An Italian Retrospective Cohort Study. Vaccines, 10(10), 1633. https://doi.org/10.3390/vaccines10101633










