Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response
Abstract
1. Introduction
2. Tfh Cells Supporting Immunoglobulin Production
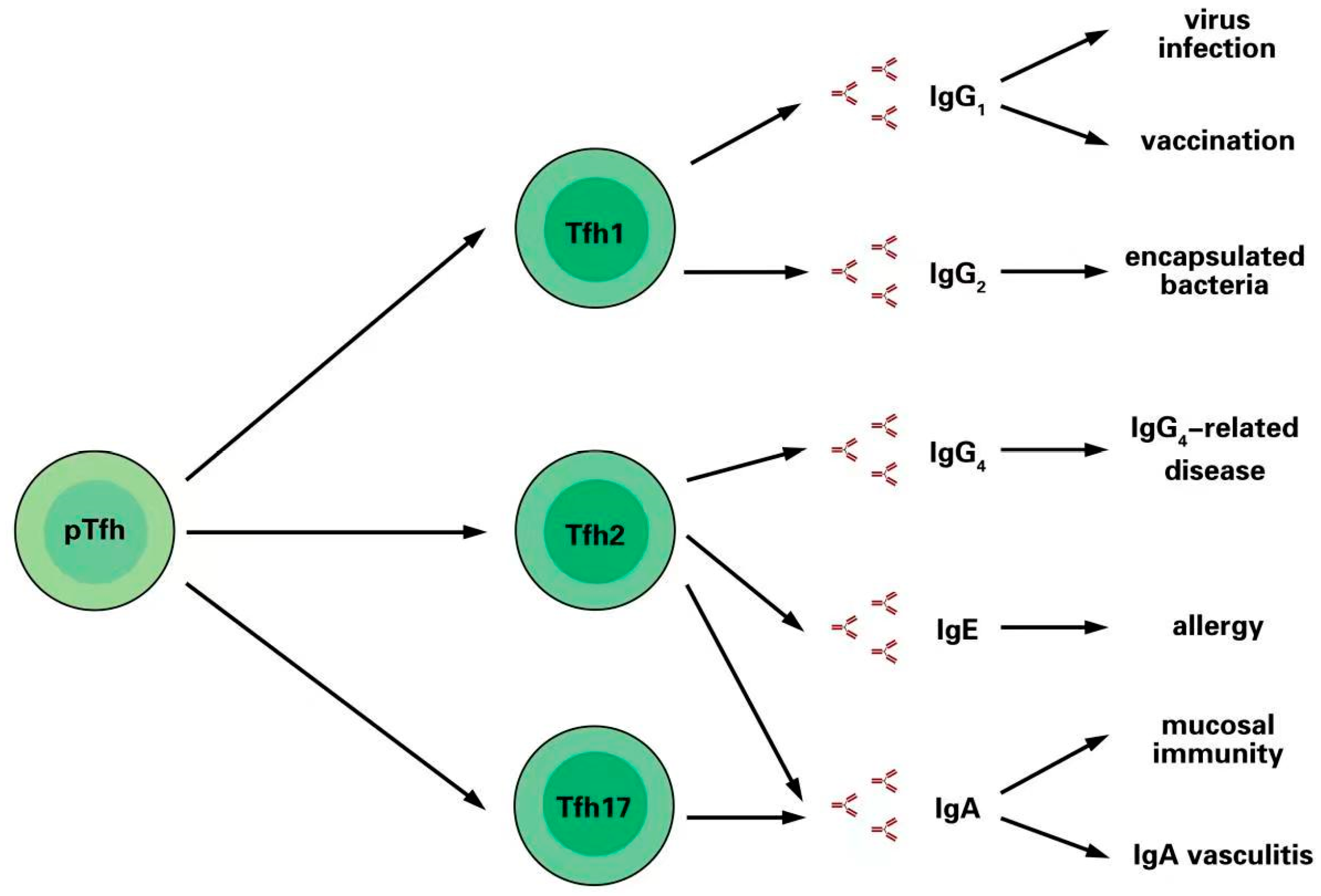
3. Different Immunoglobulins Are Promoted by Tfh Cell Subsets
3.1. Tfh1 Cells Induce IgG1 and IgG2 Production after Infection and Vaccination
3.2. Tfh2 Cells Induce IgG4 and IgE in Allergic Responses
3.3. Tfh2 and Tfh17 Cells Promote IgA Secretion in Mucosal Immunity
4. Tfh Cell Response in SARS-CoV-2 Infection
5. Tfh Cell Responses in SARS-CoV-2 Vaccination
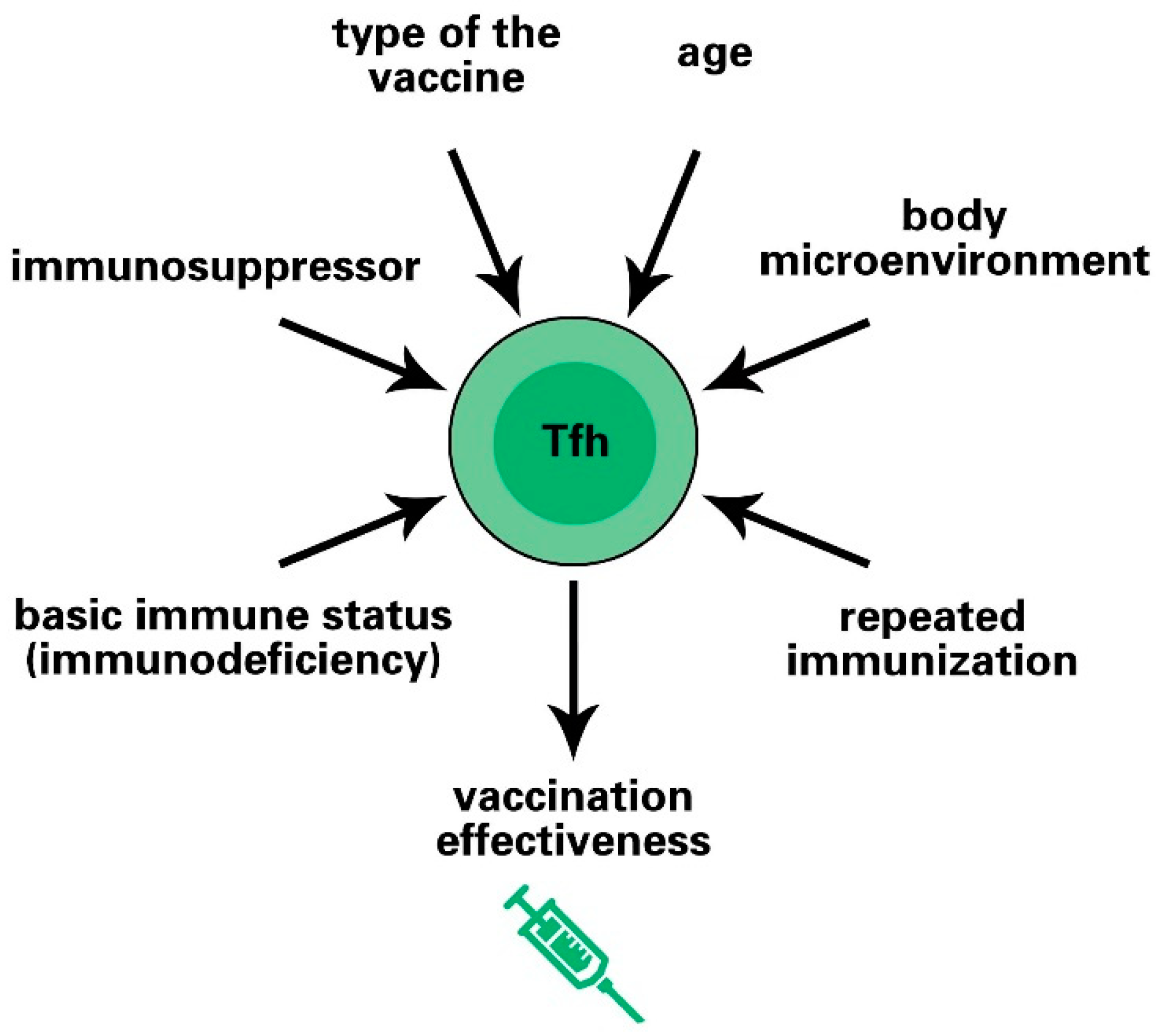
6. Factors Affecting Vaccine Effectiveness by Regulating Tfh Cell Responses
6.1. Age-Dependent Immune Response
6.2. Polarity of Tfh Cells Affects the Vaccine Effect
6.3. Initial Immune Status of Vaccines and Tfh Cell Responses
6.4. Repeated Immunization Enhances the Intensity of the Tfh Cell Response
6.5. Types of Vaccines and Inoculated Species
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Breitfeld, D.; Ohl, L.; Kremmer, E.; Ellwart, J.; Sallusto, F.; Lipp, M.; Forster, R. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J. Exp. Med. 2000, 192, 1545–1552. [Google Scholar] [CrossRef]
- Schaerli, P.; Willimann, K.; Lang, A.B.; Lipp, M.; Loetscher, P.; Moser, B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J. Exp. Med. 2000, 192, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Juno, J.A.; Hill, D.L. T follicular helper cells and their impact on humoral responses during pathogen and vaccine challenge. Curr. Opin. Immunol. 2022, 74, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Baumjohann, D.; Fazilleau, N. Antigen-dependent multistep differentiation of T follicular helper cells and its role in SARS-CoV-2 infection and vaccination. Eur. J. Immunol. 2021, 51, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Tang, Y.; Jiang, Q.; Jiang, D.; Zhang, Y.; Lv, Y.; Xu, D.; Wu, J.; Xie, J.; Wen, C.; et al. Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 731100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Q.; Ye, L. The Differentiation and Maintenance of SARS-CoV-2-Specific Follicular Helper T Cells. Front. Cell. Infect. Microbiol. 2022, 12, 953022. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Nurieva, R.I.; Chung, Y.; Martinez, G.J.; Yang, X.O.; Tanaka, S.; Matskevitch, T.D.; Wang, Y.H.; Dong, C. Bcl6 mediates the development of T follicular helper cells. Science 2009, 325, 1001–1005. [Google Scholar] [CrossRef]
- Yu, D.; Rao, S.; Tsai, L.M.; Lee, S.K.; He, Y.; Sutcliffe, E.L.; Srivastava, M.; Linterman, M.; Zheng, L.; Simpson, N.; et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009, 31, 457–468. [Google Scholar] [CrossRef]
- Johnston, R.J.; Poholek, A.C.; DiToro, D.; Yusuf, I.; Eto, D.; Barnett, B.; Dent, A.L.; Craft, J.; Crotty, S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009, 325, 1006–1010. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nakayamada, S. Multi-Source Pathways of T Follicular Helper Cell Differentiation. Front. Immunol. 2021, 12, 621105. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W., Jr.; Cavacini, L. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 2010, 125, S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Batten, M.; Ramamoorthi, N.; Kljavin, N.M.; Ma, C.S.; Cox, J.H.; Dengler, H.S.; Danilenko, D.M.; Caplazi, P.; Wong, M.; Fulcher, D.A.; et al. IL-27 supports germinal center function by enhancing IL-21 production and the function of T follicular helper cells. J. Exp. Med. 2010, 207, 2895–2906. [Google Scholar] [CrossRef] [PubMed]
- Sigal, L.H. Basic science for the clinician 58: IgG subclasses. J. Clin. Rheumatol. 2012, 18, 316–318. [Google Scholar] [CrossRef]
- Cubas, R.A.; Mudd, J.C.; Savoye, A.L.; Perreau, M.; van Grevenynghe, J.; Metcalf, T.; Connick, E.; Meditz, A.; Freeman, G.J.; Abesada-Terk, G., Jr.; et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 2013, 19, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Perreau, M.; Savoye, A.L.; De Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; De Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013, 210, 143–156. [Google Scholar] [CrossRef]
- Velu, V.; Mylvaganam, G.; Ibegbu, C.; Amara, R.R. Tfh1 Cells in Germinal Centers During Chronic HIV/SIV Infection. Front. Immunol. 2018, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Bentebibel, S.E.; Khurana, S.; Schmitt, N.; Kurup, P.; Mueller, C.; Obermoser, G.; Palucka, A.K.; Albrecht, R.A.; Garcia-Sastre, A.; Golding, H.; et al. ICOS(+)PD-1(+)CXCR3(+) T follicular helper cells contribute to the generation of high-avidity antibodies following influenza vaccination. Sci. Rep. 2016, 6, 26494. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nakayamada, S.; Kubo, S.; Sakata, K.; Yamagata, K.; Miyazaki, Y.; Yoshikawa, M.; Kitanaga, Y.; Zhang, M.; Tanaka, Y. Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis. 2018, 77, 1354–1361. [Google Scholar] [CrossRef]
- Bubier, J.A.; Sproule, T.J.; Foreman, O.; Spolski, R.; Shaffer, D.J.; Morse, H.C., 3rd; Leonard, W.J.; Roopenian, D.C. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1518–1523. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Tus, K.; Li, Q.Z.; Wang, A.; Tian, X.H.; Zhou, J.; Liang, C.; Bartov, G.; McDaniel, L.D.; Zhou, X.J.; et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc. Natl. Acad. Sci. USA 2006, 103, 9970–9975. [Google Scholar] [CrossRef]
- Lee, S.K.; Silva, D.G.; Martin, J.L.; Pratama, A.; Hu, X.; Chang, P.P.; Walters, G.; Vinuesa, C.G. Interferon-gamma excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity 2012, 37, 880–892. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Hendriks, W.; Althage, A.; Hemmi, S.; Bluethmann, H.; Kamijo, R.; Vilcek, J.; Zinkernagel, R.M.; Aguet, M. Immune response in mice that lack the interferon-gamma receptor. Science 1993, 259, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Shade, K.C.; Conroy, M.E.; Washburn, N.; Kitaoka, M.; Huynh, D.J.; Laprise, E.; Patil, S.U.; Shreffler, W.G.; Anthony, R.M. Sialylation of immunoglobulin E is a determinant of allergic pathogenicity. Nature 2020, 582, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, C.L.; Yu, D.; Liu, Z. Roles of follicular helper and regulatory T cells in allergic diseases and allergen immunotherapy. Allergy 2021, 76, 456–470. [Google Scholar] [CrossRef] [PubMed]
- Coquet, J.M.; Schuijs, M.J.; Smyth, M.J.; Deswarte, K.; Beyaert, R.; Braun, H.; Boon, L.; Karlsson Hedestam, G.B.; Nutt, S.L.; Hammad, H.; et al. Interleukin-21-Producing CD4(+) T Cells Promote Type 2 Immunity to House Dust Mites. Immunity 2015, 43, 318–330. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Tato, A.; Randall, T.D.; Lund, F.E.; Spolski, R.; Leonard, W.J.; Leon, B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity 2016, 44, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Iijima, K.; Dent, A.L.; Kita, H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J. Allergy Clin. Immunol. 2017, 139, 300–313 e307. [Google Scholar] [CrossRef] [PubMed]
- Hand, T.W.; Reboldi, A. Production and Function of Immunoglobulin A. Annu. Rev. Immunol. 2021, 39, 695–718. [Google Scholar] [CrossRef] [PubMed]
- Kato, L.M.; Kawamoto, S.; Maruya, M.; Fagarasan, S. Gut TFH and IgA: Key players for regulation of bacterial communities and immune homeostasis. Immunol. Cell Biol. 2014, 92, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Turner, J.E.; Villa, M.; Duarte, J.H.; Demengeot, J.; Steinmetz, O.M.; Stockinger, B. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 2013, 14, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Davin, J.C.; Coppo, R. Henoch-Schonlein purpura nephritis in children. Nat. Rev. Nephrol. 2014, 10, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Kiryluk, K.; Moldoveanu, Z.; Sanders, J.T.; Eison, T.M.; Suzuki, H.; Julian, B.A.; Novak, J.; Gharavi, A.G.; Wyatt, R.J. Aberrant glycosylation of IgA1 is inherited in both pediatric IgA nephropathy and Henoch-Schonlein purpura nephritis. Kidney Int. 2011, 80, 79–87. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wang, J.; Guo, L.; Liu, C.; Jiang, Y.; Wang, H.; Yang, S. Distribution of circulating T follicular helper cell subsets is altered in immunoglobulin A vasculitis in children. PLoS ONE 2017, 12, e0189133. [Google Scholar] [CrossRef]
- Chen, J.S.; Chow, R.D.; Song, E.; Mao, T.; Israelow, B.; Kamath, K.; Bozekowski, J.; Haynes, W.A.; Filler, R.B.; Menasche, B.L.; et al. High-affinity, neutralizing antibodies to SARS-CoV-2 can be made without T follicular helper cells. Sci. Immunol. 2022, 7, eabl5652. [Google Scholar] [CrossRef]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Thevarajan, I.; Nguyen, T.H.O.; Koutsakos, M.; Druce, J.; Caly, L.; van de Sandt, C.E.; Jia, X.; Nicholson, S.; Catton, M.; Cowie, B.; et al. Breadth of concomitant immune responses prior to patient recovery: A case report of non-severe COVID-19. Nat. Med. 2020, 26, 453–455. [Google Scholar] [CrossRef]
- Juno, J.A.; Tan, H.X.; Lee, W.S.; Reynaldi, A.; Kelly, H.G.; Wragg, K.; Esterbauer, R.; Kent, H.E.; Batten, C.J.; Mordant, F.L.; et al. Humoral and circulating follicular helper T cell responses in recovered patients with COVID-19. Nat. Med. 2020, 26, 1428–1434. [Google Scholar] [CrossRef]
- Shaan Lakshmanappa, Y.; Elizaldi, S.R.; Roh, J.W.; Schmidt, B.A.; Carroll, T.D.; Weaver, K.D.; Smith, J.C.; Verma, A.; Deere, J.D.; Dutra, J.; et al. SARS-CoV-2 induces robust germinal center CD4 T follicular helper cell responses in rhesus macaques. Nat. Commun. 2021, 12, 541. [Google Scholar] [CrossRef] [PubMed]
- Lipsitch, M.; Grad, Y.H.; Sette, A.; Crotty, S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat. Rev. Immunol. 2020, 20, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Rodda, L.B.; Netland, J.; Shehata, L.; Pruner, K.B.; Morawski, P.A.; Thouvenel, C.D.; Takehara, K.K.; Eggenberger, J.; Hemann, E.A.; Waterman, H.R.; et al. Functional SARS-CoV-2-Specific Immune Memory Persists after Mild COVID-19. Cell 2021, 184, 169–183.e17. [Google Scholar] [CrossRef] [PubMed]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Mathew, D.; Giles, J.R.; Baxter, A.E.; Oldridge, D.A.; Greenplate, A.R.; Wu, J.E.; Alanio, C.; Kuri-Cervantes, L.; Pampena, M.B.; D’Andrea, K.; et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020, 369, eabc8511. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Q.; Liu, Z.; Wang, Q.; Wu, J.; Hu, Y.; Bai, T.; Xie, T.; Huang, M.; Wu, T.; et al. Spike-specific circulating T follicular helper cell and cross-neutralizing antibody responses in COVID-19-convalescent individuals. Nat. Microbiol. 2021, 6, 51–58. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Xia, M.H.; Ren, L.; Zhang, Y.F.; Ao, Q.L.; Xu, S.P.; Kuang, D.; Liu, Q.; Yan, B.; Zhou, Y.W.; et al. Deficiency of Tfh Cells and Germinal Center in Deceased COVID-19 Patients. Curr. Med. Sci. 2020, 40, 618–624. [Google Scholar] [CrossRef]
- Kaneko, N.; Kuo, H.H.; Boucau, J.; Farmer, J.R.; Allard-Chamard, H.; Mahajan, V.S.; Piechocka-Trocha, A.; Lefteri, K.; Osborn, M.; Bals, J.; et al. Loss of Bcl-6-Expressing T Follicular Helper Cells and Germinal Centers in COVID-19. Cell 2020, 183, 143–157 e113.50. [Google Scholar] [CrossRef]
- Lee, J.H.; Sutton, H.; Cottrell, C.A.; Phung, I.; Ozorowski, G.; Sewall, L.M.; Nedellec, R.; Nakao, C.; Silva, M.; Richey, S.T.; et al. Long-lasting germinal center responses to a priming immunization with continuous proliferation and somatic mutation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Mudd, P.A.; Minervina, A.A.; Pogorelyy, M.V.; Turner, J.S.; Kim, W.; Kalaidina, E.; Petersen, J.; Schmitz, A.J.; Lei, T.; Haile, A.; et al. SARS-CoV-2 mRNA vaccination elicits a robust and persistent T follicular helper cell response in humans. Cell 2022, 185, 603–613.e15. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Bettini, E.; Parvathaneni, K.; Painter, M.M.; Agarwal, D.; Lundgreen, K.A.; Weirick, M.; Muralidharan, K.; Castano, D.; Goel, R.R.; et al. Germinal center responses to SARS-CoV-2 mRNA vaccines in healthy and immunocompromised individuals. Cell 2022, 185, 1008–1024 e1015. [Google Scholar] [CrossRef] [PubMed]
- Cavazzoni, C.B.; Hanson, B.L.; Podesta, M.A.; Bechu, E.D.; Clement, R.L.; Zhang, H.; Daccache, J.; Reyes-Robles, T.; Hett, E.C.; Vora, K.A.; et al. Follicular T cells optimize the germinal center response to SARS-CoV-2 protein vaccination in mice. Cell Rep. 2022, 38, 110399. [Google Scholar] [CrossRef] [PubMed]
- Alameh, M.G.; Tombacz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid nanoparticles enhance the efficacy of mRNA and protein subunit vaccines by inducing robust T follicular helper cell and humoral responses. Immunity 2022, 55, 1136–1138. [Google Scholar] [CrossRef] [PubMed]
- Wragg, K.M.; Lee, W.S.; Koutsakos, M.; Tan, H.X.; Amarasena, T.; Reynaldi, A.; Gare, G.; Konstandopoulos, P.; Field, K.R.; Esterbauer, R.; et al. Establishment and recall of SARS-CoV-2 spike epitope-specific CD4(+) T cell memory. Nat. Immunol. 2022, 23, 768–780. [Google Scholar] [CrossRef]
- Lustig, Y.; Sapir, E.; Regev-Yochay, G.; Cohen, C.; Fluss, R.; Olmer, L.; Indenbaum, V.; Mandelboim, M.; Doolman, R.; Amit, S.; et al. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: A prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir. Med. 2021, 9, 999–1009. [Google Scholar] [CrossRef]
- Lartey, S.; Zhou, F.; Brokstad, K.A.; Mohn, K.G.; Slettevoll, S.A.; Pathirana, R.D.; Cox, R.J. Live-Attenuated Influenza Vaccine Induces Tonsillar Follicular T Helper Cell Responses That Correlate with Antibody Induction. J. Infect. Dis. 2020, 221, 21–32. [Google Scholar] [CrossRef]
- Stebegg, M.; Bignon, A.; Hill, D.L.; Silva-Cayetano, A.; Krueger, C.; Vanderleyden, I.; Innocentin, S.; Boon, L.; Wang, J.; Zand, M.S.; et al. Rejuvenating conventional dendritic cells and T follicular helper cell formation after vaccination. eLife 2020, 9, e52473. [Google Scholar] [CrossRef]
- Gupta, S.; Su, H.; Agrawal, S. Immune Response to SARS-CoV-2 Vaccine in 2 Men. Int. Arch. Allergy Immunol. 2022, 183, 350–359. [Google Scholar] [CrossRef]
- Lederer, K.; Castano, D.; Gomez Atria, D.; Oguin, T.H., 3rd; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 mRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Yin, M.; Xiong, Y.; Liang, D.; Tang, H.; Hong, Q.; Liu, G.; Zeng, J.; Lian, T.; Huang, J.; Ni, J. Circulating Tfh cell and subsets distribution are associated with low-responsiveness to hepatitis B vaccination. Mol. Med. 2021, 27, 32. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Suarez, J.; de la Cruz, J.; Cedillo, A.; Llamas, P.; Duarte, R.; Jimenez-Yuste, V.; Hernandez-Rivas, J.A.; Gil-Manso, R.; Kwon, M.; Sanchez-Godoy, P.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Meyts, I.; Bucciol, G.; Quinti, I.; Neven, B.; Fischer, A.; Seoane, E.; Lopez-Granados, E.; Gianelli, C.; Robles-Marhuenda, A.; Jeandel, P.Y.; et al. Coronavirus disease 2019 in patients with inborn errors of immunity: An international study. J. Allergy Clin. Immunol. 2021, 147, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Heldman, M.R.; Kates, O.S.; Safa, K.; Kotton, C.N.; Georgia, S.J.; Steinbrink, J.M.; Alexander, B.D.; Hemmersbach-Miller, M.; Blumberg, E.A.; Multani, A.; et al. Changing trends in mortality among solid organ transplant recipients hospitalized for COVID-19 during the course of the pandemic. Am. J. Transplant. 2022, 22, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ponsford, M.J.; Ward, T.J.C.; Stoneham, S.M.; Dallimore, C.M.; Sham, D.; Osman, K.; Barry, S.M.; Jolles, S.; Humphreys, I.R.; Farewell, D. A Systematic Review and Meta-Analysis of Inpatient Mortality Associated with Nosocomial and Community COVID-19 Exposes the Vulnerability of Immunosuppressed Adults. Front. Immunol. 2021, 12, 744696. [Google Scholar] [CrossRef] [PubMed]
- Hagin, D.; Freund, T.; Navon, M.; Halperin, T.; Adir, D.; Marom, R.; Levi, I.; Benor, S.; Alcalay, Y.; Freund, N.T. Immunogenicity of Pfizer-BioNTech COVID-19 vaccine in patients with inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Delmonte, O.M.; Bergerson, J.R.E.; Burbelo, P.D.; Durkee-Shock, J.R.; Dobbs, K.; Bosticardo, M.; Keller, M.D.; McDermott, D.H.; Rao, V.K.; Dimitrova, D.; et al. Antibody responses to the SARS-CoV-2 vaccine in individuals with various inborn errors of immunity. J. Allergy Clin. Immunol. 2021, 148, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Hassold, N.; Brichler, S.; Ouedraogo, E.; Leclerc, D.; Carroue, S.; Gater, Y.; Alloui, C.; Carbonnelle, E.; Bouchaud, O.; Mechai, F.; et al. Impaired antibody response to COVID-19 vaccination in advanced HIV infection. AIDS 2022, 36, F1–F5. [Google Scholar] [CrossRef]
- Spinelli, M.A.; Peluso, M.J.; Lynch, K.L.; Yun, C.; Glidden, D.V.; Henrich, T.J.; Deeks, S.G.; Gandhi, M. Differences in Post-mRNA Vaccination SARS-CoV-2 IgG Concentrations and Surrogate Virus Neutralization Test Response by HIV Status and Type of Vaccine: A Matched Case-Control Observational Study. Clin. Infect. Dis. 2021, 75, e916–e919. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: A multicentre study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, S.A.; Kakara, M.; Painter, M.M.; Goel, R.R.; Mathew, D.; Lenzi, K.; Rezk, A.; Patterson, K.R.; Espinoza, D.A.; Kadri, J.C.; et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021, 27, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Painter, M.M.; Mathew, D.; Goel, R.R.; Apostolidis, S.A.; Pattekar, A.; Kuthuru, O.; Baxter, A.E.; Herati, R.S.; Oldridge, D.A.; Gouma, S.; et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021, 54, 2133–2142.e3. [Google Scholar] [CrossRef] [PubMed]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung. Transplant 2022, 41, 148–157. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients with Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Kamar, N.; Abravanel, F.; Marion, O.; Romieu-Mourez, R.; Couat, C.; Del Bello, A.; Izopet, J. Assessment of 4 Doses of SARS-CoV-2 Messenger RNA-Based Vaccine in Recipients of a Solid Organ Transplant. JAMA Netw. Open 2021, 4, e2136030. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Tan, H.X.; Juno, J.A.; Lee, W.S.; Barber-Axthelm, I.; Kelly, H.G.; Wragg, K.M.; Esterbauer, R.; Amarasena, T.; Mordant, F.L.; Subbarao, K.; et al. Immunogenicity of prime-boost protein subunit vaccine strategies against SARS-CoV-2 in mice and macaques. Nat. Commun. 2021, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Huang, X.; Yuan, L.; Wang, S.; Zhang, Y.; Xiong, H.; Chen, R.; Ma, J.; Qi, R.; Nie, M.; et al. A recombinant spike protein subunit vaccine confers protective immunity against SARS-CoV-2 infection and transmission in hamsters. Sci. Transl. Med. 2021, 13, eabg1143. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Ella, R.; Reddy, S.; Jogdand, H.; Sarangi, V.; Ganneru, B.; Prasad, S.; Das, D.; Raju, D.; Praturi, U.; Sapkal, G.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: Interim results from a double-blind, randomised, multicentre, phase 2 trial, and 3-month follow-up of a double-blind, randomised phase 1 trial. Lancet Infect. Dis. 2021, 21, 950–961. [Google Scholar] [CrossRef]
- Tauzin, A.; Nayrac, M.; Benlarbi, M.; Gong, S.Y.; Gasser, R.; Beaudoin-Bussieres, G.; Brassard, N.; Laumaea, A.; Vezina, D.; Prevost, J.; et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe 2021, 29, 1137–1150.e6. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Lan, L.Y.; Andrews, S.F.; Henry, C.; Rojas, K.T.; Neu, K.E.; Huang, M.; Huang, Y.; DeKosky, B.; Palm, A.E.; et al. Low CD21 expression defines a population of recent germinal center graduates primed for plasma cell differentiation. Sci. Immunol. 2017, 2, eaai8153. [Google Scholar] [CrossRef] [PubMed]
| Vaccine Types | Species | Location | Tfh-Related Response | Antibody | Reference | |
|---|---|---|---|---|---|---|
| 1 | NVX-CoV2373 protein vaccine | mice, baboons | blood, spleen | Elicits multifunctional CD4+ and CD8+ T cells, Tfh cells, and antigen-specific GC B cells | IgG | [75] |
| 2 | Spike protein subunit vaccine and Receptor-Binding Domain protein subunit vaccine | macaques | blood, lymph node | Elicits potent antibody responses and serum neutralizing activity | IgG | [76] |
| 3 | Spike protein subunit vaccine | mice | blood, lymph node | Elicits robust GC B cell and Tfh cell responses | IgG | [76] |
| 4 | StriFK-FH002C protein vaccine | mice, hamsters, cynomolgus monkeys | blood, lymph node | Generates substantially higher neutralizing antibody titers | IgG, IgG1, IgG2a, IgG2b | [77] |
| 5 | Protein vaccine formulated with MF59-like adjuvant | mice | blood | Triggers Th2-skewed Tfh cell profile | IgG1 | [59] |
| 6 | BBIBP-CorV protein vaccine | human | blood | Induces rapid, robust humoral responses | No data | [79] |
| 7 | CoronaVac protein vaccine | human | blood | Induces rapid, robust humoral responses | IgG | [80] |
| 8 | BBV152 protein vaccine | human | blood | Induces high neutralizing antibody responses; enhances humoral and cell-mediated immune responses | IgG | [82] |
| 9 | SARS-CoV-2 mRNA vaccine | mice | blood, lymph node, bone marrow, spleen | Elicits potent GC B and Tfh cell responses with superior ability specific to SARS-CoV-2 | IgG1, IgG2a, IgG2b | [59] |
| 10 | mRNA-1273 vaccine | rhesus macaques | blood, lung | Induces Th1 and Tfh cell responses; elicits robust SARS-CoV-2 neutralizing activity | IgA, IgG | [78] |
| 11 | BNT162b2 mRNA vaccine | human | blood | Induces cTfh cells; the frequency of cTfh cells is positively correlated with anti-Spike-specific IgA and IgG antibody titers | IgA, IgG | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chi, X.; Gu, J.; Ma, X. Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response. Vaccines 2022, 10, 1623. https://doi.org/10.3390/vaccines10101623
Chi X, Gu J, Ma X. Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response. Vaccines. 2022; 10(10):1623. https://doi.org/10.3390/vaccines10101623
Chicago/Turabian StyleChi, Xuyang, Jia Gu, and Xiaoxue Ma. 2022. "Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response" Vaccines 10, no. 10: 1623. https://doi.org/10.3390/vaccines10101623
APA StyleChi, X., Gu, J., & Ma, X. (2022). Characteristics and Roles of T Follicular Helper Cells in SARS-CoV-2 Vaccine Response. Vaccines, 10(10), 1623. https://doi.org/10.3390/vaccines10101623






