Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Statistical Analysis
3. Results
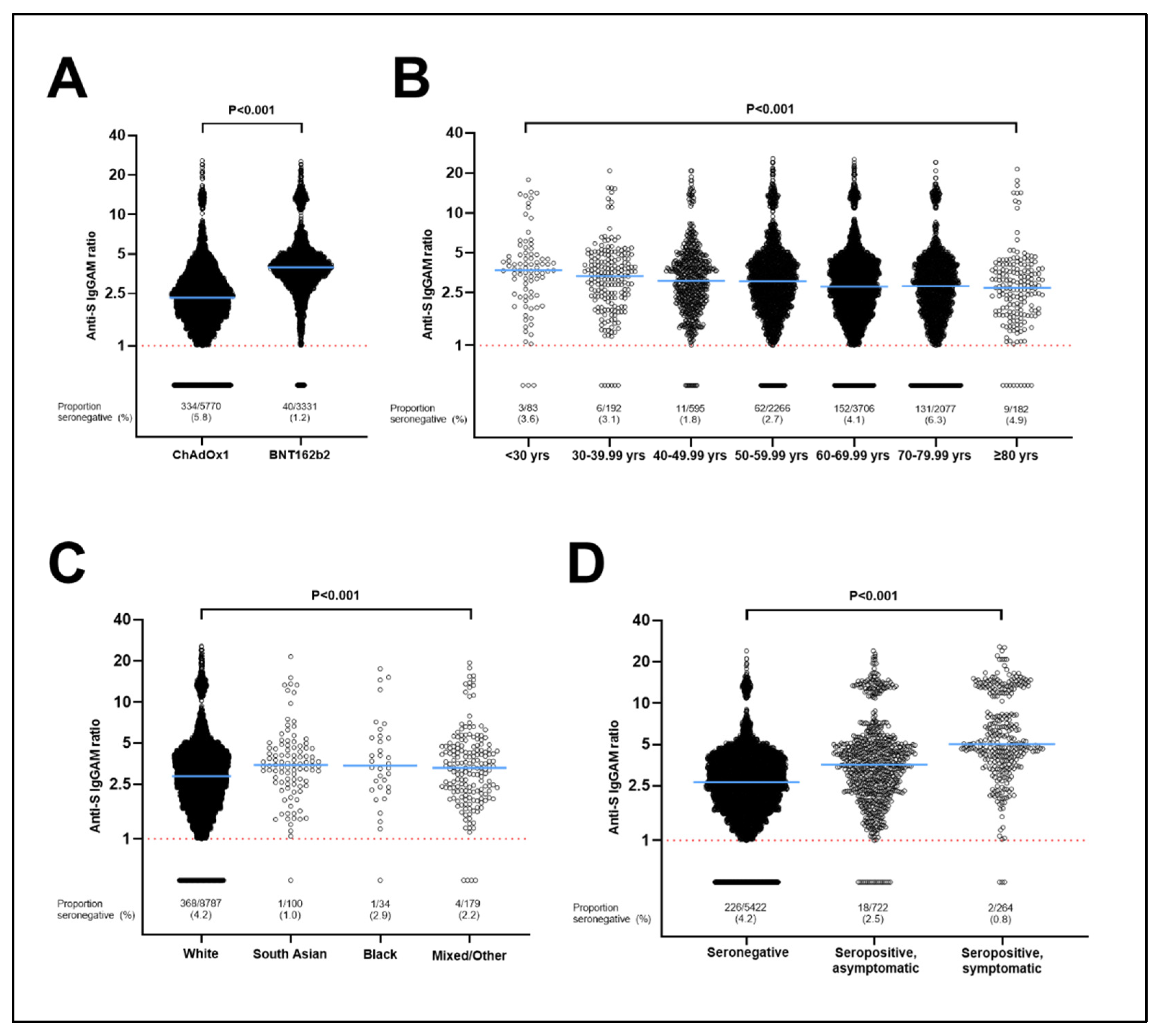
3.1. Determinants of Seronegativity following a Primary Course of SARS-CoV-2 Vaccination
3.2. Determinants of Post-Vaccination Antibody Titres in Subset of Individuals Who Were Seropositive following a Primary Course of SARS-CoV-2 Vaccination
3.3. Stratification of Antibody Responses by Vaccine Type
3.4. Influence of Post-Vaccination Paracetamol/NSAIDs on Antibody Response to Primary Course of Vaccination
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. COVID-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 385, 1474–1484. [Google Scholar] [CrossRef]
- de la Monte, S.M.; Long, C.; Szczepanski, N.; Griffin, C.; Fitzgerald, A.; Chapin, K. Heterogeneous Longitudinal Antibody Responses to COVID-19 mRNA Vaccination. Clin. Pathol. 2021, 14, 2632010X211049255. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Stoesser, N.; Matthews, P.C.; Ayoubkhani, D.; Studley, R.; Bell, I.; Bell, J.I.; Newton, J.N.; Farrar, J.; Diamond, I.; et al. Antibody responses to SARS-CoV-2 vaccines in 45,965 adults from the general population of the United Kingdom. Nat. Microbiol. 2021, 6, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.P.; Longet, S.; Austin, J.A.; Skelly, D.T.; Dejnirattisai, W.; Adele, S.; Meardon, N.; Faustini, S.; Al-Taei, S.; Moore, S.C.; et al. Immunogenicity of standard and extended dosing intervals of BNT162b2 mRNA vaccine. Cell 2021, 184, 5699–5714.e11. [Google Scholar] [CrossRef]
- Amirthalingam, G.; Bernal, J.L.; Andrews, N.J.; Whitaker, H.; Gower, C.; Stowe, J.; Tessier, E.; Subbarao, S.; Ireland, G.; Baawuah, F.; et al. Serological responses and vaccine effectiveness for extended COVID-19 vaccine schedules in England. Nat. Commun. 2021, 12, 7217. [Google Scholar] [CrossRef]
- Kertes, J.; Gez, S.B.; Saciuk, Y.; Supino-Rosin, L.; Stein, N.S.; Mizrahi-Reuveni, M.; Zohar, A.E. Effectiveness of mRNA BNT162b2 Vaccine 6 Months after Vaccination among Patients in Large Health Maintenance Organization, Israel. Emerg. Infect. Dis. 2021, 28, 338–346. [Google Scholar] [CrossRef]
- Papadopoli, R.; De Sarro, C.; Palleria, C.; Gallelli, L.; Pileggi, C.; De Sarro, G. Serological Response to SARS-CoV-2 Messenger RNA Vaccine: Real-World Evidence from Italian Adult Population. Vaccines 2021, 9, 1494. [Google Scholar] [CrossRef]
- Abu Jabal, K.; Ben-Amram, H.; Beiruti, K.; Batheesh, Y.; Sussan, C.; Zarka, S.; Edelstein, M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Eurosurveillance 2021, 26, 2100096. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, M.; Almendro-Vazquez, P.; Carretero, O.; Ruiz-Merlo, T.; Laguna-Goya, R.; San Juan, R.; Lopez-Medrano, F.; Garcia-Rios, E.; Mas, V.; Moreno-Batenero, M.; et al. Discordance Between SARS-CoV-2-specific Cell-mediated and Antibody Responses Elicited by mRNA-1273 Vaccine in Kidney and Liver Transplant Recipients. Transplant. Direct 2021, 7, e794. [Google Scholar] [CrossRef]
- Parry, H.; McIlroy, G.; Bruton, R.; Ali, M.; Stephens, C.; Damery, S.; Otter, A.; McSkeane, T.; Rolfe, H.; Faustini, S.; et al. Antibody responses after first and second COVID-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J. 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Manisty, C.; Otter, A.D.; Treibel, T.A.; McKnight, A.; Altmann, D.M.; Brooks, T.; Noursadeghi, M.; Boyton, R.J.; Semper, A.; Moon, J.C. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet 2021, 397, 1057–1058. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer-BioNTech and Oxford-AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef]
- Long, J.E.; Drayson, M.T.; Taylor, A.E.; Toellner, K.M.; Lord, J.M.; Phillips, A.C. Morning vaccination enhances antibody response over afternoon vaccination: A cluster-randomised trial. Vaccine 2016, 34, 2679–2685. [Google Scholar] [CrossRef]
- Rayman, M.P.; Calder, P.C. Optimising COVID-19 vaccine efficacy by ensuring nutritional adequacy. Br. J. Nutr. 2021, 126, 1919–1920. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Cedernaes, J. Could a good night’s sleep improve COVID-19 vaccine efficacy? Lancet Respir. Med. 2021, 9, 447–448. [Google Scholar] [CrossRef]
- Calina, D.; Hartung, T.; Mardare, I.; Mitroi, M.; Poulas, K.; Tsatsakis, A.; Rogoveanu, I.; Docea, A.O. COVID-19 pandemic and alcohol consumption: Impacts and interconnections. Toxicol. Rep. 2021, 8, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, P.; Ponticelli, D.; Aguero, F.; Caci, G.; Vitale, A.; Borrelli, M.; Schiavone, B.; Antonazzo, I.C.; Mantovani, L.G.; Tomaselli, V.; et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Public Health 2022, 203, 97–99. [Google Scholar] [CrossRef] [PubMed]
- Prymula, R.; Siegrist, C.A.; Chlibek, R.; Zemlickova, H.; Vackova, M.; Smetana, J.; Lommel, P.; Kaliskova, E.; Borys, D.; Schuerman, L. Effect of prophylactic paracetamol administration at time of vaccination on febrile reactions and antibody responses in children: Two open-label, randomised controlled trials. Lancet 2009, 374, 1339–1350. [Google Scholar] [CrossRef]
- Holt, H.; Talaei, M.; Greenig, M.; Zenner, D.; Symons, J.; Relton, C.; Young, K.S.; Davies, M.R.; Thompson, K.N.; Ashman, J.; et al. Risk factors for developing COVID-19: A population-based longitudinal study (COVIDENCE UK). Thorax 2021, 77, 900–912. [Google Scholar] [CrossRef]
- Morley, G.L.; Taylor, S.; Jossi, S.; Perez-Toledo, M.; Faustini, S.E.; Marcial-Juarez, E.; Shields, A.M.; Goodall, M.; Allen, J.D.; Watanabe, Y.; et al. Sensitive Detection of SARS-CoV-2-Specific Antibodies in Dried Blood Spot Samples. Emerg. Infect. Dis. 2020, 26, 2970–2973. [Google Scholar] [CrossRef] [PubMed]
- Shields, A.M.; Faustini, S.E.; Kristunas, C.A.; Cook, A.M.; Backhouse, C.; Dunbar, L.; Ebanks, D.; Emmanuel, B.; Crouch, E.; Kroger, A.; et al. COVID-19: Seroprevalence and Vaccine Responses in UK Dental Care Professionals. J. Dent. Res. 2021, 100, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Vivaldi, G.; Jolliffe, D.A.; Faustini, S.E.; Holt, H.; Perdek, N.; Talaei, M.; Tydeman, F.; Chambers, E.S.; Cai, W.; Li, W.; et al. Correlation between post-vaccination titres of IgG, IgA and IgM anti-Spike antibodies and protection against breakthrough SARS-CoV-2 infection: A population-based longitudinal study (COVIDENCE UK). medRxiv 2022. [Google Scholar] [CrossRef]
- Talaei, M.; Faustini, S.E.; Holt, H.; Jolliffe, D.A.; Vivaldi, G.; Greenig, M.; Perdek, N.; Maltby, S.; Bigogno, C.M.; Symons, J.; et al. Determinants of pre-vaccination antibody responses to SARS-CoV-2: A population-based longitudinal study (COVIDENCE UK). BMC Med. 2022, 20, 87. [Google Scholar] [CrossRef]
- Saleh, E.; Moody, M.A.; Walter, E.B. Effect of antipyretic analgesics on immune responses to vaccination. Hum. Vaccin. Immunother. 2016, 12, 2391–2402. [Google Scholar] [CrossRef]
- Parry, H.; Bruton, R.; Stephens, C.; Brown, K.; Amirthalingam, G.; Otter, A.; Hallis, B.; Zuo, J.; Moss, P. Differential immunogenicity of BNT162b2 or ChAdOx1 vaccines after extended-interval homologous dual vaccination in older people. Immun. Ageing 2021, 18, 34. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M.; Turner, C.T.; Shih, B.B.; Trahair, H.; Pollara, G.; Tsaliki, E.; Rustin, M.; Freeman, T.C.; Mabbott, N.A.; et al. Vitamin D3 replacement enhances antigen-specific immunity in older adults. Immunother. Adv. 2020, 1, ltaa008. [Google Scholar] [CrossRef]
- Dopico, X.C.; Evangelou, M.; Ferreira, R.C.; Guo, H.; Pekalski, M.L.; Smyth, D.J.; Cooper, N.; Burren, O.S.; Fulford, A.J.; Hennig, B.J.; et al. Widespread seasonal gene expression reveals annual differences in human immunity and physiology. Nat. Commun. 2015, 6, 7000. [Google Scholar] [CrossRef] [PubMed]
- Wyse, C.; O’Malley, G.; Coogan, A.N.; McConkey, S.; Smith, D.J. Seasonal and daytime variation in multiple immune parameters in humans: Evidence from 329,261 participants of the UK Biobank cohort. iScience 2021, 24, 102255. [Google Scholar] [CrossRef]
- Linder, N.; Abudi, Y.; Abdalla, W.; Badir, M.; Amitai, Y.; Samuels, J.; Mendelson, E.; Levy, I. Effect of season of inoculation on immune response to rubella vaccine in children. J. Trop. Pediatr. 2011, 57, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Martins, C.; Garly, M.L.; Bale, C.; Rodrigues, A.; Njie-Jobe, J.; Benn, C.S.; Whittle, H.; Aaby, P. Measles virus antibody responses in children randomly assigned to receive standard-titer edmonston-zagreb measles vaccine at 4.5 and 9 months of age, 9 months of age, or 9 and 18 months of age. J. Infect. Dis. 2014, 210, 693–700. [Google Scholar] [CrossRef]
- Abreu, T.C.; Boshuizen, H.; Mollema, L.; Berbers, G.A.M.; Korthals, A.H. Association between season of vaccination and antibody levels against infectious diseases. Epidemiol. Infect. 2020, 148, e276. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.E.; Collinson, A.C.; Fulford, A.J.; Jalil, F.; Siegrist, C.A.; Goldblatt, D.; Hanson, L.Å.; Prentice, A.M. Effect of month of vaccine administration on antibody responses in The Gambia and Pakistan. Trop. Med. Int. Health 2006, 11, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Pant, N.; Pinna, N.K.; Bhar, S.; Dutta, A.; Mande, S.S. Does immune recognition of SARS-CoV2 epitopes vary between different ethnic groups? Virus Res. 2021, 305, 198579. [Google Scholar] [CrossRef]
- Mishra, S.K.; Pradhan, S.K.; Pati, S.; Sahu, S.; Nanda, R.K. Waning of Anti-spike Antibodies in AZD1222 (ChAdOx1) Vaccinated Healthcare Providers: A Prospective Longitudinal Study. Cureus 2021, 13, e19879. [Google Scholar] [CrossRef]
- Uysal, E.B.; Gumus, S.; Bektore, B.; Bozkurt, H.; Gozalan, A. Evaluation of antibody response after COVID-19 vaccination of healthcare workers. J. Med. Virol. 2021, 94, 1060–1066. [Google Scholar] [CrossRef]
- Lee, S.W.; Moon, J.Y.; Lee, S.K.; Lee, H.; Moon, S.; Chung, S.J.; Yeo, Y.; Park, T.S.; Park, D.W.; Kim, T.H.; et al. Anti-SARS-CoV-2 Spike Protein RBD Antibody Levels After Receiving a Second Dose of ChAdOx1 nCov-19 (AZD1222) Vaccine in Healthcare Workers: Lack of Association With Age, Sex, Obesity, and Adverse Reactions. Front. Immunol. 2021, 12, 779212. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Liu, D.; Zeng, Q.; Li, L.; Zhou, Q.; Li, M.; Mei, J.; Yang, N.; Mo, S.; et al. Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res. 2021, 31, 1215–1217. [Google Scholar] [CrossRef]
- Wang, W.; Balfe, P.; Eyre, D.W.; Lumley, S.F.; O’Donnell, D.; Warren, F.; Crook, D.W.; Jeffery, K.; Matthews, P.C.; Klerman, E.B.; et al. Time of Day of Vaccination Affects SARS-CoV-2 Antibody Responses in an Observational Study of Health Care Workers. J. Biol. Rhythms 2022, 37, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Picchianti-Diamanti, A.; Aiello, A.; Lagana, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Farroni, C.; Lapa, D.; Najafi Fard, S.; Cuzzi, G.; et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 mRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021, 12, 740249. [Google Scholar] [CrossRef] [PubMed]
- Farroni, C.; Picchianti-Diamanti, A.; Aiello, A.; Nicastri, E.; Lagana, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Colavita, F.; Cuzzi, G.; et al. Kinetics of the B- and T-Cell Immune Responses After 6 Months From SARS-CoV-2 mRNA Vaccination in Patients With Rheumatoid Arthritis. Front. Immunol. 2022, 13, 846753. [Google Scholar] [CrossRef]
- Tortorella, C.; Aiello, A.; Gasperini, C.; Agrati, C.; Castilletti, C.; Ruggieri, S.; Meschi, S.; Matusali, G.; Colavita, F.; Farroni, C.; et al. Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies. Neurology 2022, 98, e541–e554. [Google Scholar] [CrossRef] [PubMed]
- Petrone, L.; Petruccioli, E.; Vanini, V.; Cuzzi, G.; Najafi Fard, S.; Alonzi, T.; Castilletti, C.; Palmieri, F.; Gualano, G.; Vittozzi, P.; et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin. Microbiol. Infect. 2021, 27, 286-e7. [Google Scholar] [CrossRef]
- Petruccioli, E.; Najafi Fard, S.; Navarra, A.; Petrone, L.; Vanini, V.; Cuzzi, G.; Gualano, G.; Pierelli, L.; Bertoletti, A.; Nicastri, E.; et al. Exploratory analysis to identify the best antigen and the best immune biomarkers to study SARS-CoV-2 infection. J. Transl. Med. 2021, 19, 272. [Google Scholar] [CrossRef]
- Ward, H.; Atchison, C.; Whitaker, M.; Ainslie, K.E.C.; Elliott, J.; Okell, L.; Redd, R.; Ashby, D.; Donnelly, C.A.; Barclay, W.; et al. SARS-CoV-2 antibody prevalence in England following the first peak of the pandemic. Nat. Commun. 2021, 12, 905. [Google Scholar] [CrossRef]
- Chadeau-Hyam, M.; Bodinier, B.; Elliott, J.; Whitaker, M.D.; Tzoulaki, I.; Vermeulen, R.; Kelly-Irving, M.; Delpierre, C.; Elliott, P. Risk factors for positive and negative COVID-19 tests: A cautious and in-depth analysis of UK biobank data. Int. J. Epidemiol. 2020, 49, 1454–1467. [Google Scholar] [CrossRef]
- Rozenfeld, Y.; Beam, J.; Maier, H.; Haggerson, W.; Boudreau, K.; Carlson, J.; Medows, R. A model of disparities: Risk factors associated with COVID-19 infection. Int. J. Equity Health 2020, 19, 126. [Google Scholar] [CrossRef]
- Mathur, R.; Rentsch, C.T.; Morton, C.E.; Hulme, W.J.; Schultze, A.; MacKenna, B.; Eggo, R.M.; Bhaskaran, K.; Wong, A.Y.S.; Williamson, E.J.; et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: An observational cohort study using the OpenSAFELY platform. Lancet 2021, 397, 1711–1724. [Google Scholar] [CrossRef]
- Cook, A.M.; Faustini, S.E.; Williams, L.J.; Cunningham, A.F.; Drayson, M.T.; Shields, A.M.; Kay, D.; Taylor, L.; Plant, T.; Huissoon, A.; et al. Validation of a combined ELISA to detect IgG, IgA and IgM antibody responses to SARS-CoV-2 in mild or moderate non-hospitalised patients. J. Immunol. Methods 2021, 494, 113046. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
| Characteristic | ChAdOx1 (n = 5770) | BNT162b2 (n = 3331) | Overall (n = 9101) | |
|---|---|---|---|---|
| Age | Median age, years (IQR) | 63.5 (57.0–68.9) | 65.5 (57.4–71.6) | 64.2 (57.1–69.9) |
| Age range, years | 17.4 89.4 | 16.6 90.5 | 16.6 90.5 | |
| Sex, n (%) | Male | 1671 (29.0) | 956 (28.7) | 2627 (28.9) |
| Female | 4099 (71.0) | 2375 (71.3) | 6474 (71.1) | |
| Ethnicity, n (%) 1 | White | 5565 (96.4) | 3222 (96.7) | 8787 (96.5) |
| South Asian | 119 (2.1) | 60 (1.8) | 179 (2) | |
| Black/African/Caribbean/Black British | 62 (1.1) | 38 (1.1) | 100 (1.1) | |
| Mixed/Multiple/Other | 24 (0.4) | 10 (0.3) | 34 (0.4) | |
| Nation of residence 2 | England | 5177 (89.8) | 2906 (87.3) | 8083 (88.8) |
| Northern Ireland | 39 (0.7) | 78 (2.3) | 117 (1.3) | |
| Scotland | 358 (6.2) | 199 (6.0) | 557 (6.1) | |
| Wales | 194 (3.4) | 147 (4.4) | 341 (3.8) | |
| Body mass index, kg/m2, n (%) 3 | <25 | 2845 (49.3) | 1596 (47.9) | 4441 (48.8) |
| 25–30 | 1881 (32.6) | 1097 (32.9) | 2978 (32.7) | |
| >30 | 1034 (17.9) | 636 (19.1) | 1670 (18.3) | |
| Highest educational level attained, n (%) 4 | Primary/Secondary | 636 (11) | 401 (12) | 1037 (11.4) |
| Higher/Further (A levels) | 857 (14.9) | 441 (13.2) | 1298 (14.3) | |
| College | 2540 (44) | 1500 (45) | 4040 (44.4) | |
| Post-graduate | 1736 (30.1) | 984 (29.5) | 2720 (29.9) | |
| Quantiles of IMD rank, n (%) 5 | Q1 (most deprived) | 1172 (20.3) | 786 (23.6) | 1958 (21.5) |
| Q2 | 1417 (24.6) | 782 (23.5) | 2199 (24.2) | |
| Q3 | 1537 (26.6) | 868 (26.1) | 2405 (26.4) | |
| Q4 (least deprived) | 1639 (28.4) | 893 (26.8) | 2532 (27.8) | |
| Tobacco smoking, n (%) | Non-current/never smoker | 5546 (96.1) | 3194 (95.9) | 8740 (96) |
| Current smoker | 224 (3.9) | 137 (4.1) | 361 (4) | |
| Alcohol consumption/week, units, n (%) | None | 1505 (26.1) | 896 (26.9) | 2401 (26.4) |
| 1–7 | 2018 (35) | 1212 (36.4) | 3230 (35.5) | |
| 8–14 | 1186 (20.6) | 695 (20.9) | 1881 (20.7) | |
| 15–21 | 593 (10.3) | 310 (9.3) | 903 (9.9) | |
| 22–28 | 263 (4.6) | 127 (3.8) | 390 (4.3) | |
| >28 | 205 (3.6) | 91 (2.7) | 296 (3.3) | |
| Self-assessed general health | Excellent | 1183 (20.5) | 654 (19.6) | 1837 (20.2) |
| Very good | 2336 (40.5) | 1315 (39.5) | 3651 (40.1) | |
| Good | 1441 (25.0) | 925 (27.8) | 2366 (26.0) | |
| Fair | 637 (11.0) | 344 (10.3) | 981 (10.8) | |
| Poor | 173 (3.0) | 93 (2.8) | 266 (2.9) | |
| Pre-vaccination anti-spike IgG/A/M serostatus | Negative | 3789 (65.7) | 1770 (53.1) | 5559 (61.1) |
| Positive | 696 (12.1) | 325 (9.8) | 1021 (11.2) | |
| Unknown | 1285 (22.3) | 1236 (37.1) | 2521 (27.7) | |
| Post-vaccination anti-spike IgG/A/M serostatus | Negative | 334 (5.8) | 40 (1.2) | 374 (4.1) |
| Positive | 5436 (94.2) | 3291 (98.8) | 8727 (95.9) | |
| Median inter-dose interval, weeks (IQR) | 11.0 (10.0–11.2) | 10.7 (9.5–11.1) | 11.0 (9.8–11.1) | |
| Median time from date of second vaccine dose to date of sampling, weeks (IQR) | 7.6 (5.7–9.6) | 10.1 (8.3–13.1) | 8.6 (6.4–10.7) | |
| Predictor | n Seronegative (%) | Minimally Adjusted Odds Ratio (95% CI) 1 | Pairwise p Value | Fully Adjusted Odds Ratio (95% CI) 2 | Pairwise p Value | P for Trend | |
|---|---|---|---|---|---|---|---|
| Vaccine type and timing | |||||||
| Vaccine type | ChAdOx1 | 334/5770 (5.8) | 5.49 (3.94, 7.66) | <0.001 | 6.62 (4.21, 10.42) | <0.001 * | - |
| BNT162b2 | 40/3331 (1.2) | Referent | |||||
| Time from date of second vaccine dose to date of sampling, weeks (IQR) | <2 | 3/34 (8.8) | 2.85 (0.80, 10.20) | 0.11 | |||
| 2–4 | 19/593 (3.2) | Referent | |||||
| 5–8 | 127/3878 (3.3) | 0.87 (0.53, 1.42) | 0.57 | ||||
| 9–16 | 213/4155 (5.1) | 1.27 (0.78, 2.07) | 0.33 | ||||
| >16 | 12/441 (2.7) | 0.68 (0.33, 1.43) | 0.31 | ||||
| Inter-dose interval, weeks | <6 | 25/474 (5.3) | 1.52 (0.99, 2.34) | 0.054 | 2.56 (1.22, 5.37) | 0.013 * | <0.001 |
| 6–10 | 142/2959 (4.8) | 1.47 (1.17, 1.83) | 0.001 | 1.60 (1.20, 2.14) | 0.001 * | ||
| >10 | 207/5668 (3.7) | Referent | |||||
| Time of second vaccine dose | Before 12 p.m. | 148/3692 (4.0) | Referent | ||||
| 12 p.m.–2 p.m. | 74/1561 (4.7) | 1.21 (0.91, 1.61) | 0.19 | ||||
| 2 p.m.–5 p.m. | 103/2573 (4.0) | 1.01 (0.78, 1.31) | 0.92 | ||||
| After 5 p.m. | 30/941 (3.2) | 0.84 (0.57, 1.26) | 0.41 | ||||
| Quarter of second vaccine dose | Q1 | 56/1318 (4.2) | 1.02 (0.76, 1.37) | 0.87 | 1.08 (0.61, 1.92) | 0.79 | 0.005 † |
| Q2 | 313/7755 (4.0) | Referent | |||||
| Q3 | 2/13 (15.4) | 4.10 (0.89, 18.77) | 0.07 | 12.64 (2.21, 72.27) | 0.004 * | ||
| Q4 | 3/15 (20.0) | 5.82 (1.60, 21.17) | 0.007 | 21.83 (1.74, 273.33) | 0.017 * | ||
| Socio-demographic factors | |||||||
| Age, years | <30 | 3/83 (3.6) | Referent | <0.001 | |||
| 30–39.99 | 6/192 (3.1) | 0.87 (0.21, 3.57) | 0.85 | 0.96 (0.09, 10.65) | 0.97 | ||
| 40–49.99 | 11/595 (1.8) | 0.51 (0.14, 1.85) | 0.30 | 0.53 (0.06, 4.97) | 0.58 | ||
| 50–59.99 | 62/2266 (2.7) | 0.75 (0.23, 2.44) | 0.63 | 0.88 (0.10, 7.41) | 0.90 | ||
| 60–69.99 | 152/3706 (4.1) | 1.11 (0.35, 3.55) | 0.86 | 1.64 (0.20, 13.67) | 0.65 | ||
| 70–79.99 | 131/2077 (6.3) | 1.69 (0.52, 5.42) | 0.38 | 2.86 (0.34, 24.18) | 0.33 | ||
| ≥80 | 9/182 (4.9) | 1.25 (0.33, 4.76) | 0.74 | 3.38 (0.25, 46.30) | 0.36 | ||
| Sex | Female | 234/6474 (3.6) | Referent | ||||
| Male | 140/2627 (5.3) | 1.33 (1.07, 1.66) | 0.01 | 1.32 (0.98, 1.77) | 0.06 | - | |
| Ethnicity | White | 368/8787 (4.2) | Referent | ||||
| Mixed/Multiple/Other | 4/179 (2.2) | 0.59 (0.22, 1.59) | 0.30 | ||||
| South Asian | 1/100 (1.0) | 0.27 (0.04, 1.94) | 0.19 | ||||
| Black/African/Caribbean/ Black British | 1/34 (2.9) | 0.85 (0.12, 6.28) | 0.88 | ||||
| BMI, kg/m2 | <25 | 164/4441 (3.7) | Referent | 0.71 | |||
| 25–30 | 135/2978 (4.5) | 1.20 (0.95, 1.52) | 0.13 | 1.15 (0.85, 1.55) | 0.37 | ||
| >30 | 74/1670 (4.4) | 1.30 (0.98, 1.72) | 0.07 | 1.05 (0.70, 1.56) | 0.82 | ||
| Highest educational level attained | Primary/Secondary | 54/1036 (5.2) | 1.12 (0.80, 1.57) | 0.50 | |||
| Higher/further (A levels) | 56/1298 (4.3) | 0.99 (0.72, 1.38) | 0.97 | ||||
| College | 150/4041 (3.7) | 0.86 (0.67, 1.10) | 0.23 | ||||
| Post-graduate | 114/2720 (4.2) | Referent | |||||
| Quantiles of IMD rank | Q1 (most deprived) | 85/1958 (4.3) | 1.20 (0.89, 1.61) | 0.24 | |||
| Q2 | 94/2199 (4.3) | 1.12 (0.84, 1.49) | 0.45 | ||||
| Q3 | 96/2405 (4.0) | 1.01 (0.76, 1.35) | 0.92 | ||||
| Q4 (least deprived) | 99/2532 (3.9) | Referent | |||||
| Lifestyle factors | |||||||
| Tobacco smoking | No | 363/8740 (4.2) | Referent | ||||
| Yes | 11/361 (3.0) | 0.77 (0.42, 1.42) | 0.40 | ||||
| Vaping | No | 364/8881 (4.1) | Referent | ||||
| Yes | 9/196 (4.6) | 1.16 (0.59, 2.29) | 0.67 | ||||
| Alcohol, units/week | None | 118/2401 (4.9) | Referent | 0.81 | |||
| 1–7 | 103/3230 (3.2) | 0.63 (0.48, 0.82) | 0.001 | 0.71 (0.50, 1.01) | 0.06 | ||
| 8–14 | 84/1880 (4.5) | 0.85 (0.64, 1.14) | 0.29 | 1.10 (0.76, 1.60) | 0.61 | ||
| 15–21 | 40/903 (4.4) | 0.83 (0.57, 1.20) | 0.31 | 1.00 (0.63, 1.60) | 0.99 | ||
| 22–28 | 17/391 (4.3) | 0.78 (0.46, 1.32) | 0.35 | 1.15 (0.61, 2.17) | 0.68 | ||
| >28 | 12/296 (4.1) | 0.71 (0.38, 1.30) | 0.27 | 0.60 (0.25, 1.46) | 0.26 | ||
| Light exercise, hours/week | 0–4 | 140/2824 (5.0) | 1.52 (1.18, 1.96) | 0.001 | 1.19 (0.86, 1.67) | 0.30 | 0.31 |
| 5–9 | 120/2987 (4.0) | 1.20 (0.93, 1.56) | 0.17 | 1.09 (0.79, 1.50) | 0.61 | ||
| ≥10 | 114/3268 (3.5) | Referent | |||||
| Vigorous exercise, hours/week | 0 | 155/3458 (4.5) | 1.19 (0.91, 1.56) | 0.20 | |||
| 1–3 | 132/3387 (3.9) | 1.04 (0.79, 1.37) | 0.79 | ||||
| ≥4 | 87/2230 (3.9) | Referent | |||||
| Sleep, hours/night | ≤5 | 43/797 (5.4) | 1.26 (0.87, 1.81) | 0.22 | 1.29 (0.81, 2.05) | 0.29 | 0.54 |
| 6 | 93/2215 (4.2) | 0.93 (0.70, 1.23) | 0.60 | 0.91 (0.63, 1.32) | 0.62 | ||
| 7 | 133/3750 (3.6) | 0.77 (0.59, 1.00) | 0.05 | 0.84 (0.60, 1.17) | 0.29 | ||
| ≥8 | 105/2336 (4.5) | Referent | |||||
| Self-assessed general health | Excellent | 52/1837 (2.8) | Referent | 0.002 | |||
| Very good | 136/3652 (3.7) | 1.31 (0.95, 1.82) | 0.10 | 1.57 (1.03, 2.40) | 0.036 | ||
| Good | 100/2365 (4.2) | 1.54 (1.09, 2.16) | 0.01 | 1.87 (1.19, 2.95) | 0.007 * | ||
| Fair | 60/981 (6.1) | 2.35 (1.60, 3.44) | <0.001 | 2.00 (1.15, 3.47) | 0.014 * | ||
| Poor | 26/266 (9.8) | 3.85 (2.35, 6.28) | <0.001 | 3.12 (1.39, 6.96) | 0.006 * | ||
| Anxiety or depression | No | 288/6910 (4.2) | Referent | ||||
| Yes | 86/2187 (3.9) | 1.00 (0.78, 1.29) | 0.98 | ||||
| Food choice | None | 362/8607 (4.2) | Referent | ||||
| Vegetarian | 9/380 (2.4) | 0.59 (0.30, 1.15) | 0.12 | ||||
| Vegan | 3/114 (2.6) | 0.63 (0.20, 2.01) | 0.44 | ||||
| Medical conditions | |||||||
| Heart disease 3 | No | 351/8707 (4.0) | Referent | ||||
| Yes | 23/394 (5.8) | 1.26 (0.81, 1.97) | 0.30 | ||||
| Arterial disease 4 | No | 340/8572 (4.0) | Referent | ||||
| Yes | 34/529 (6.4) | 1.45 (1.00, 2.11) | 0.05 | 0.50 (0.23, 1.09) | 0.08 | - | |
| Hypertension | No | 246/6902 (3.6) | Referent | ||||
| Yes | 128/2199 (5.8) | 1.56 (1.25, 1.95) | <0.001 | 0.89 (0.58, 1.37) | 0.61 | - | |
| Immunodeficiency disorder 5 | No | 364/9042 (4.0) | Referent | ||||
| Yes | 10/59 (16.9) | 4.62 (2.32, 9.23) | <0.001 | 6.48 (2.53, 16.59) | <0.001 * | - | |
| Major neurological condition 6 | No | 350/8833 (4.0) | Referent | ||||
| Yes | 24/268 (9.0) | 2.23 (1.44, 3.44) | <0.001 | 1.79 (0.82, 3.91) | 0.15 | - | |
| Cancer | Never | 330/8128 (4.1) | Referent | ||||
| Past (cured or in remission) | 40/888 (4.5) | 1.04 (0.74, 1.47) | 0.80 | ||||
| Present (active) | 4/85 (4.7) | 1.00 (0.36, 2.77) | 0.99 | ||||
| Asthma | No | 303/7652 (4.0) | Referent | ||||
| Yes | 71/1449 (4.9) | 1.30 (1.00, 1.70) | 0.05 | 0.99 (0.69, 1.41) | 0.94 | - | |
| COPD | No | 364/8905 (4.1) | Referent | ||||
| Yes | 10/196 (5.1) | 1.19 (0.62, 2.27) | 0.60 | ||||
| Diabetic status | No diabetes | 317/8334 (3.8) | Referent | 0.06 | |||
| Pre-diabetes | 16/296 (5.4) | 1.38 (0.82, 2.31) | 0.23 | 1.12 (0.57, 2.22) | 0.74 | ||
| Type 1 diabetes | 4/69 (5.8) | 1.59 (0.57, 4.41) | 0.37 | 1.87 (0.52, 6.75) | 0.34 | ||
| Type 2 diabetes | 34/385 (8.8) | 2.26 (1.56, 3.28) | <0.001 | 2.02 (0.94, 4.33) | 0.07 | ||
| Atopy 7 | No | 281/6794 (4.1) | Referent | ||||
| Yes | 93/2307 (4.0) | 1.01 (0.80, 1.29) | 0.92 | ||||
| Pre-vaccination SARS-CoV-2 status | Seronegative | 226/5422 (4.2) | Referent | <0.001 | |||
| Seropositive, asymptomatic | 18/722 (2.5) | 0.58 (0.35, 0.94) | 0.03 | 0.53 (0.32, 0.89) | 0.015 * | ||
| Seropositive, symptomatic | 2/264 (0.8) | 0.20 (0.05, 0.81) | 0.02 | 0.16 (0.04, 0.56) | 0.004 * | ||
| Nutritional supplements | |||||||
| Multivitamin | No | 299/7200 (4.2) | Referent | ||||
| Yes | 75/1901 (3.9) | 0.98 (0.75, 1.26) | 0.86 | ||||
| Vitamin A | No | 371/9053 (4.1) | Referent | ||||
| Yes | 3/48 (6.3) | 1.56 (0.48, 5.04) | 0.46 | ||||
| Vitamin C | No | 346/8192 (4.2) | Referent | ||||
| Yes | 28/909 (3.1) | 0.73 (0.49, 1.08) | 0.11 | ||||
| Vitamin D | No | 204/4455 (4.6) | Referent | ||||
| Yes | 170/4646 (3.7) | 0.80 (0.65, 0.98) | 0.03 | 0.66 (0.51, 0.87) | 0.003 * | - | |
| Zinc | No | 357/8664 (4.1) | Referent | ||||
| Yes | 17/437 (3.9) | 0.94 (0.57, 1.55) | 0.82 | ||||
| Selenium | No | 372/9003 (4.1) | Referent | ||||
| Yes | 2/98 (2.0) | 0.49 (0.12, 2.01) | 0.32 | ||||
| Iron | No | 368/8816 (4.2) | Referent | ||||
| Yes | 6/285 (2.1) | 0.53 (0.23, 1.19) | 0.12 | ||||
| Probiotics | No | 359/8528 (4.2) | Referent | ||||
| Yes | 15/573 (2.6) | 0.64 (0.38, 1.09) | 0.10 | ||||
| Omega-3 fatty acids | No | 336/7975 (4.2) | Referent | ||||
| Yes | 38/1126 (3.4) | 0.80 (0.57, 1.12) | 0.19 | ||||
| Cod liver oil | No | 341/8279 (4.1) | Referent | ||||
| Yes | 33/822 (4.0) | 0.93 (0.64, 1.34) | 0.68 | ||||
| Garlic | No | 364/8907 (4.1) | Referent | ||||
| Yes | 10/194 (5.2) | 1.20 (0.63, 2.30) | 0.57 | ||||
| Medications | |||||||
| Beta-2 adrenergic agonists | No | 334/8275 (4.0) | Referent | ||||
| Yes | 40/826 (4.8) | 1.23 (0.87, 1.72) | 0.24 | ||||
| Beta blockers | No | 334/8398 (4.0) | Referent | ||||
| Yes | 40/703 (5.7) | 1.35 (0.96, 1.89) | 0.09 | 1.01 (0.62, 1.65) | 0.96 | - | |
| Statins | No | 277/7337 (3.8) | Referent | ||||
| Yes | 97/1764 (5.5) | 1.31 (1.02, 1.68) | 0.03 | 0.92 (0.64, 1.32) | 0.65 | - | |
| ACE inhibitors | No | 320/8122 (3.9) | Referent | ||||
| Yes | 54/979 (5.5) | 1.30 (0.96, 1.76) | 0.09 | 1.12 (0.70, 1.80) | 0.64 | - | |
| Proton pump inhibitors | No | 287/7723 (3.7) | Referent | ||||
| Yes | 87/1378 (6.3) | 1.69 (1.32, 2.16) | <0.001 | 0.91 (0.63, 1.33) | 0.63 | - | |
| H2-receptor antagonists | No | 373/9040 (4.1) | Referent | ||||
| Yes | 1/61 (1.6) | 0.39 (0.05, 2.80) | 0.35 | ||||
| Inhaled corticosteroids | No | 349/8505 (4.1) | Referent | ||||
| Yes | 25/596 (4.2) | 1.01 (0.66, 1.53) | 0.98 | ||||
| Bronchodilators | No | 333/8240 (4.0) | Referent | ||||
| Yes | 41/861 (4.8) | 1.20 (0.85, 1.67) | 0.30 | ||||
| Systemic Immunosuppressants | No | 327/8630 (3.8) | Referent | ||||
| Yes | 47/471 (10.0) | 2.86 (2.08, 3.95) | <0.001 | 3.71 (2.41, 5.69) | <0.001 * | - | |
| Angiotensin receptor blockers | No | 331/8493 (3.9) | Referent | ||||
| Yes | 43/608 (7.1) | 1.75 (1.25, 2.43) | <0.001 | 0.98 (0.58, 1.68) | 0.95 | - | |
| SSRI antidepressants | No | 350/8533 (4.1) | Referent | ||||
| Yes | 24/568 (4.2) | 1.11 (0.73, 1.70) | 0.62 | ||||
| Non-SSRIs antidepressants | No | 352/8726 (4.0) | Referent | ||||
| Yes | 22/375 (5.9) | 1.57 (1.01, 2.46) | 0.05 | 0.73 (0.35, 1.50) | 0.39 | - | |
| Calcium channel blockers | No | 314/8100 (3.9) | Referent | ||||
| Yes | 60/1001 (6.0) | 1.45 (1.09, 1.94) | 0.01 | 1.00 (0.65, 1.55) | 0.99 | - | |
| Thiazide diuretics | No | 359/8765 (4.1) | Referent | ||||
| Yes | 15/336 (4.5) | 1.04 (0.61, 1.76) | 0.89 | ||||
| Vitamin K antagonists | No | 370/9027 (4.1) | Referent | ||||
| Yes | 4/74 (5.4) | 1.17 (0.42, 3.23) | 0.76 | ||||
| SGLT-2 inhibitors | No | 369/9054 (4.1) | Referent | ||||
| Yes | 5/47 (10.6) | 2.56 (1.01, 6.54) | 0.05 | 2.99 (0.92, 9.65) | 0.07 | - | |
| Anticholinergics | No | 354/8656 (4.1) | Referent | ||||
| Yes | 20/445 (4.5) | 1.08 (0.67, 1.72) | 0.76 | ||||
| Metformin | No | 353/8827 (4.0) | Referent | ||||
| Yes | 21/274 (7.7) | 1.83 (1.16, 2.90) | 0.01 | 0.61 (0.24, 1.55) | 0.30 | - | |
| Bisphosphonates | No | 362/8912 (4.1) | Referent | ||||
| Yes | 12/189 (6.3) | 1.72 (0.94, 3.12) | 0.08 | 0.77 (0.31, 1.89) | 0.57 | - | |
| Anti-platelet drugs | No | 328/8453 (3.9) | Referent | ||||
| Yes | 46/648 (7.1) | 1.67 (1.21, 2.32) | 0.002 | 2.79 (1.06, 7.38) | 0.038 | - | |
| Sex hormone therapy | No | 347/8376 (4.1) | Referent | ||||
| Yes | 27/725 (3.7) | 1.04 (0.69, 1.56) | 0.87 | ||||
| Aspirin 8 | No | 341/8593 (4.0) | Referent | ||||
| Yes | 33/508 (6.5) | 1.47 (1.01, 2.14) | 0.04 | 0.37 (0.14, 1.01) | 0.05 | - | |
| Paracetamol 8 | No | 345/8677 (4.0) | Referent | ||||
| Yes | 29/424 (6.8) | 1.77 (1.19, 2.62) | 0.005 | 0.84 (0.47, 1.51) | 0.56 | - | |
| BCG vaccinated | No | 44/1075 (4.1) | Referent | ||||
| Yes | 280/7166 (3.9) | 0.99 (0.71, 1.37) | 0.13 | ||||
| Predictor | Median IgGAM Ratio (IQR) | Minimally Adjusted % Difference (95% CI) 1 | p Value | Fully Adjusted % Difference (95% CI) 2 | Pairwise p Value | P for Trend | |
|---|---|---|---|---|---|---|---|
| Vaccine type | ChAdOx1 | 2.39 (1.76, 3.30) | −39.35 (−40.65, −38.02) | <0.001 | −43.31 (−44.8, −41.78) | <0.001 * | - |
| BNT162b2 | 3.96 (3.15, 4.86) | Referent | |||||
| Time from second vaccine dose to sampling, weeks | <2 | 2.96 (2.23, 13.62) | 36.68 (11.82, 67.05) | 0.002 | 1.98 (−17.97, 26.79) | 0.86 | <0.001 |
| 2–4 | 2.86 (2.06, 4.05) | Referent | |||||
| 5–8 | 2.81 (1.95, 3.99) | −0.59 (−5.37, 4.43) | 0.81 | −7.41 (−11.62, −3.00) | 0.001 * | ||
| 9–16 | 3.13 (2.10, 4.24) | 7.54 (2.33, 13.01) | 0.004 | −12.68 (−16.94, −8.20) | <0.001 * | ||
| >16 | 2.93 (2.03, 4.43) | 7.14 (−0.08, 14.89) | 0.05 | −7.83 (−16.12, 1.28) | 0.09 | ||
| Inter−dose interval, weeks | <6 | 2.91 (1.94, 4.12) | −1.23 (−6.38, 4.21) | 0.65 | −10.43 (−16.69, −3.70) | 0.003 * | <0.001 |
| 6–10 | 2.89 (2.00, 4.01) | −5.12 (−7.51, −2.66) | <0.001 | −5.85 (−8.3, −3.34) | <0.001 * | ||
| >10 | 2.99 (2.06, 4.21) | Referent | |||||
| Time of second vaccine dose | Before 12 p.m. | 2.92 (2.02, 4.10) | Referent | 0.96 † | |||
| 12 p.m.–2 p.m. | 2.91 (2.00, 4.07) | −1.12 (−4.39, 2.26) | 0.51 | −2.87 (−6.00, 0.36) | 0.08 | ||
| 2 p.m.–5 p.m. | 3.03 (2.06, 4.21) | 2.88 (−0.01, 5.86) | 0.05 | 1.15 (−1.61, 3.99) | 0.42 | ||
| After 5 p.m. | 2.98 (2.07, 4.07) | 0.54 (−3.45, 4.70) | 0.79 | −2.39 (−6.2, 1.58) | 0.24 | ||
| Quarter of second vaccine dose | Q1 | 3.44 (2.42, 4.43) | 15.46 (11.70, 19.34) | <0.001 | −8.07 (−12.69, −3.22) | 0.001 * | 0.004 † |
| Q2 | 2.88 (1.99, 4.09) | Referent | |||||
| Q3 | 3.17 (1.36, 4.47) | −3.37 (−30.38, 34.12) | 0.84 | 31.63 (−22.10, 122.40) | 0.30 | ||
| Q4 | 1.93 (1.28, 3.24) | −24.08 (−44.54, 3.92) | 0.09 | −47.69 (−69.11, −11.39) | 0.016 * | ||
| Age, years | <30 | 3.79 (2.48, 4.85) | Referent | <0.001 | |||
| 30–39.99 | 3.43 (2.34, 4.55) | −12.71 (−24.56, 1.00) | 0.07 | −5.94 (−20.45, 11.21) | 0.47 | ||
| 40–49.99 | 3.13 (2.16, 4.31) | −16.59 (−26.77, −5.01) | 0.006 | −4.12 (−17.60, 11.58) | 0.59 | ||
| 50–59.99 | 3.10 (2.14, 4.30) | −16.58 (−26.32, −5.55) | 0.004 | 0.70 (−13.04, 16.61) | 0.93 | ||
| 60–69.99 | 2.85 (1.98, 4.05) | −22.79 (−31.75, −12.65) | <0.001 | −5.39 (−18.27, 9.53) | 0.46 | ||
| 70–79.99 | 2.92 (1.96, 4.03) | −23.08 (−32.09, −12.87) | <0.001 | −10.54 (−22.81, 3.69) | 0.14 | ||
| ≥80 | 2.80 (1.84, 3.78) | −24.71 (−35.06, −12.72) | <0.001 | −19.62 (−34.77, −0.95) | 0.040 | ||
| Sex | Female | 3.01 (2.07, 4.19) | Referent | ||||
| Male | 2.87 (1.95, 4.04) | −3.17 (−5.68, −0.58) | 0.02 | −2.48 (−5.05, 0.17) | 0.07 | - | |
| Ethnicity | White | 2.94 (2.02, 4.11) | Referent | <0.001 † | |||
| Mixed/Multiple/Other | 3.35 (2.24, 4.73) | 12.78 (3.75, 22.59) | 0.005 | 11.83 (2.85, 21.59) | 0.009 * | ||
| South Asian | 3.46 (2.58, 4.64) | 16.26 (4.10, 29.85) | 0.008 | 16.21 (3.02, 31.10) | 0.015 * | ||
| Black/African/ Caribbean/Black British | 3.54 (2.39, 5.46) | 26.76 (4.79, 53.33) | 0.015 | 12.31 (−6.94, 35.54) | 0.23 | ||
| BMI, kg/m2 | <25 | 2.90 (2.03, 4.03) | Referent | 0.038 | |||
| 25–30 | 2.96 (2.04, 4.15) | 3.62 (0.91, 6.40) | 0.009 | 2.89 (0.18, 5.67) | 0.037 | ||
| >30 | 3.17 (2.02, 4.36) | 4.87 (1.56, 8.29) | 0.004 | 2.62 (−0.85, 6.21) | 0.14 | ||
| Highest educational level attained | Primary/Secondary | 3.02 (1.93, 4.29) | 1.05 (1.01, 1.10) | 0.018 | 1.73 (−2.36, 5.99) | 0.41 | 0.37 |
| Higher/further (A levels) | 2.98 (2.07, 4.14) | 1.03 (1.00, 1.07) | 0.07 | 1.59 (−2.14, 5.45) | 0.41 | ||
| College | 2.99 (2.07, 4.11) | 1.04 (1.01, 1.07) | 0.009 | 3.31 (0.52, 6.17) | 0.020 * | ||
| Post-graduate | 2.89 (1.98, 4.10) | Referent | |||||
| Quantiles of IMD rank | Q1 (most deprived) | 3.06 (2.12, 4.24) | 2.28 (−1.10, 5.79) | 0.19 | |||
| Q2 | 2.97 (1.98, 4.19) | 0.53 (−2.68, 3.85) | 0.75 | ||||
| Q3 | 2.90 (2.04, 4.01) | −1.34 (−4.42, 1.84) | 0.41 | ||||
| Q4 (least deprived) | 2.95 (2.01, 4.14) | Referent | |||||
| Tobacco smoking | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.20 (2.24, 4.20) | 1.22 (−4.65, 7.46) | 0.69 | ||||
| Vaping | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.37 (2.18, 4.37) | 5.47 (−2.73, 14.37) | 0.20 | ||||
| Alcohol, units/week | None | 2.97 (2.05, 4.20) | Referent | 0.71 | |||
| 1–7 | 3.03 (2.06, 4.14) | −0.68 (−3.63, 2.35) | 0.66 | 1.36 (−1.66, 4.48) | 0.38 | ||
| 8–14 | 2.94 (2.02, 4.16) | −1.87 (−5.21, 1.58) | 0.28 | 0.9 (−2.57, 4.48) | 0.62 | ||
| 15–21 | 2.91 (1.98, 4.01) | −3.31 (−7.46, 1.03) | 0.13 | 0.3 (−3.97, 4.76) | 0.89 | ||
| 22–28 | 2.65 (1.91, 3.94) | −6.32 (−11.90, −0.39) | 0.04 | −4.21 (−9.79, 1.71) | 0.16 | ||
| >28 | 2.84 (2.03, 4.18) | −3.06 (−9.56, 3.90) | 0.38 | 0.01 (−6.59, 7.07) | 1.00 | ||
| Light exercise, hours/week | 0–4 | 3.07 (2.10, 4.26) | 5.22 (2.21, 8.31) | 0.001 | 0.18 (−2.81, 3.26) | 0.91 | 0.92 |
| 5–9 | 2.95 (2.02, 4.13) | 1.29 (−1.54, 4.20) | 0.38 | −1.02 (−3.77, 1.82) | 0.48 | ||
| ≥10 | 2.88 (1.99, 4.06) | Referent | |||||
| Vigorous exercise, hours/week | 0 | 3.02 (2.05, 4.21) | 4.97 (1.82, 8.21) | 0.002 | 1.34 (−1.82, 4.6) | 0.41 | 0.45 |
| 1–3 | 2.98 (2.05, 4.12) | 3.09 (−0.01, 6.28) | 0.05 | 0.01 (−2.96, 3.06) | 1.00 | ||
| ≥4 | 2.86 (1.99, 4.06) | Referent | |||||
| Sleep, hours/night | ≤5 | 3.07 (2.13, 4.29) | 5.03 (0.31, 9.98) | 0.04 | 0.64 (−3.96, 5.45) | 0.79 | 0.94 |
| 6 | 3.00 (2.04, 4.19) | 1.12 (−2.18, 4.52) | 0.51 | −1.20 (−4.41, 2.11) | 0.47 | ||
| 7 | 2.92 (2.00, 4.08) | −1.82 (−4.66, 1.11) | 0.22 | −2.48 (−5.28, 0.39) | 0.39 | ||
| ≥8 | 2.96 (2.05, 4.13) | Referent | |||||
| Self−assessed general health | Excellent | 2.92 (2.05, 4.07) | Referent | 0.27 | |||
| Very good | 2.92 (2.01, 4.09) | −0.20 (−3.32, 3.02) | 0.90 | −2.35 (−5.36, 0.75) | 0.14 | ||
| Good | 3.03 (2.06, 4.23) | 4.73 (1.17, 8.42) | 0.009 | −0.86 (−4.34, 2.74) | 0.63 | ||
| Fair | 3.07 (2.02, 4.15) | 2.38 (−2.07, 7.03) | 0.30 | −1.96 (−6.45, 2.74) | 0.41 | ||
| Poor | 2.95 (2.04, 4.39) | 5.60 (−2.04, 13.84) | 0.16 | −6.73 (−14.02, 1.17) | 0.09 | ||
| Anxiety or depression | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 2.99 (2.03, 4.17) | 0.68 (−2.06, 3.50) | 0.63 | ||||
| Food choice | None | 2.96 (2.03, 4.13) | Referent | ||||
| Vegetarian | 2.92 (2.04, 4.13) | −2.98 (−8.46, 2.83) | 0.31 | ||||
| Vegan | 2.61 (2.00, 4.33) | −2.04 (−11.76, 8.74) | 0.70 | ||||
| Heart disease 3 | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.76 (1.93, 4.17) | 1.33 (−4.46, 7.48) | 0.66 | ||||
| Arterial disease 4 | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.96 (1.94, 4.14) | 2.02 (−3.09, 7.40) | 0.45 | ||||
| Hypertension | No | 2.98 (2.06, 4.16) | Referent | ||||
| Yes | 2.89 (1.92, 4.08) | −2.62 (−5.32, 0.14) | 0.06 | −4.09 (−6.94, −1.14) | 0.007 * | - | |
| Immunodeficiency disorder 5 | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.85 (1.98, 3.96) | −0.17 (−14.65, 16.77) | 0.98 | ||||
| Major neurological condition 6 | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.90 (1.84, 4.10) | −0.74 (−7.55, 6.57) | 0.84 | ||||
| Cancer | Never | 2.95 (2.04, 4.13) | Referent | ||||
| Past (cured or in remission) | 2.99 (2.00, 4.15) | 1.24 (−2.72, 5.35) | 0.55 | ||||
| Present (active) | 2.93 (1.89, 3.82) | −5.33 (−16.23, 7.00) | 0.38 | ||||
| Asthma | No | 2.97 (2.03, 4.14) | Referent | ||||
| Yes | 2.91 (2.06, 4.10) | −0.74 (−3.88, 2.51) | 0.65 | ||||
| COPD | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (1.98, 4.12) | 2.97 (−5.06, 11.67) | 0.48 | ||||
| Diabetic status | No diabetes | 2.97 (2.04, 4.14) | Referent | ||||
| Pre-diabetes | 2.68 (1.87, 3.93) | −4.13 (−10.31, 2.46) | 0.21 | ||||
| Type 1 diabetes | 3.13 (1.95, 4.10) | −1.81 (−14.31, 12.52) | 0.79 | ||||
| Type 2 diabetes | 2.95 (1.88, 4.30) | 0.77 (−5.09, 6.98) | 0.80 | ||||
| Atopy 7 | No | 2.95 (2.02, 4.14) | Referent | ||||
| Yes | 2.97 (2.05, 4.11) | −0.14 (−2.80, 2.59) | 0.92 | ||||
| Pre−vaccination SARS−COV−2 status | Seronegative | 2.74 (1.92, 3.80) | Referent | <0.001 | |||
| Seropositive, asymptomatic | 3.62 (2.40, 5.01) | 37.37 (31.83, 43.13) | <0.001 | 39.77 (34.73, 45.00) | <0.001 * | ||
| Seropositive, symptomatic | 5.50 (4.17, 12.62 | 126.30 (112.07, 141.5) | <0.001 | 105.06 (94.13, 116.60) | <0.001 * | ||
| Multivitamin | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.91 (2.05, 4.17) | −0.64 (−3.46, 2.26) | 0.66 | ||||
| Vitamin A | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.90 (1.94, 3.93) | −4.11 (−18.56, 12.91) | 0.61 | ||||
| Vitamin C | No | 2.95 (2.02, 4.11) | Referent | ||||
| Yes | 3.13 (2.10, 4.26) | 4.07 (0.10, 8.19) | 0.04 | 1.47 (−2.42, 5.52) | 0.46 | - | |
| Vitamin D | No | 2.98 (2.05, 4.12) | Referent | ||||
| Yes | 2.94 (2.02, 4.15) | −0.87 (−3.16, 1.49) | 0.47 | ||||
| Zinc | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.91 (2.07, 4.18) | 1.48 (−3.92, 7.18) | 0.60 | ||||
| Selenium | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.57 (1.85, 4.06) | −6.21 (−16.16, 4.92) | 0.26 | ||||
| Iron | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.80 (1.88, 4.10) | −3.18 (−9.42, 3.49) | 0.34 | ||||
| Probiotics | No | 2.97 (2.03, 4.13) | Referent | ||||
| Yes | 2.87 (2.08, 4.27) | 0.52 (−4.18, 5.45) | 0.83 | ||||
| Omega−3 fatty acids | No | 2.95 (2.02, 4.13) | Referent | ||||
| Yes | 3.01 (2.12, 4.21) | 3.08 (−0.51, 6.80) | 0.09 | 2.09 (−1.48, 5.79) | 0.26 | - | |
| Cod liver oil | No | 2.96 (2.04, 4.13) | Referent | ||||
| Yes | 2.97 (2.00, 4.12) | 1.15 (−2.90, 5.38) | 0.58 | ||||
| Garlic | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (2.26, 4.42) | 6.51 (−1.83, 15.55) | 0.13 | ||||
| Beta−2 adrenergic agonists | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.94 (2.05, 4.13) | 0.83 (−3.21, 5.04) | 0.69 | ||||
| Beta blockers | No | 2.97 (2.04, 4.13) | Referent | ||||
| Yes | 2.87 (1.94, 4.11) | −1.17 (−5.47, 3.32) | 0.60 | ||||
| Statins | No | 2.98 (2.06, 4.17) | Referent | ||||
| Yes | 2.86 (1.91, 4.01) | −2.52 (−5.51, 0.57) | 0.11 | ||||
| ACE inhibitors | No | 2.97 (2.05, 4.14) | Referent | ||||
| Yes | 2.89 (1.85, 4.09) | −1.85 (−5.55, 2.00) | 0.34 | ||||
| Proton pump inhibitors | No | 2.97 (2.05, 4.13) | Referent | ||||
| Yes | 2.91 (1.92, 4.16) | 0.26 (−3.00, 3.63) | 0.88 | ||||
| H2−receptor antagonists | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.02 (2.09, 4.20) | 1.57 (−11.84, 17.01) | 0.83 | ||||
| Inhaled corticosteroids | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.88 (2.03, 4.11) | −0.44 (−5.04, 4.39) | 0.86 | ||||
| Bronchodilators | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.97 (2.08, 4.18) | 2.06 (−1.95, 6.24) | 0.32 | ||||
| Systemic immunosuppressants | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.80 (1.99, 3.91) | −4.33 (−9.40, 1.02) | 0.11 | ||||
| Angiotensin receptor blockers | No | 2.96 (2.04, 4.14) | Referent | ||||
| Yes | 2.94 (1.93, 4.03) | −2.33 (−6.89, 2.45) | 0.33 | ||||
| SSRI antidepressants | No | 2.95 (2.03, 4.11) | Referent | ||||
| Yes | 3.17 (2.08, 4.33) | 5.15 (0.17, 10.39) | 0.04 | 0.18 (−4.69, 5.29) | 0.94 | - | |
| Non−SSRIs antidepressants | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 2.98 (2.01, 4.18) | 2.24 (−3.66, 8.51) | 0.46 | ||||
| Calcium channel blockers | No | 2.96 (2.05, 4.14) | Referent | ||||
| Yes | 2.94 (1.92, 4.11) | −1.54 (−5.23, 2.29) | 0.43 | ||||
| Thiazide diuretics | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.75 (1.84, 3.95) | −5.56 (−11.27, 0.52) | 0.07 | −2.77 (−8.88, 3.76) | 0.40 | - | |
| Vitamin K antagonists | No | 2.96 (2.03, 4.14) | Referent | ||||
| Yes | 2.94 (1.89, 3.81) | −5.97 (−17.55, 7.23) | 0.36 | ||||
| SGLT2 inhibitors | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.93 (2.44, 4.86) | 10.26 (−6.90, 30.57) | 0.26 | ||||
| Anticholinergics | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 3.04 (2.11, 4.18) | 3.08 (−2.38, 8.84) | 0.27 | ||||
| Metformin | No | 2.95 (2.03, 4.12) | Referent | ||||
| Yes | 3.20 (2.02, 4.46) | 6.77 (−0.44, 14.50) | 0.07 | 0.36 (−6.45, 7.66) | 0.92 | - | |
| Bisphosphonates | No | 2.96 (2.03, 4.13) | Referent | ||||
| Yes | 2.73 (1.99, 4.16) | −1.09 (−8.99, 7.50) | 0.80 | ||||
| Anti−platelet drugs | No | 2.97 (2.04, 4.14) | Referent | ||||
| Yes | 2.87 (1.88, 4.05) | 0.49 (−4.12, 5.32) | 0.84 | ||||
| Sex hormone therapy | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.06 (2.08, 4.18) | 0.53 (−3.77, 5.03) | 0.81 | ||||
| Aspirin 8 | No | 2.97 (2.04, 4.13) | Referent | ||||
| Yes | 2.84 (1.89, 4.11) | 0.01 (−5.09, 5.39) | 1.00 | ||||
| Paracetamol 8 | No | 2.95 (2.03, 4.13) | Referent | ||||
| Yes | 3.03 (1.99, 4.23) | 2.81 (−2.82, 8.76) | 0.34 | ||||
| BCG vaccinated | No | 2.86 (2.05, 4.07) | Referent | ||||
| Yes | 3.00 (2.05, 4.17) | 2.77 (−0.92, 6.59) | 0.65 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jolliffe, D.A.; Faustini, S.E.; Holt, H.; Perdek, N.; Maltby, S.; Talaei, M.; Greenig, M.; Vivaldi, G.; Tydeman, F.; Symons, J.; et al. Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK). Vaccines 2022, 10, 1601. https://doi.org/10.3390/vaccines10101601
Jolliffe DA, Faustini SE, Holt H, Perdek N, Maltby S, Talaei M, Greenig M, Vivaldi G, Tydeman F, Symons J, et al. Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK). Vaccines. 2022; 10(10):1601. https://doi.org/10.3390/vaccines10101601
Chicago/Turabian StyleJolliffe, David A., Sian E. Faustini, Hayley Holt, Natalia Perdek, Sheena Maltby, Mohammad Talaei, Matthew Greenig, Giulia Vivaldi, Florence Tydeman, Jane Symons, and et al. 2022. "Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK)" Vaccines 10, no. 10: 1601. https://doi.org/10.3390/vaccines10101601
APA StyleJolliffe, D. A., Faustini, S. E., Holt, H., Perdek, N., Maltby, S., Talaei, M., Greenig, M., Vivaldi, G., Tydeman, F., Symons, J., Davies, G. A., Lyons, R. A., Griffiths, C. J., Kee, F., Sheikh, A., Shaheen, S. O., Richter, A. G., & Martineau, A. R. (2022). Determinants of Antibody Responses to SARS-CoV-2 Vaccines: Population-Based Longitudinal Study (COVIDENCE UK). Vaccines, 10(10), 1601. https://doi.org/10.3390/vaccines10101601





