Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Vaccination
2.4. Pregnancy Outcomes and Events of Interest
2.5. Statistical Methods
3. Results
Outcomes
4. Discussion
4.1. Main Findings
4.2. Strengths and Limitations
4.3. Interpretation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and control of seasonal influenza with vaccines: Recommendations of the advisory committee on immunization practices—United States, 2020–2021 influenza season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetrics and Gynecology. ACOG committee opinion no. 732: Influenza vaccination during pregnancy. Obstet. Gynecol. 2018, 131, e109–e114. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Blanton, L.H.; Ferdinands, J.M.; Chung, J.R.; Broder, K.R.; Talbot, H.K.; Morgan, R.L.; Fry, A.M. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022–2023 Influenza Season. MMWR Recomm. Rep. 2022, 71, 1–28. [Google Scholar] [CrossRef]
- Phadke, V.K.; Omer, S.B. Maternal vaccination for the prevention of influenza: Current status and hopes for the future. Expert. Rev. Vaccines 2016, 15, 1255–1280. [Google Scholar] [CrossRef]
- Holstein, R.; Dawood, F.S.; O’Halloran, A.; Cummings, C.; Ujamaa, D.; Kirley, P.D.; Yousey-Hindes, K.; Fawcett, E.; Monroe, M.L.; Kim, S.; et al. Characteristics and outcomes of hospitalized pregnant women with influenza, 2010 to 2019: A repeated cross-sectional study. Ann. Intern. Med. 2022, 175, 149–158. [Google Scholar] [CrossRef]
- Rasmussen, S.A.; Jamieson, D.J.; Uyeki, T.M. Effects of influenza on pregnant women and infants. Am. J. Obstet. Gynecol. 2012, 207, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, E.O.; Vazquez-Benitez, G.; Romitti, P.A.; Naleway, A.L.; Cheetham, T.C.; Lipkind, H.S.; Klein, N.P.; Lee, G.; Jackson, M.L.; Hambidge, S.J.; et al. First trimester influenza vaccination and risks for major structural birth defects in offspring. J. Pediatr. 2017, 187, 234–239.e4. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.G.; Kieke, B.A.; King, J.P.; Mascola, M.A.; Shimabukuro, T.T.; DeStefano, F.; Hanson, K.E.; McClure, D.L.; Olaiya, O.; Glanz, J.M.; et al. Inactivated influenza vaccine and spontaneous abortion in the vaccine safety datalink in 2012–13, 2013–2014, and 2014–15. Vaccine 2019, 37, 6673–6681. [Google Scholar] [CrossRef]
- Louik, C.; Kerr, S.; Van Bennekom, C.M.; Chambers, C.; Jones, K.L.; Schatz, M.; Mitchell, A.A. Safety of the 2011–12, 2012–2013, and 2013–14 seasonal influenza vaccines in pregnancy: Preterm delivery and specific malformations, a study from the case-control arm of VAMPSS. Vaccine 2016, 34, 4450–4459. [Google Scholar] [CrossRef]
- Zerbo, O.; Modaressi, S.; Chan, B.; Goddard, K.; Lewis, N.; Bok, K.; Fireman, B.; Klein, N.P.; Baxter, R. No association between influenza vaccination during pregnancy and adverse birth outcomes. Vaccine 2017, 35, 3186–3190. [Google Scholar] [CrossRef]
- Chambers, C.D.; Johnson, D.L.; Xu, R.; Luo, Y.J.; Louik, C.; Mitchell, A.A.; Schatz, M.; Jones, K.L.; Otis Collaborative Research Group. Safety of the 2010–11, 2011–2012, 2012–13, and 2013–14 seasonal influenza vaccines in pregnancy: Birth defects, spontaneous abortion, preterm delivery, and small for gestational age infants, a study from the cohort arm of VAMPSS. Vaccine 2016, 34, 4443–4449. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi, H.; Kahn, K.E.; Black, C.L.; Lindley, M.C.; Jatlaoui, T.C.; Fiebelkorn, A.P.; Havers, F.P.; D’Angelo, D.V.; Cheung, A.; Ruther, N.A.; et al. Influenza and tdap vaccination coverage among pregnant women—United States, April 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; McLean, H.Q. Influenza vaccine effectiveness: Defining the H3N2 problem. Clin. Infect. Dis. 2019, 69, 1817–1823. [Google Scholar] [CrossRef]
- Flannery, B.; Kondor, R.J.G.; Chung, J.R.; Gaglani, M.; Reis, M.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S.; et al. Spread of Antigenically Drifted Influenza A(H3N2) Viruses and Vaccine Effectiveness in the United States During the 2018–2019 Season. J. Infect. Dis. 2020, 221, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Boikos, C.; Gelone, D.K.; Gandhi, A. Influenza vaccines: The potential benefits of cell-culture isolation and manufacturing. Ther. Adv. Vaccines Immunother. 2020, 8, 2515135520908121. [Google Scholar] [CrossRef] [PubMed]
- Zost, S.J.; Parkhouse, K.; Gumina, M.E.; Kim, K.; Diaz Perez, S.; Wilson, P.C.; Treanor, J.J.; Sant, A.J.; Cobey, S.; Hensley, S.E. Contemporary H3N2 influenza viruses have a glycosylation site that alters binding of antibodies elicited by egg-adapted vaccine strains. Proc. Natl Acad Sci. USA 2017, 114, 12578–12583. [Google Scholar] [CrossRef] [PubMed]
- Rajaram, S.; Van Boxmeer, J.; Leav, B.; Suphaphiphat, P.; Iheanacho, I.; Kistler, K. Retrospective evaluation of mismatch from egg-based isolation of influenza strains compared to cell-based isolation and the possible implications for vaccine effectiveness. In Proceedings of the IDWeek 2018, San Francisco, CA, USA, 3–7 October 2018. [Google Scholar]
- Katz, J.M.; Naeve, C.W.; Webster, R.G. Host cell-mediated variation in H3N2 influenza viruses. Virology 1987, 156, 386–395. [Google Scholar] [CrossRef]
- Rocha, E.P.; Xu, X.; Hall, H.E.; Allen, J.R.; Regnery, H.L.; Cox, N.J. Comparison of 10 influenza A (H1N1 and H3N2) haemagglutinin sequences obtained directly from clinical specimens to those of MDCK cell- and egg-grown viruses. J. Gen. Virol. 1993, 74 Pt 11, 2513–2518. [Google Scholar] [CrossRef]
- Izurieta, H.S.; Chillarige, Y.; Kelman, J.; Wei, Y.; Lu, Y.; Xu, W.; Lu, M.; Pratt, D.; Chu, S.; Wernecke, M.; et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J. Infect. Dis. 2019, 220, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Eick-Cost, A.; Hu, Z. Relative effectiveness of cell-based influenza vaccines compared to egg-based influenza vaccines, active component U.S. Service members, 2017–2018 season. In Proceedings of the International Conference on Emerging Infectious Diseases, Atlanta, GA, USA, 26–29 August 2018; p. 54. [Google Scholar]
- Klein, N.P.; Fireman, B.; Goddard, K.; Zerbo, O.; Asher, J.; Zhou, J.; King, J.; Lewis, N. LB15. Vaccine effectiveness of flucelvax relative to inactivated influenza vaccine during the 2017–18 influenza season in Northern California. Open Forum Infect. Dis. 2018, 5, S764. [Google Scholar] [CrossRef]
- Barr, I.G.; Donis, R.O.; Katz, J.M.; McCauley, J.W.; Odagiri, T.; Trusheim, H.; Tsai, T.F.; Wentworth, D.E. Cell culture-derived influenza vaccines in the severe 2017–2018 epidemic season: A step towards improved influenza vaccine effectiveness. NPJ Vaccines 2018, 3, 44. [Google Scholar] [CrossRef]
- Boikos, C.; Sylvester, G.C.; Sampalis, J.S.; Mansi, J.A. Relative effectiveness of the cell-cultured quadrivalent influenza vaccine compared to standard, egg-derived quadrivalent influenza vaccines in preventing influenza-like illness in 2017–2018. Clin. Infect. Dis. 2020, 71, e665–e671. [Google Scholar] [CrossRef] [PubMed]
- Boikos, C.; Fischer, L.; O’Brien, D.; Vasey, J.; Sylvester, G.C.; Mansi, J.A. Relative effectiveness of the cell-derived inactivated quadrivalent influenza vaccine versus egg-derived inactivated quadrivalent influenza vaccines in preventing influenza-related medical encounters during the 2018–2019 influenza season in the United States. Clin. Infect. Dis. 2021, 73, e692–e698. [Google Scholar] [CrossRef] [PubMed]
- Seqirus, Inc. Flucelvax Quadrivalent (Influenza Vaccine) Prescribing Information; Seqirus USA Inc.: Summit, NJ, USA, 2021. [Google Scholar]
- US Food and Drug Administration. Guidance for Industry: Establishing Pregnancy Exposure Registries. Available online: https://www.fda.gov/media/75607/download (accessed on 22 April 2022).
- Public Policy Committee International Society of Pharmacoepidemiology. Guidelines for good pharmacoepidemiology practice (GPP). Pharmacoepidemiol. Drug Saf. 2016, 25, 2–10. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull. World Health Organ. 2001, 79, 373–374. [Google Scholar]
- Centers for Disease Control and Prevention. Metropolitan Atlanta Congenital Defects Program (MACDP). Available online: https://www.cdc.gov/ncbddd/birthdefects/macdp.html (accessed on 9 February 2022).
- Correa, A.; Cragan, J.D.; Kucik, J.E.; Alverson, C.J.; Gilboa, S.M.; Balakrishnan, R.; Strickland, M.J.; Duke, C.W.; O’Leary, L.A.; Riehle-Colarusso, T.; et al. Reporting birth defects surveillance data 1968–2003. Birth Defects Res. Clin. Mol. Teratol. 2007, 79, 65–186. [Google Scholar] [CrossRef]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.; Curtin, S.C.; Matthews, T.J. Births: Final data for 2013. Natl. Vital Stat. Rep. 2015, 64, 1–65. [Google Scholar] [PubMed]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K. Births in the United States, 2019. NCHS Data Brief 2020, 346, 1–8. [Google Scholar]
- Hoyert, D.L.; Gregory, E.C.W. Cause-of-death data from the fetal death file, 2015–2017. Natl. Vital Stat. Rep. 2020, 69, 1–20. [Google Scholar]
- Martin, J.A.; Hamilton, B.E.; Osterman, M.J.K.; Driscoll, A.K. Births: Final data for 2018. Natl. Vital Stat. Rep. 2019, 68, 1–47. [Google Scholar]
- Covington, D.; Veley, K.; Buus, R.; Churchill, P. Factors associated with losses to follow-up in pregnancyexposure registries. Pharmacoepidemiol. Drug Saf. 2020, 29, 536. [Google Scholar]
- American College of Obstetricians Gynecologists’ Committee on Practice Bulletins-Gynecology. ACOG practice bulletin no. 200: Early pregnancy loss. Obstet. Gynecol. 2018, 132, e197–e207. [Google Scholar] [CrossRef] [PubMed]
- U. S. Census Bureau. Quick Facts. Available online: https://www.census.gov/quickfacts/fact/table/US/PST045219 (accessed on 27 April 2022).
- Office of Disease Prevention and Health Promotion. IID-12.10 Increase the Percentage of Pregnant Women Who Are Vaccinated against Seasonal Influenza. Available online: https://www.healthypeople.gov/node/4660/data_details (accessed on 27 April 2022).
| Outcome, n, % (95% CI) | Vaccine Exposure | Overall | ||
|---|---|---|---|---|
| First Trimester a | Second Trimester | Third Trimester | ||
| All enrolled persons | n = 196 | n = 286 | n = 211 | N = 693 |
| Primary analysis population | n = 178 | n = 277 | n = 210 | N = 665 |
| Enrollment < 20 weeks of gestation | n = 147 | n = 64 | N/A | N = 211 |
| Live birth, n % (95% CI) | 172 96.6 (92.8–98.8) | 277 100 (98.7–100) | 210 100 (98.3–100) | 659 99.1 (98.0–99.7) |
| Stillbirth | 0 0 (0.0–2.1) | 0 0 (0.0–1.3) | 0 0 (0.0–1.7) | 0 0 (0.0–0.6) |
| Spontaneous abortion b | 4 2.7 (0.7–6.8) | 0 0 (0.0–5.6) | N/A | 4 1.9 (0.5–4.8) |
| Elective termination b | 1 0.7 (0.0–3.7) | 0 0 (0.0–5.6) | N/A | 1 0.5 (0.0–2.6) |
| Outcome | Vaccine Exposure | Overall | ||
|---|---|---|---|---|
| First Trimester a | Second Trimester | Third Trimester | ||
| Persons | n = 178 | n = 277 | n = 210 | n = 665 |
| Infants | n = 178 | n = 282 | n = 212 | n = 673 |
| Sex, n (%) | ||||
| Male | 84 (47.2) | 136 (48.2) | 111 (52.4) | 331 (49.2) |
| Female | 88 (49.4) | 146 (51.8) | 101 (47.6) | 335 (49.8) |
| Missing data | 6 (3.4) | 0 | 0 | 7 (1.0) a |
| Birthweight | n = 172 | n = 278 | n = 211 | n = 661 |
| Mean ± SD, g | 3295.4 ± 562.3 | 3236.1 ± 561.3 | 3313.4 ± 506.3 | 3276.2 ± 544.9 |
| Mean gestational age at outcome ± SD, weeks | 37.9 (5.1) | 38.5 (2.1) | 39.1 (1.2) | 38.5 (3.0) |
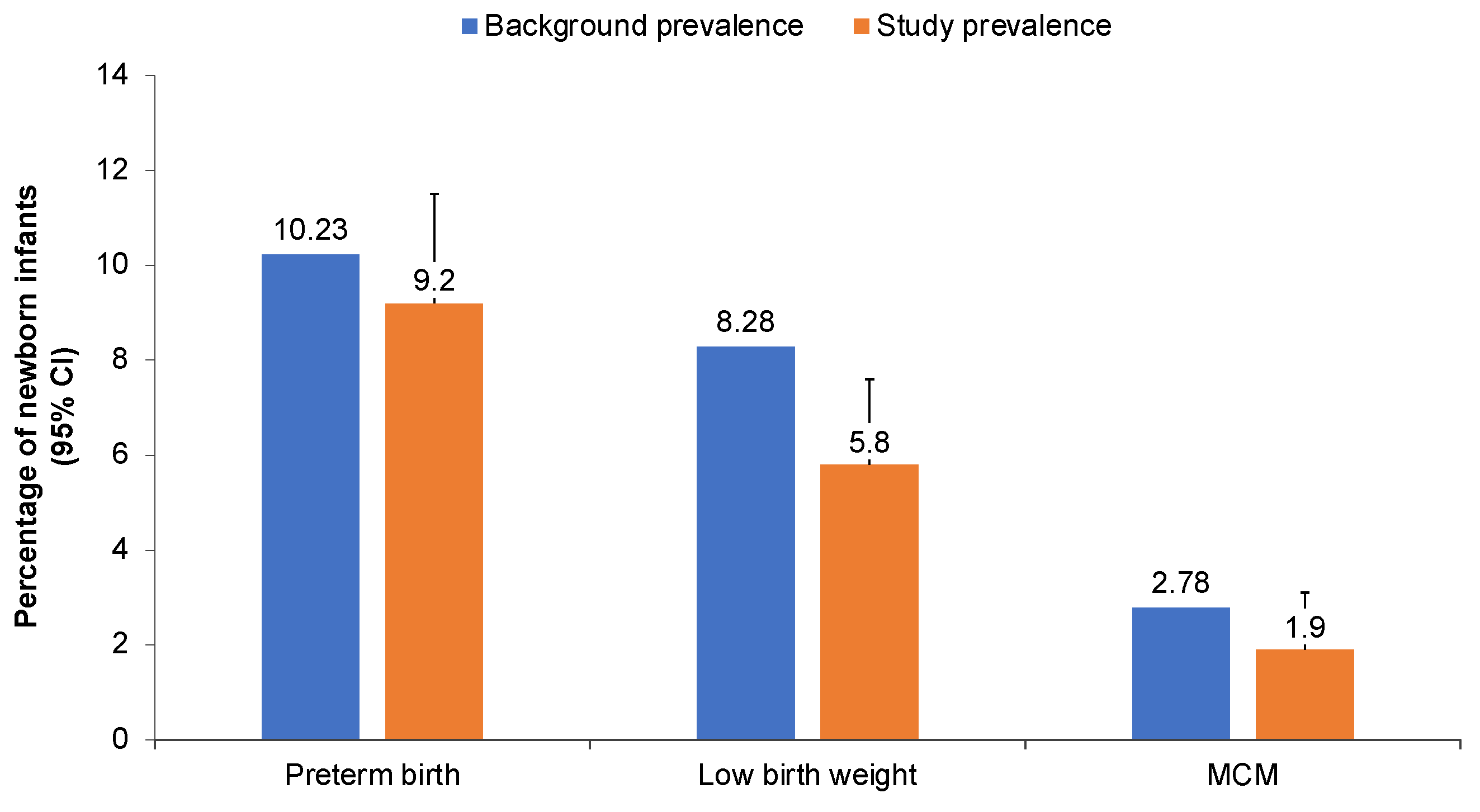
| Preterm birth b | n = 166 | n = 250 | n = 149 | n = 565 |
| n, % (95% upper CI) | 17 10.3 (15.1) | 27 10.8 (14.6) | 8 5.4 (9.5) | 52 9.2 (11.5) |
| Low birthweight c | n = 170 | n = 264 | n = 203 | n = 637 |
| n, % (95% upper CI) | 14 8.3 (12.6) | 15 5.7 (8.6) | 8 3.9 (7.0) | 37 5.8 (7.6) |
| MCMs (live-born infants) | n = 173 | n = 282 | n = 212 | n = 667 |
| n, % (95% upper CI) | 1 0.6 (2.7) | 7 2.5 (4.6) | 5 2.4 (4.9) | 13 1.9 (3.1) |
| Participant | Timing of Exposure (Week of Gestation) | Preferred Term |
|---|---|---|
| 1 | 5.4 | Sex chromosome: XYY in a male |
| 2 | 10.7 | Talipes equinovarus |
| 3 | 16.1 | Polycystic kidneys |
| 4 | 16.1 | Renal agenesis, right: MACDP term “Kidney—absence, agenesis, dysplasia, or hypoplasia unilateral, right” |
| 5 | 16.4 | Clubfoot |
| Cardiomegaly | ||
| Aorta malformation a | ||
| Hypoplasia of upper limb, hypoplasia of lower limb | ||
| 6 | 18.1 | Situs inversus abdominus |
| 7 | 19.9 | Hirschsprung’s disease |
| 8 | 23 | Fluid around kidneys a |
| 9 | 24.9 | Micropenis |
| Microphthalmos | ||
| 10 | 30.3 | Transposition of great vessels |
| 11 | 32.1 | Trisomy 21 |
| Atrial septal defect | ||
| Patent ductus arteriosus | ||
| 12 | 33 | Absent foreskin |
| 13 | 33.3 | Hypospadias |
| 14 | 33.4 | Absent forearm only, left |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, C.; Van Boxmeer, J.; Tilson, H.; Scialli, A.; Vanchiere, J.A.; Ides, E.; Sawlwin, D.; Molrine, D.; Hohenboken, M.; Edelman, J.; et al. Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study. Vaccines 2022, 10, 1600. https://doi.org/10.3390/vaccines10101600
Robinson C, Van Boxmeer J, Tilson H, Scialli A, Vanchiere JA, Ides E, Sawlwin D, Molrine D, Hohenboken M, Edelman J, et al. Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study. Vaccines. 2022; 10(10):1600. https://doi.org/10.3390/vaccines10101600
Chicago/Turabian StyleRobinson, Christopher, Josephine Van Boxmeer, Hugh Tilson, Anthony Scialli, John A. Vanchiere, Ellis Ides, Daphne Sawlwin, Deborah Molrine, Matthew Hohenboken, Jonathan Edelman, and et al. 2022. "Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study" Vaccines 10, no. 10: 1600. https://doi.org/10.3390/vaccines10101600
APA StyleRobinson, C., Van Boxmeer, J., Tilson, H., Scialli, A., Vanchiere, J. A., Ides, E., Sawlwin, D., Molrine, D., Hohenboken, M., Edelman, J., & Albano, J. D. (2022). Outcomes in Pregnant Persons Immunized with a Cell-Based Quadrivalent Inactivated Influenza Vaccine: A Prospective Observational Cohort Study. Vaccines, 10(10), 1600. https://doi.org/10.3390/vaccines10101600






