Abstract
In China, the vaccination strategy against pertussis is started from 3 months of age, with no booster dose used after the booster given at two years. Despite a high vaccination coverage, pertussis has been increasingly reported since the last decade. This study evaluates the prevalence of serum anti-pertussis toxin (PT) IgG antibodies in adults at childbearing age and infants before the age of primary immunization in Beijing, China. A total of 1175 serum samples randomly selected from individuals who attended an annual health examination at the Sixth Medical Center of the PLA General Hospital, Beijing, in 2019, was included. The geometric mean concentration (GMC) and median concentration of anti-PT IgG antibodies among adults aged 20–39 years were 3.81 IU/mL and 3.24 IU/mL, and the corresponding concentrations were 1.72 IU/mL and 1.43 IU/mL among infants under 3 months of age. The seroprevalence of PT IgG antibodies ≥ 40 IU/mL in adults and infants was 2.0% (15/735) and 1.1% (5/440). In total, 65.99% (485/735) of adults and 83.41% (367/440) of infants had non-detectable pertussis-specific antibodies (<5 IU/mL). Our results showed that the majority of adults at a reproductive age and young infants are vulnerable to pertussis, suggesting that booster vaccinations in adults should be considered in this country.
1. Introduction
Whooping cough, or pertussis, is an acute respiratory infection and is mainly caused by the Gram-negative bacillus Bordetella pertussis. Infants too young to be fully vaccinated are at the highest risk of contracting pertussis, and hospitalizations and mortality rates are high in infants under 3 months of age [1]. In China, whole-cell pertussis vaccines (WPVs) were introduced in the 1960s. Since the implementation of the national immunization program in 1978, the incidence and mortality of pertussis have decreased significantly. A combined diphtheria–tetanus–acellular pertussis (DTaP) vaccine has been in use from 2007, and completely replaced the diphtheria–tetanus–whole pertussis (DTwP) vaccine by 2013. However, over the following few years, the incidence of pertussis has continued to rise (Figure 1) [2,3]. The Chinese vaccination strategy is primarily administered with three doses of DTaP vaccines at the age of 3, 4, and 5 months, with a booster dose given at 18–24 months. After the booster dose at two years of age, no booster dose is given. This vaccination strategy has remained unchanged since the 1980s. Therefore, most adults at childbearing age and young infants may not have enough protective antibodies such as anti-pertussis toxin (PT) IgG antibodies. A recent cross-sectional study conducted in Beijing, China, compared the seroprevalence of anti-PT IgG antibodies in samples collected during two study periods (2010 and 2015/2016) and found that the seroprevalence of anti-PT IgG antibodies (≥40 IU/mL indicative of a pertussis infection within past two years) was 5.1% in 2010 and 4.0% in 2015/2016. Moreover, the rate of undetectable anti-PT IgG antibodies (<5 IU/mL) has significantly increased between the two periods [4]. It is well known that parents and other family members are the main source of infection for infants and young children [5,6]. Newborns are mainly protected from maternal antibodies [7,8]. The concentrations of anti-pertussis antibodies in cord blood samples are equal to or even higher than those observed in the mothers. However, Meng et al. compared 194 paired maternal and cord blood samples collected in Beijing from 2016 to 2017 and found that most pregnant women (70.1%) and newborns (74.8%) were generally lacking protective antibodies to pertussis [9]. In general, data on the occurrence of pertussis between adults at childbearing age and young infants are quite limited. Even in the above-mentioned studies, the number of subjects was not sizable.
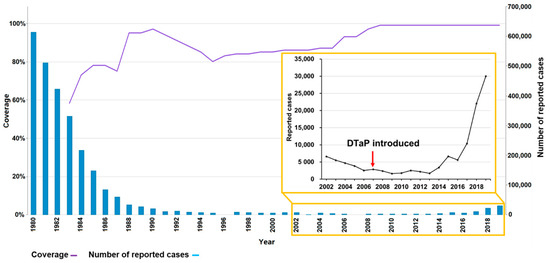
Figure 1.
DTP3 vaccination coverage and number of reported pertussis cases in China, 1980–2019 [2,3]. DTP3: diphtheria and tetanus toxoids and pertussis (DTP)-containing vaccine, 3rd dose. This is an adaptation of an original work “Comparison of Immunization coverage for Diphtheria Tetanus Toxoid and Pertussis (DTP) vaccination coverage and Reported cases for Pertussis. Geneva: World Health Organization (WHO); 2021. Licence: CC BY-NC-SA 3.0 IGO”.
In this present study, we aim to evaluate the level of serum anti-PT IgG antibodies in adults at childbearing age and infants under 3 months of age in Beijing to better understand the seroepidemiology of pertussis in these populations, and to provide important information on how effective the current immunization strategy in China is.
2. Materials and Methods
2.1. Study Subjects and Serum Samples
Serum samples were collected from adults and infants who attended annual health examinations at the Sixth Medical Center of People Liberation Army General Hospital, Beijing, in 2019. Altogether, 1631 sera from adults at a childbearing age (20–39 years old) and 440 sera from infants under 3 months of age were collected. Of these, 735 (45.06%) sera from adults and 440 (100%) sera from infants were included in this study. The only information collected from the study subjects was age, gender, and date of sampling. The information on the vaccination status of these subjects was not collected. Because the first dose of DTaP vaccine is given at 3 months of age, all infants were considered unvaccinated. Since pertussis vaccination was introduced in 1960s, many adults should have received pertussis vaccines.
2.2. Serological Testing
Commercial ELISA kits (Institut Virion/Serion GmbH, Würzburg, Germany) were used to determine concentration of anti-PT IgG antibodies according to the manufacturer’s instructions. The interpretation of the results was described previously [10]. Antibodies greater than or equal to 100 IU/mL indicated a recent infection within one year, and a value between 40 and 100 IU/mL indicated an infection within the past few years. When the concentration values were below 5 IU/mL, it was considered that no antibodies were detected. When the concentration was between 5 and 40 IU/mL, it was considered seronegative.
2.3. Statistical Analysis
Data were analyzed using the GraphPad Prism 7 version (San Diego, CA, USA) and SPSS version 25.0 (SPSS Inc., Chicago, IL, USA). Serum anti-PT IgG concentrations in different age groups and genders were analyzed by a normality test. Normally distributed continuous variables of the two groups were compared using a Student’s t-test. Non-normally distributed continuous variables of the two groups were compared using the Mann–Whitney U test. Seroprevalence and the proportion of subjects with non-detectable anti-PT IgG between adults and infants were compared by the Chi-square test. Two-tailed p values < 0.05 were considered statistically significant.
3. Results
A total of 1175 serum samples was included in this study. Of them, 735 included 328 females and 407 males aged 20–39 years, and 440 included 218 females and 222 males aged under 3 months old (Table 1). Among the infant samples, 248 cases were one month old, and 192 cases were two months old.

Table 1.
Characteristics of 1175 study subjects and their serum anti-PT IgG antibodies.
The geometric mean concentration (GMC) and median concentration of anti-PT IgG antibodies among adult subjects were 3.81 IU/mL and 3.24 IU/mL. No difference was found between men and women. Altogether, 15 (2.0%) subjects had anti-PT IgG antibodies higher than 40 IU/mL. There was no statistical difference between men and women (p = 0.37) (Table 1). Among these 15 subjects, 14 (1.9%), including 9 men and 4 women, had a concentration between about 40 and 100 IU/mL, and 1 (0.1%) had a concentration ≥ 100 IU/mL. The non-detectable rate of anti-PT IgG antibodies was 66.0% (Figure 2). No statistical difference in the prevalence of non-detectable anti-PT IgG antibodies was noticed between men and women (p = 0.48).
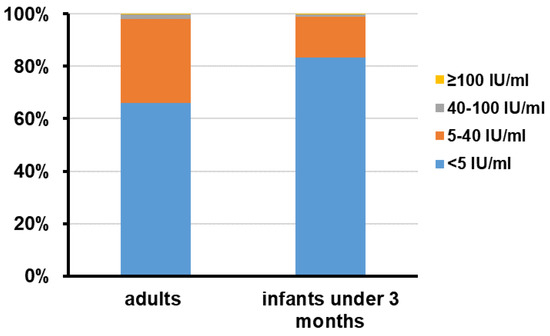
Figure 2.
Distribution of serum PT IgG antibodies concentrations in adults at childbearing age and infants under 3 months old. The number of serum specimens with PT IgG antibodies concentrations ≥ 100 IU/mL, 40~100 IU/mL, 5~40 IU/mL, and <5 IU/mL in each group was calculated and the data are shown as percentage.
Among 440 infants, the GMC and median concentrations of serum anti-PT IgG antibodies were 1.72 IU/mL and 1.43 IU/mL (Table 1). There were five (1.1%) subjects who had antibodies higher than 40 IU/mL. Among these five subjects, four (0.9%) had a concentration between about 40 and 100 IU/mL, and one (0.2%) had a concentration ≥ 100 IU/mL. There were 367 (83.4%) infants who had non-detectable anti-PT IgG antibodies (Figure 2). The GMC and median concentration of anti-PT IgG antibodies were 1.73 IU/mL and 1.43 IU/mL among one-month-old infants, and 1.71 IU/mL and 3.17 IU/mL among two-month-old infants. However, no differences were observed between the two groups (p = 0.56) (Figure 3).
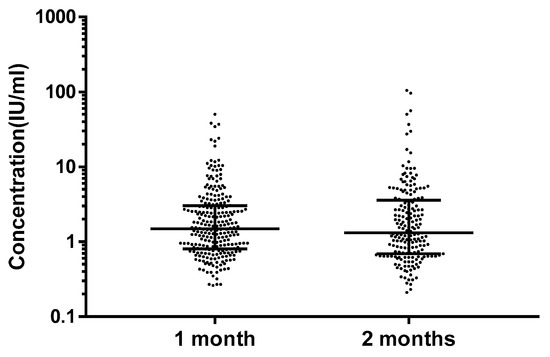
Figure 3.
Serum PT IgG antibodies concentrations of one-month-old and two-month-old infants. The data are shown as median interquartile range. No statistical difference was observed between the two groups (p = 0.56).
Although there was no difference in the level of serum anti-PT IgG antibodies between groups of infants and adults (p = 0.25), the non-detectable rate of anti-PT IgG antibodies was significantly higher in infants than in adults (83.4% vs. 66.0%, p < 0.001).
4. Discussion
Pertussis is still a significant public health problem throughout the world. It is well known that pertussis is no longer just a childhood disease. Many studies have reported the occurrence of adult pertussis [4,11,12,13]. Typical clinical characteristics of pertussis include a paroxysmal cough and whooping. In adults, however, the typical symptoms of pertussis are not often present, and atypical symptoms such as a persistent cough are not uncommon [14,15]. Due to the increase in atypical clinical cases and asymptomatic infections, as with many countries, the incidence of pertussis in China is also most likely under-reported [16,17,18,19]. Similarly, we also found the seroprevalence of 2.0% in adults aged 20~39 years old in our study. It has been more than 18 years since they were vaccinated. Therefore, those adults who are at a childbearing age who had antibodies ≥ 40 IU/mL were considered to have a real B. pertussis infection and are probably the main source of infant pertussis.
In this study, we found that, in 2019, 66% of adults at a childbearing age and 83.4% of young infants before the age of the first dose of pertussis vaccination did not have detectable anti-PT IgG antibodies, showing that they were vulnerable to pertussis. This finding was in line with a recent study carried out in Beijing during the period of 2016–2017, in which 70.1% (95% CI: 63.6–76.1%) of mothers and 74.8% (95% CI: 68.2–80.3%) of newborns did not have detectable serum anti-PT IgG antibodies [9].
Several earlier studies have shown that the level of anti-PT IgG antibodies in cord bloods is close to or higher than that of maternal antibodies. The level gradually decreases in the first few months after birth, and the antibody concentration becomes negligible by 2–4 months [20,21]. In a recent study, the half-life of pertussis-specific antibodies in infants induced by the maternal Tdap vaccination (29–36 days) was shorter than previously reported data in the pre-Tdap era (5–6 weeks) [22]. In our study, although 1.1% of infants under three months of age had positive PT IgG antibodies, we could not differentiate whether they had a real pertussis infection, or if they were from maternal antibodies. No statistical difference was found in the level of antibody concentrations between one-month-old and two-month-old infants. In addition, we did not observe differences between men and women.
Vaccination is the most effective way to prevent pertussis. Although the percentages of DTaP vaccination coverage in children aged 3, 4, and 5 months were very high in China in 2019 (99%), pertussis protection in terms of the prevalence of children in the target vaccination population with vaccine-induced pertussis protection (83.8%) was not sufficient to establish herd immunity and block the transmission of B. pertussis with values of Ro ≥ 10 in the community [23]. Since pertussis is common in adolescents, a booster immunization in the age group is recommended in many industrial countries. In response to nationwide outbreaks in 2012, maternal immunization with the tetanus, diphtheria, and acellular pertussis (Tdap) vaccine was introduced in the UK and USA [24]. The use of maternal immunization clearly provides protection to infants who are too young to be vaccinated [25,26,27]. At present, several countries with a high incidence of pertussis have implemented the strategy of maternal immunizations [28]. However, so far, no booster immunizations in China have been recommended [29].
5. Conclusions
Our results showed that, in China, about two-thirds of adults at childbearing age and more than 80% of infants before the age of primary pertussis immunizations did not have pertussis-specific antibodies, suggesting that they were vulnerable to pertussis. To protect against pertussis in these populations, booster vaccinations in adults should be considered in China.
Author Contributions
Conceptualization, Q.H.; methodology, Z.C., J.P., N.Z. and N.C.; resources, Y.D.; writing—original draft preparation, Z.C. and J.P.; writing—review and editing, Q.H.; project administration, N.C.; funding acquisition, Q.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Beijing Natural Science Foundation and Haidian Inovation Joint Project, grant number 19L2043.
Institutional Review Board Statement
The study was approved by the Ethics Committee of Capital Medical University, Beijing, China (2020SY008).
Informed Consent Statement
Not applicable.
Data Availability Statement
Reported pertussis cases and DTP3 coverage in China during 1980–2019 were summarized and visualized according to data from the WHO Immunization data pool at https://immunizationdata.who.int/compare.html?COMPARISON=type1__WIISE/MT_AD_COV_LONG+type2__WIISE/MT_AD_INC_LONG+option1__DTP_coverage+option2__PERTUSSIS_cases&CODE=CHN&YEAR= (accessed on 13 September 2021), and the National Health Commission of the People’s Republic of China at http://www.nhc.gov.cn/jkj/s7923/202108/7337fd75c8b749309a2de28aec1a03bd.shtml (accessed on 13 September 2021). Other data presented in this study are available on request from the corresponding author. The data are not publicly available due to intellectual property considerations.
Acknowledgments
We would like to thank Tom Hamilton for English editing of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Masseria, C.; Martin, C.K.; Krishnarajah, G.; Becker, L.K.; Buikema, A.; Tan, T.Q. Incidence and Burden of Pertussis among Infants Less Than 1 Year of Age. Pediatr. Infect. Dis. J. 2017, 36, e54–e61. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Immunization Data: Comparison of Immunization Coverage for DTP Vaccination Coverage and Reported Cases for Pertussis. Available online: https://immunizationdata.who.int/compare.html?COMPARISON=type1__WIISE/MT_AD_COV_LONG+type2__WIISE/MT_AD_INC_LONG+option1__DTP_coverage+option2__PERTUSSIS_cases&CODE=CHN&YEAR= (accessed on 13 September 2021).
- National Health Commission of the People’s Republic of China. Epidemic Situations of Legal Infectious Diseases in China. Available online: http://www.nhc.gov.cn/jkj/s7923/202108/7337fd75c8b749309a2de28aec1a03bd.shtml (accessed on 13 September 2021).
- Zhang, Y.; Chen, Z.; Zhao, J.; Zhang, N.; Chen, N.; Zhang, J.; Li, S.; He, Q. Increased susceptibility to pertussis in adults at childbearing age as determined by comparative seroprevalence study, China 2010–2016. J. Infect. 2019, 79, 1–6. [Google Scholar] [CrossRef]
- Skoff, T.H.; Kenyon, C.; Cocoros, N.; Liko, J.; Miller, L.; Kudishet, K.; Baumbach, J.; Zansky, S.; Faulkner, A.; Martin, S.W. Sources of Infant Pertussis Infection in the United States. Pediatrics 2015, 136, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Katfy, K.; Diawara, I.; Maaloum, F.; Aziz, S.; Guiso, N.; Fellah, H.; Slaoui, B.; Zerouali, K.; Belabbes, H.; Elmdaghri, N. Pertussis in infants, in their mothers and other contacts in Casablanca, Morocco. BMC Infect. Dis. 2020, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Perrett, K.P.; Nolan, T.M. Immunization During Pregnancy: Impact on the Infant. Paediatr. Drugs 2017, 19, 313–324. [Google Scholar] [CrossRef]
- Furuta, M.; Sin, J.; Ng, E.S.W.; Wang, K. Efficacy and safety of pertussis vaccination for pregnant women—A systematic review of randomised controlled trials and observational studies. BMC Pregnancy Childbirth 2017, 17, 390. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.H.; Liu, Y.; Yu, J.Q.; Li, L.J.; Shi, W.; Shen, Y.J.; Li, L.; Zhan, S.N.; Yang, F.; Wang, Y.J.; et al. Seroprevalence of Maternal and Cord Antibodies Specific for Diphtheria, Tetanus, Pertussis, Measles, Mumps and Rubella in Shunyi, Beijing. Sci. Rep. 2018, 8, 13021. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, J.; Cao, L.; Zhang, N.; Zhu, J.; Ping, G.; Zhao, J.; Li, S.; He, Q. Seroprevalence of pertussis among adults in China where whole cell vaccines have been used for 50 years. J. Infect. 2016, 73, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Garcia-Cenoz, M.; Toledo, D.; Carmona, G.; Caylà, J.A.; Alsedà, M.; Àlvarez, J.; Barrabeig, I.; Camps, N.; Plans, P.; et al. Factors influencing the spread of pertussis in households: A prospective study, Catalonia and Navarre, Spain, 2012 to 2013. Euro Surveill. 2016, 21, 30393. [Google Scholar] [CrossRef]
- Mbayei, S.A.; Faulkner, A.; Miner, C.; Edge, K.; Cruz, V.; Peña, S.A.; Kudish, K.; Coleman, J.; Pradhan, E.; Thomas, S.; et al. Severe Pertussis Infections in the United States, 2011–2015. Clin. Infect. Dis. 2019, 69, 218–226. [Google Scholar] [CrossRef]
- Fiasca, F.; Gabutti, G.; Mattei, A. Trends in Hospital Admissions for Pertussis Infection: A Nationwide Retrospective Observational Study in Italy, 2002–2016. Int. J. Environ. Res. Public Health 2019, 16, 4531. [Google Scholar] [CrossRef]
- Macina, D.; Evans, K.E. Bordetella pertussis in School-Age Children, Adolescents, and Adults: A Systematic Review of Epidemiology, Burden, and Mortality in Asia. Infect. Dis. Ther. 2021, 10, 1115–1140. [Google Scholar] [CrossRef]
- Siriyakorn, N.; Leethong, P.; Tantawichien, T.; Sripakdee, S.; Kerdsin, A.; Dejsirilert, S.; Paitoonpong, L. Adult pertussis is unrecognized public health problem in Thailand. BMC Infect. Dis. 2016, 16, 25. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, H.; Liu, M.; Han, K.; Shu, J.; Wu, C.; Xu, N.; He, Q.; Luo, H. The Seroepidemiology of Immunoglobulin G Antibodies against Pertussis Toxin in China: A Cross Sectional Study. BMC Infect. Dis. 2012, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, M.; Zhang, T.; Li, J.; Zeng, Y.; Lu, L. Seroepidemiology of diphtheria and pertussis in Beijing, China: A cross-sectional study. Hum. Vaccin. Immunother. 2015, 11, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, L.; Xu, J.; Wang, X.; Wei, C.; Luo, P.; Ma, X.; Hou, Q.; Wang, J. Seroprevalence of pertussis in China: Need to improve vaccination strategies. Hum. Vaccin. Immunother. 2014, 10, 192–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, N.; Zeng, Q.; Wang, Q. Seroepidemiology of diphtheria and pertussis in Chongqing, China: Serology-based evidence of Bordetella pertussis infection. Public Health 2018, 156, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Healy, C.M.; Munoz, F.M.; Rench, M.A.; Halasa, N.B.; Edwards, K.M.; Baker, C.J. Prevalence of pertussis antibodies in maternal delivery, cord, and infant serum. J. Infect. Dis. 2004, 190, 335–340. [Google Scholar] [CrossRef]
- Van Savage, J.; Decker, M.D.; Edwards, K.M.; Sell, S.H.; Karzon, D.T. Natural history of pertussis antibody in the infant and effect on vaccine response. J. Infect. Dis. 1990, 161, 487–492. [Google Scholar] [CrossRef]
- Healy, C.M.; Rench, M.A.; Swaim, L.S.; Timmins, A.; Vyas, A.; Sangi-Haghpeykar, H.; Ng, N.; Rajam, G.; Havers, F.; Schiffer, J.; et al. Kinetics of maternal pertussis-specific antibodies in infants of mothers vaccinated with tetanus, diphtheria and acellular pertussis (Tdap) during pregnancy. Vaccine 2020, 38, 5955–5961. [Google Scholar] [CrossRef]
- Plans-Rubió, P. Vaccination Coverage for Routine Vaccines and Herd Immunity Levels against Measles and Pertussis in the World in 2019. Vaccines 2021, 9, 256. [Google Scholar] [CrossRef] [PubMed]
- CDC. Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. Morb. Mortal. Wkly. Rep. 2011, 60, 1424–1426. [Google Scholar]
- Baxter, R.; Bartlett, J.; Fireman, B.; Lewis, E.; Klein, N.P. Effectiveness of Vaccination during Pregnancy to Prevent Infant Pertussis. Pediatrics 2017, 139, e20164091. [Google Scholar] [CrossRef] [PubMed]
- Skoff, T.H.; Blain, A.E.; Watt, J.; Scherzinger, K.; McMahon, M.; Zansky, S.M.; Kudish, K.; Cieslak, P.R.; Lewis, M.; Shang, N.; et al. Impact of the US Maternal Tetanus, Diphtheria, and Acellular Pertussis Vaccination Program on Preventing Pertussis in Infants <2 Months of Age: A Case-Control Evaluation. Clin. Infect. Dis. 2017, 65, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Langley, J.M.; Ye, L.; MacKinnon-Cameron, D.; Elsherif, M.; Allen, V.M.; Smith, B.; Halperin, B.A.; McNeil, S.A.; Vanderkooi, O.G.; et al. A Randomized Controlled Trial of the Safety and Immunogenicity of Tetanus, Diphtheria, and Acellular Pertussis Vaccine Immunization during Pregnancy and Subsequent Infant Immune Response. Clin. Infect. Dis. 2018, 67, 1063–1071. [Google Scholar] [CrossRef]
- Pertussis: Halting the Epidemic by Protecting Infants. Available online: http://www.bpac.org.nz/magazine/2013/march/docs/BPJ51-pages-34-38.pdf (accessed on 13 September 2021).
- Li, Y.T.; Luo, X.Q.; Zhong, X.B.; Cai, L.M.; Zhu, L.P.; Chen, X.Q.; Wang, K.C.; Chen, Z.G. Seroprevalences of antibodies against pertussis, diphtheria, tetanus, measles, mumps and rubella: A cross-sectional study in children following vaccination procedure in Guangzhou, China. Vaccine 2020, 38, 3960–3967. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).