Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Dietary Intervention
2.4. Vaccination Procedure and Blood Collection
2.5. Biochemical Measures
2.6. Anthropometric and Body Composition Assessment
2.7. Adverse Events
2.8. Statistics
3. Results
3.1. Study Population
3.2. Dietary Intervention Metabolic Efficacy
3.3. Dietary Intervention Safety
3.4. Vaccine Adverse Events
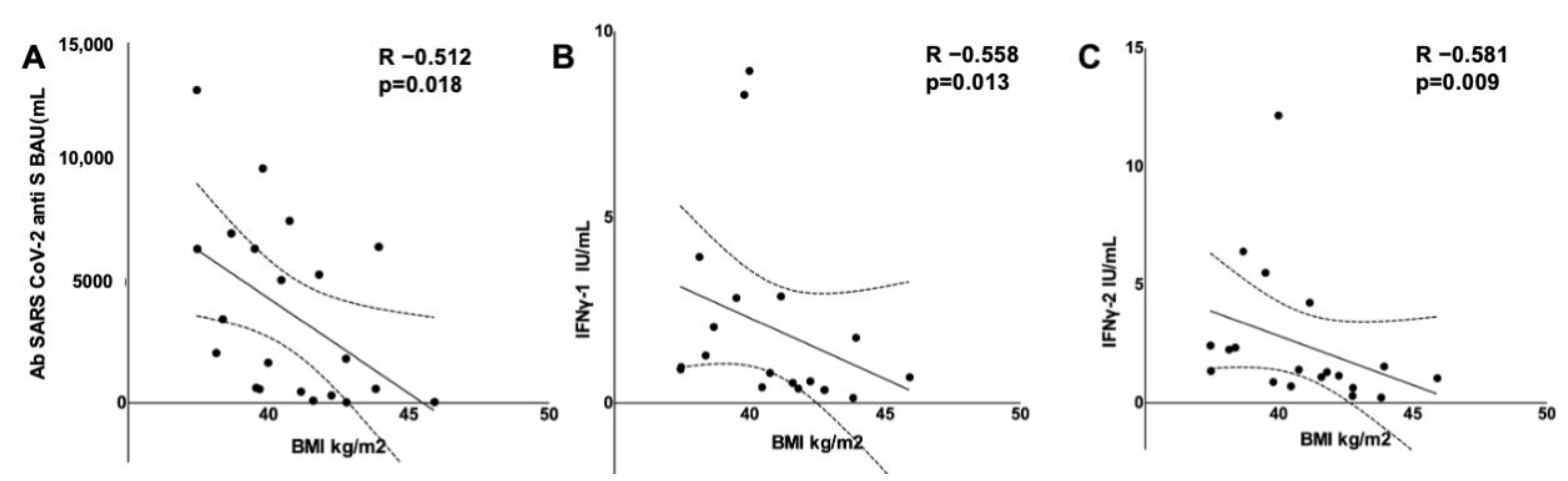
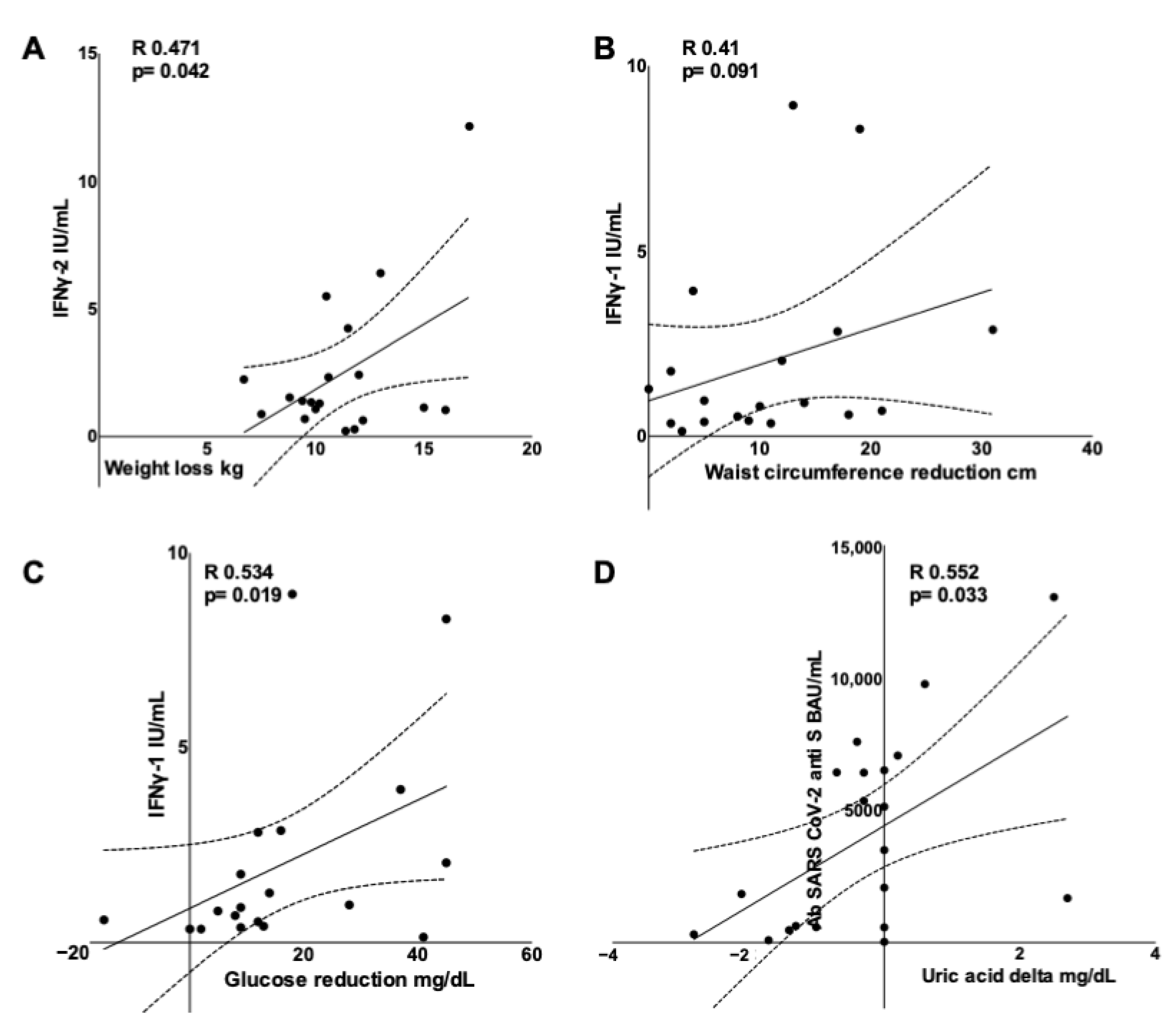
3.5. Adaptive Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watanabe, M.; Risi, R.; Tuccinardi, D.; Baquero, C.J.; Manfrini, S.; Gnessi, L. Obesity and SARS-CoV-2: A population to safeguard. Diabetes Metab. Res. Rev. 2020, 36, e3325. [Google Scholar] [CrossRef]
- Kass, A.D.; Duggal, P.; Cingolani, O. Obesity could shift severe COVID-19 disease to younger ages. Lancet 2020, 395, 1544–1545. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, Y.; Zhang, J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. BMC Public Heath 2021, 21, 1505. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Caruso, D.; Tuccinardi, D.; Risi, R.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Tarallo, M.; Strigari, L.; Manfrini, S.; et al. Visceral fat shows the strongest association with the need of intensive care in patients with COVID-19. Metabolism 2020, 111, 154319. [Google Scholar] [CrossRef]
- Pranata, R.; Lim, M.A.; Huang, I.; Yonas, E.; Henrina, J.; Vania, R.; Lukito, A.A.; Nasution, S.A.; Alwi, I.; Siswanto, B.B. Visceral adiposity, subcutaneous adiposity, and severe coronavirus disease-2019 (COVID-19): Systematic review and meta-analysis. Clin. Nutr. ESPEN 2021, 43, 163–168. [Google Scholar] [CrossRef]
- McLarnon, A. Influenza immunity impaired in obesity. Nat. Rev. Endocrinol. 2011, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.J.; Murphy, E.K.; Fernandez, M.L. Impact of obesity and metabolic syndrome on immunity. Adv. Nutr. Int. Rev. J. 2016, 7, 66–75. [Google Scholar] [CrossRef]
- Painter, S.D.; Ovsyannikova, I.G.; Poland, G.A. The weight of obesity on the human immune response to vaccination. Vaccine 2015, 33, 4422–4429. [Google Scholar] [CrossRef] [PubMed]
- Westheim, A.J.F.; Bitorina, A.V.; Theys, J.; Shiri-Sverdlov, R. COVID-19 infection, progression, and vaccination: Focus on obesity and related metabolic disturbances. Obes. Rev. 2021, 22, 13313. [Google Scholar] [CrossRef]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, A.M. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef]
- Watanabe, M.; Balena, A.; Tuccinardi, D.; Tozzi, R.; Risi, R.; Masi, D.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Filippi, V.; et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab. Res. Rev. 2021, e3465. [Google Scholar] [CrossRef]
- DeUgarte, D.A.; DeUgarte, C.M.; Sahakian, V. Surrogate obesity negatively impacts pregnancy rates in third-party reproduction. Fertil. Steril. 2010, 93, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- NHS. Better Health Campaign. Available online: https://campaignresources.phe.gov.uk/resources/campaigns/109-better-health---adult-obesity (accessed on 1 December 2021).
- Nieman, D.C.; Nehlsen-Cannarella, I.S.; Henson, A.D.; Butterworth, E.D.; Fagoaga, O.R.; Warren, B.J.; Rainwater, M.K. Immune response to obesity and moderate weight loss. Int. J. Obes. 1996, 20, 353–360. [Google Scholar]
- El-Kader, S.M.A.; Al-Jiffri, O.H. Impact of weight reduction on selected immune system response among Hepatitis C virus Saudi patients. Afr. Heath Sci. 2018, 18, 417–427. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A biometric study of human basal metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef]
- Perkmann, T.; Perkmann-Nagele, N.; Breyer, M.-K.; Breyer-Kohansal, R.; Burghuber, O.C.; Hartl, S.; Aletaha, D.; Sieghart, D.; Quehenberger, P.; Marculescu, R.; et al. Side-by-side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin. Chem. 2020, 66, 1405–1413. [Google Scholar] [CrossRef]
- Frasca, D.; Ferracci, F.; Diaz, A.; Romero, M.; Lechner, S.; Blomberg, B.B. Obesity decreases B cell responses in young and elderly individuals. Obesity 2016, 24, 615–625. [Google Scholar] [CrossRef]
- Miyara, M.; Sakaguchi, S. Natural regulatory T cells: Mechanisms of suppression. Trends Mol. Med. 2007, 13, 108–116. [Google Scholar] [CrossRef] [PubMed]
- De Rosa, V.; Procaccini, C.; Calì, G.; Pirozzi, G.; Fontana, S.; Zappacosta, S.; La Cava, A.; Matarese, G. A key role of Leptin in the control of regulatory T cell proliferation. Immunity 2007, 26, 241–255. [Google Scholar] [CrossRef]
- Tsai, S.; Clemente-Casares, X.; Zhou, A.C.; Lei, H.; Ahn, J.J.; Chan, Y.T.; Choi, O.; Luck, H.; Woo, M.; Dunn, S.E.; et al. Insulin receptor-mediated stimulation boosts T cell immunity during inflammation and infection. Cell Metab. 2018, 28, 922–934.e4. [Google Scholar] [CrossRef]
- Viardot, A.; Grey, S.T.; Mackay, F.; Chisholm, D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology 2007, 148, 346–353. [Google Scholar] [CrossRef]
- Njajou, O.T.; Cawthon, R.M.; Blackburn, E.H.; Harris, T.B.; Li, R.; Sanders, J.L.; Newman, A.B.; Nalls, M.; Cummings, S.R.; Hsueh, W.C. Shorter telomeres are associated with obesity and weight gain in the elderly. Int. J. Obes. 2012, 36, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Duong, M.T.; Sahin, E. RAP1: Protector of telomeres, defender against obesity. Cell Rep. 2013, 3, 1757–1758. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Guo, Z.; Dong, C. Influences of obesity on the immunogenicity of Hepatitis B vaccine. Hum. Vaccines Immunother. 2017, 13, 1014–1017. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef]
- Shaw, F.; Guess, H.; Roets, J.; Mohr, F.; Coleman, P.; Mandel, E.; Roehm, R.; Talley, W.; Hadler, S. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989, 7, 425–430. [Google Scholar] [CrossRef]
- Ozdemir, R.; Canpolat, F.E.; Yurttutan, S.; Oncel, M.Y.; Erdeve, O.; Dilmen, U. Effect of needle length for response to hepatitis B vaccine in macrosomic neonates: A prospective randomized study. Vaccine 2012, 30, 3155–3158. [Google Scholar] [CrossRef][Green Version]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Early onset of SARS-CoV-2 antibodies after first dose of BNT162b2: Correlation with age, gender and BMI. Vaccines 2021, 9, 685. [Google Scholar] [CrossRef]
- Pellini, R.; Venuti, A.; Pimpinelli, F.; Abril, E.; Blandino, G.; Campo, F.; Conti, L.; De Virgilio, A.; De Marco, F.; Di Domenico, E.G.; et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021, 36, 100928. [Google Scholar] [CrossRef]
- Bruxvoort, K.J.; Sy, L.S.; Qian, L.; Ackerson, B.K.; Luo, Y.; Lee, G.S.; Tian, Y.; Florea, A.; Takhar, H.S.; Tubert, J.E.; et al. Real-world effectiveness of the mRNA-1273 vaccine against COVID-19: Interim results from a prospective observational cohort study. Lancet Reg. Health Am. 2021, 100134. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Muscogiuri, G.; El Ghoch, M.; Colao, A.; Hassapidou, M.; Yumuk, V.; Busetto, L.; Obesity Management Task Force (OMTF) of the European Association for the Study of Obesity (EASO). European guidelines for obesity management in adults with a very low-calorie ketogenic diet: A systematic review and meta-analysis. Obes. Facts 2021, 14, 222–245. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tuccinardi, D.; Ernesti, I.; Basciani, S.; Mariani, S.; Genco, A.; Manfrini, S.; Lubrano, C.; Gnessi, L. Scientific evidence underlying contraindications to the ketogenic diet: An update. Obes. Rev. 2020, 21, e13053. [Google Scholar] [CrossRef]
- McMurray, R.W.; Bradsher, R.W.; Steele, R.W.; Pilkington, N.S. Effect of prolonged modified fasting in obese persons on in vitro markers of immunity: Lymphocyte function and serum effects on normal neutrophils. Am. J. Med. Sci. 1990, 299, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.; Berkley, J.A. Severe acute malnutrition and infection. Paediatr. Int. Child Health 2014, 34, S1–S29. [Google Scholar] [CrossRef]
- Collins, N. Dietary regulation of memory T cells. Int. J. Mol. Sci. 2020, 21, 4363. [Google Scholar] [CrossRef]
- Basciani, S.; Camajani, E.; Contini, S.; Persichetti, A.; Risi, R.; Bertoldi, L.; Strigari, L.; Prossomariti, G.; Watanabe, M.; Mariani, S.; et al. Very-low-calorie ketogenic diets with whey, vegetable, or animal protein in patients with obesity: A randomized pilot study. J. Clin. Endocrinol. Metab. 2020, 105, 2939–2949. [Google Scholar] [CrossRef]
- Basciani, S.; Costantini, D.; Contini, S.; Persichetti, A.; Watanabe, M.; Mariani, S.; Lubrano, C.; Spera, G.; Lenzi, A.; Gnessi, L. Safety and efficacy of a multiphase dietetic protocol with meal replacements including a step with very low calorie diet. Endocrine 2015, 48, 863–870. [Google Scholar] [CrossRef]
- Bruci, A.; Tuccinardi, D.; Tozzi, R.; Balena, A.; Santucci, S.; Frontani, R.; Mariani, S.; Basciani, S.; Spera, G.; Gnessi, L.; et al. Very low-calorie ketogenic diet: A safe and effective tool for weight loss in patients with obesity and mild kidney failure. Nutrients 2020, 12, 333. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Risi, R.; Camajani, E.; Contini, S.; Persichetti, A.; Tuccinardi, D.; Ernesti, I.; Mariani, S.; Lubrano, C.; Genco, A.; et al. Baseline HOMA IR and circulating FGF21 levels predict NAFLD improvement in patients undergoing a low carbohydrate dietary intervention for weight loss: A prospective observational pilot study. Nutrients 2020, 12, 2141. [Google Scholar] [CrossRef]
- Fontana, L.; Eagon, J.C.; Colonna, M.; Klein, S. Impaired mononuclear cell immune function in extreme obesity is corrected by weight loss. Rejuvenation Res. 2007, 10, 41–46. [Google Scholar] [CrossRef]
- Moulin, C.M.; Rizzo, L.V.; Halpern, A. Effect of surgery-induced weight loss on immune function. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 617–619. [Google Scholar] [CrossRef]
- Mehrdad, M.; Norouzy, A.; Safarian, M.; Nikbakht, H.A.; Gholamalizadeh, M.; Mahmoudi, M. The antiviral immune defense may be adversely influenced by weight loss through a calorie restriction program in obese women. Am. J. Transl. Res. 2021, 13, 10404–10412. [Google Scholar] [PubMed]
- Viardot, A.; Lord, R.V.; Samaras, K. the effects of weight loss and gastric banding on the innate and adaptive immune system in type 2 diabetes and prediabetes. J. Clin. Endocrinol. Metab. 2010, 95, 2845–2850. [Google Scholar] [CrossRef]
- Tsigos, C.; Kyrou, I.; Chala, E.; Tsapogas, P.; Stavridis, J.C.; Raptis, S.A.; Katsilambros, N. Circulating tumor necrosis factor alpha concentrations are higher in abdominal versus peripheral obesity. Metabolism 1999, 48, 1332–1335. [Google Scholar] [CrossRef]
- Dandona, P.; Weinstock, R.; Thusu, K.; Abdel-Rahman, E.; Aljada, A.; Wadden, T. Tumor necrosis factor-α in sera of obese patients: Fall with weight loss. J. Clin. Endocrinol. Metab. 1998, 83, 2907–2910. [Google Scholar] [CrossRef] [PubMed]
- Lussier, D.M.; Woolf, E.C.; Johnson, J.L.; Brooks, K.S.; Blattman, J.N.; Scheck, A.C. Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet. BMC Cancer 2016, 16, 310. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, E.L.; Molony, R.D.; Kudo, E.; Sidorov, S.; Kong, Y.; Dixit, V.D.; Iwasaki, A. Ketogenic diet activates protective γδ T cell responses against influenza virus infection. Sci. Immunol. 2019, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Shchukina, I.; Youm, Y.-H.; Qing, H.; Hilliard, B.; Dlugos, T.; Zhang, X.; Yasumoto, Y.; Booth, C.J.; Fernández-Hernando, C.; et al. Ketogenic diet restrains aging-induced exacerbation of coronavirus infection in mice. eLife 2021, 10, e66522. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Bassetti, M. Induction of ketosis as a potential therapeutic option to limit hyperglycemia and prevent cytokine storm in COVID-19. Nutrition 2020, 79–80, 110967. [Google Scholar] [CrossRef]
- Sukkar, S.G.; Cogorno, L.; Pisciotta, L.; Pasta, A.; Vena, A.; Gradaschi, R.; Dentone, C.; Guiddo, E.; Martino, E.; Beltramini, S.; et al. Clinical efficacy of eucaloric ketogenic nutrition in the COVID-19 cytokine storm: A retrospective analysis of mortality and intensive care unit admission. Nutrition 2021, 89, 111236. [Google Scholar] [CrossRef]
- Shomali, N.; Mahmoudi, J.; Mahmoodpoor, A.; Zamiri, R.E.; Akbari, M.; Xu, H.; Shotorbani, S.S. Harmful effects of high amounts of glucose on the immune system: An updated review. Biotechnol. Appl. Biochem. 2021, 68, 404–410. [Google Scholar] [CrossRef]
- Wofford, J.A.; Wieman, H.L.; Jacobs, S.R.; Zhao, Y.; Rathmell, J.C. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood 2008, 111, 2101–2111. [Google Scholar] [CrossRef]
- MacIver, N.J.; Jacobs, S.R.; Wieman, H.L.; Wofford, J.A.; Coloff, J.L.; Rathmell, J.C. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J. Leukoc. Biol. 2008, 84, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Siska, P.J.; Rathmell, J.C. T cell metabolic fitness in antitumor immunity. Trends Immunol. 2015, 36, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Cabău, G.; Crișan, T.O.; Klück, V.; Popp, R.A.; Joosten, L.A.B. Urate-induced immune programming: Consequences for gouty arthritis and hyperuricemia. Immunol. Rev. 2020, 294, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Jiang, Y.; Wang, Y.; Qu, X.; Li, L.; Lou, X.; Wang, Y.; Guo, H.; Liu, Y. Male asymptomatic hyperuricemia patients display a lower number of NKG2D+ NK cells before and after a low-purine diet. Medicine 2018, 97, e13668. [Google Scholar] [CrossRef] [PubMed]
- Baey, C.; Yang, J.; Ronchese, F.; Harper, J.L. Hyperuricaemic UrahPlt2/Plt2 mice show altered T cell proliferation and defective tumor immunity after local immunotherapy with Poly I:C. PLoS ONE 2018, 13, e0206827. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, J.C.; Gluud, C.; Wetterslev, J.; Winkel, P. When and how should multiple imputation be used for handling missing data in randomised clinical trials—A practical guide with flowcharts. BMC Med. Res. Methodol. 2017, 17, 162. [Google Scholar] [CrossRef] [PubMed]
| Pre | Post | p | |||||
|---|---|---|---|---|---|---|---|
| n | 21 | 21 | |||||
| Female, n (%) | 15(71.4) | ||||||
| Age (years) | 51.50 | 41.50 | 55.25 | ||||
| Weight (kg) | 111.00 | 106.25 | 117.88 | 100.15 | 94.33 | 106.30 | 0.001 |
| BMI (Kg/m2) | 40.95 | 39.30 | 42.76 | 36.83 | 34.93 | 38.23 | 0.001 |
| Waist circumference (cm) | 122.50 | 117.75 | 136.25 | 114.00 | 106.00 | 119.25 | 0.001 |
| Hip circumference (cm) | 132.00 | 125.75 | 134.75 | 121.00 | 115.50 | 128.00 | 0.001 |
| Waist-to-hip ratio | 0.96 | 0.85 | 1.04 | 0.96 | 0.83 | 1.03 | 0.093 |
| Systolic BP (mmHg) | 130.00 | 120.00 | 140.00 | 120.00 | 110.00 | 130.00 | 0.024 |
| Diastolic BP (mmHg) | 80.00 | 70.00 | 91.25 | 75.00 | 70.00 | 80.00 | 0.325 |
| Heart rate (bpm) | 77.50 | 70.75 | 85.00 | 70.00 | 63.50 | 78.50 | 0.345 |
| Glucose (mg/dL) | 99 | 97 | 117 | 86 | 85 | 96.5 | 0.000 |
| Insulin (µU/mL) | 24.2 | 16.35 | 35.05 | 11.5 | 9.275 | 15.775 | 0.000 |
| BUN (mg/dL) | 31.8 | 25.05 | 37.95 | 33.6 | 27 | 37.5 | 0.191 |
| Creatinine (mg/dL) | 0.79 | 0.695 | 0.8975 | 0.82 | 0.7675 | 0.905 | 0.013 |
| Sodium (mmoL/L) | 140 | 137.75 | 141.25 | 141 | 137.75 | 143 | 0.274 |
| Potassium (mmoL/L) | 4.35 | 3.875 | 4.5 | 4.3 | 4.125 | 4.475 | 0.809 |
| AST (U/L) | 19 | 16.75 | 21 | 20 | 16 | 23.75 | 0.822 |
| ALT (U/L) | 24 | 18 | 28.25 | 21 | 17.25 | 25 | 0.156 |
| Total cholesterol (mg/dL) | 197 | 173.75 | 221.75 | 179 | 152.75 | 207.5 | 0.000 |
| LDL cholesterol (mg/dL) | 115 | 99.5 | 141 | 113 | 88.75 | 151 | 0.360 |
| HDL cholesterol (mg/dL) | 49 | 39.25 | 59.5 | 43 | 34 | 50.75 | 0.001 |
| Triglycerides (mg/dL) | 116 | 77 | 179.75 | 97 | 68 | 113.5 | 0.002 |
| Uric acid (mg/dL) | 5.5 | 5.275 | 6.25 | 6.7 | 5.85 | 6.8 | 0.177 |
| Capillary BHB (mmoL/L) | 0.78 | 0.63 | 0.97 | ||||
| U. acetoacetate (mmoL/L) | 0.67 | 0.46 | 2.42 | ||||
| IFNγ-1 (IU/mL) | 0.86 | 0.41 | 2.84 | ||||
| IFNγ-2 (IU/mL) | 1.32 | 0.83 | 2.87 | ||||
| Anti S Abs (BAU/mL) | 2070 | 519 | 6464 | ||||
| Diabetes, n (%) | 2(9.5) | ||||||
| Hypertension, n (%) | 11(52.4) | ||||||
| Dyslipidemia, n (%) | 13(61.9) | ||||||
| Smoking habit, n (%) | 5(23.8) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Balena, A.; Masi, D.; Tozzi, R.; Risi, R.; Caputi, A.; Rossetti, R.; Spoltore, M.E.; Biagi, F.; Anastasi, E.; et al. Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine. Vaccines 2022, 10, 79. https://doi.org/10.3390/vaccines10010079
Watanabe M, Balena A, Masi D, Tozzi R, Risi R, Caputi A, Rossetti R, Spoltore ME, Biagi F, Anastasi E, et al. Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine. Vaccines. 2022; 10(1):79. https://doi.org/10.3390/vaccines10010079
Chicago/Turabian StyleWatanabe, Mikiko, Angela Balena, Davide Masi, Rossella Tozzi, Renata Risi, Alessandra Caputi, Rebecca Rossetti, Maria Elena Spoltore, Filippo Biagi, Emanuela Anastasi, and et al. 2022. "Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine" Vaccines 10, no. 1: 79. https://doi.org/10.3390/vaccines10010079
APA StyleWatanabe, M., Balena, A., Masi, D., Tozzi, R., Risi, R., Caputi, A., Rossetti, R., Spoltore, M. E., Biagi, F., Anastasi, E., Angeloni, A., Mariani, S., Lubrano, C., Tuccinardi, D., & Gnessi, L. (2022). Rapid Weight Loss, Central Obesity Improvement and Blood Glucose Reduction Are Associated with a Stronger Adaptive Immune Response Following COVID-19 mRNA Vaccine. Vaccines, 10(1), 79. https://doi.org/10.3390/vaccines10010079












