A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Epidemiological Model
2.2. Cost Parameters
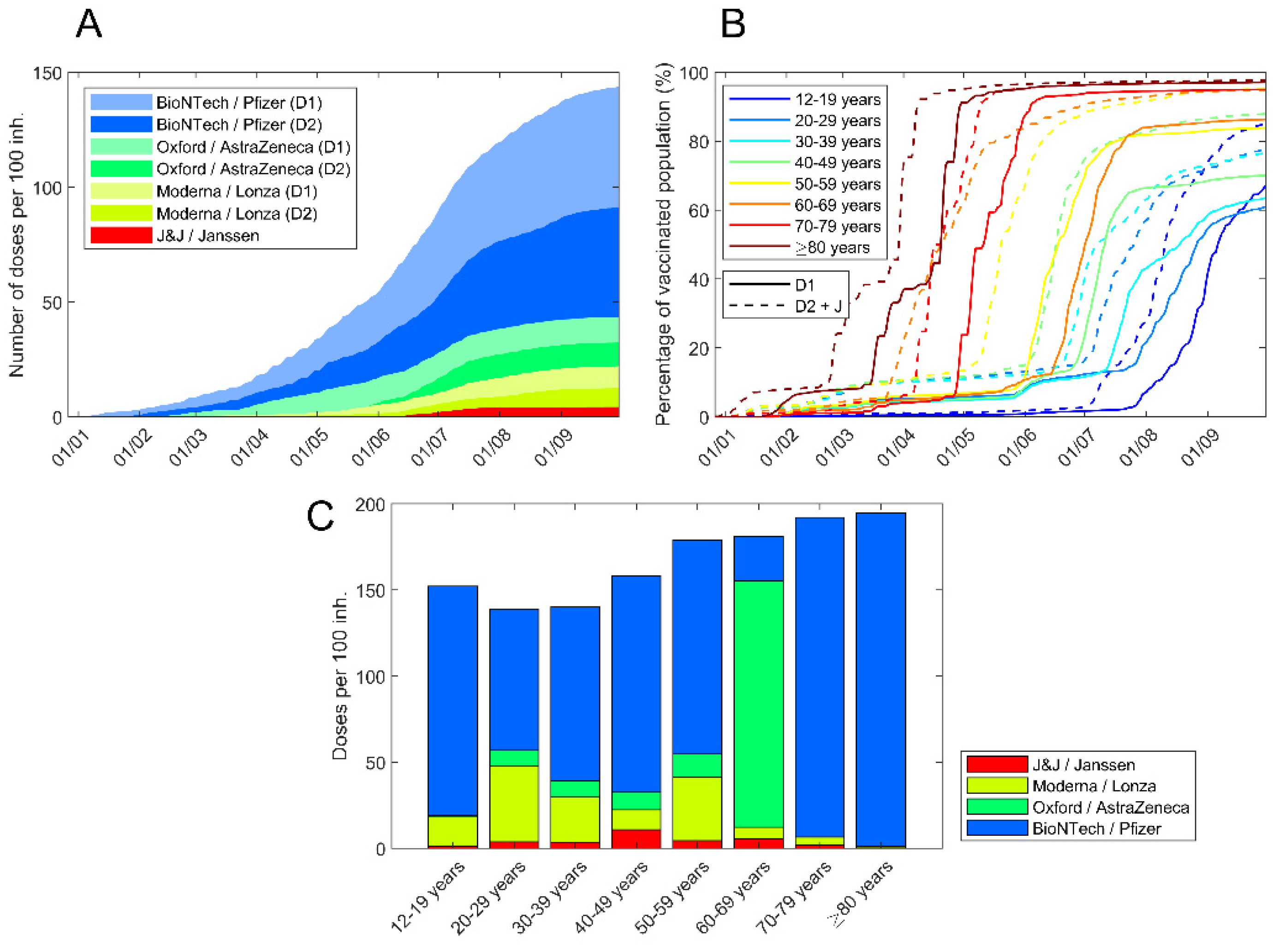
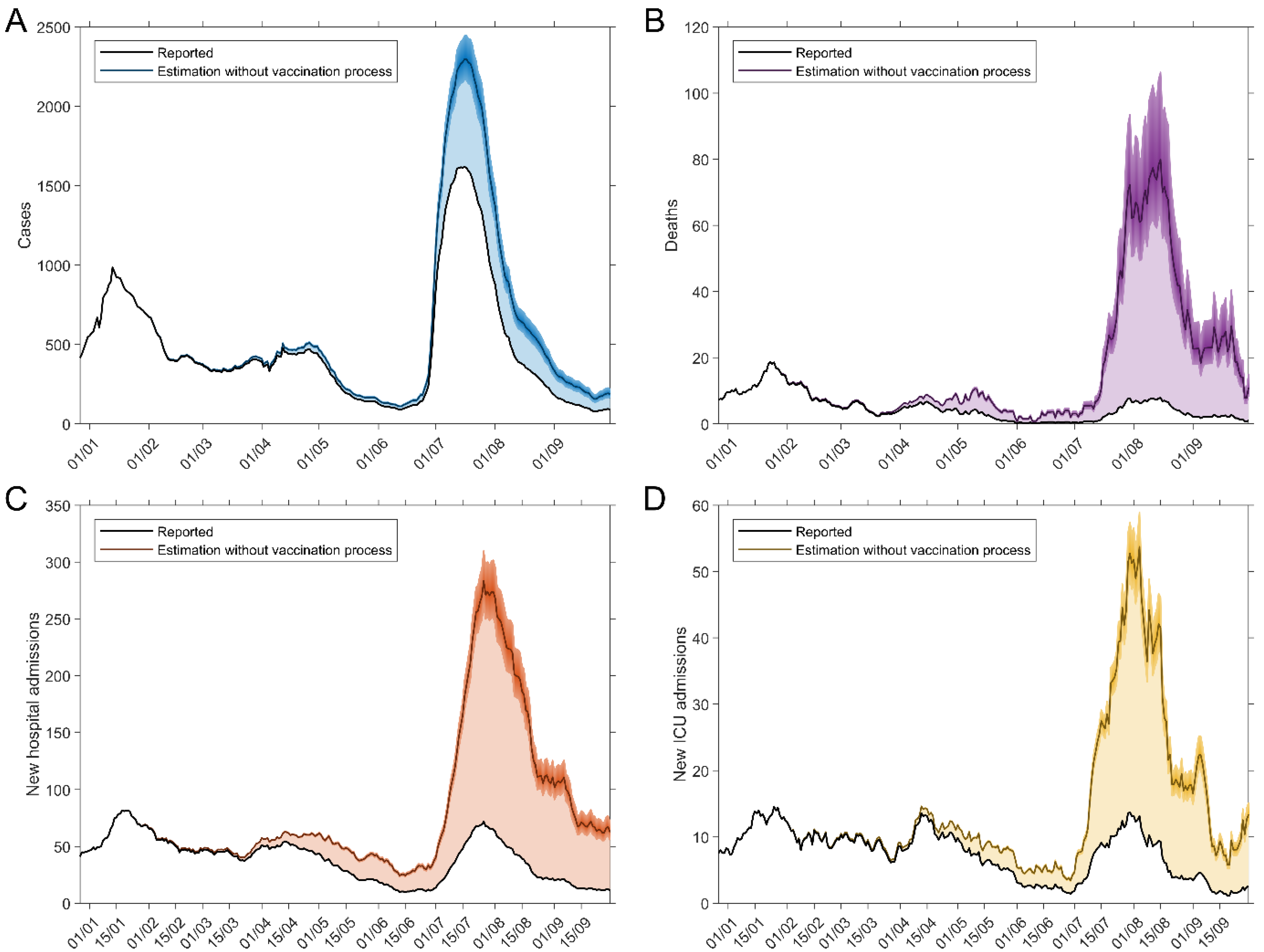
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McGill; COVID-19 Vaccine Tracker Team. “COVID-19 Vaccine Tracker”. Available online: https://covid19.trackvaccines.org/ (accessed on 8 November 2021).
- Català, M. On Short-Term Scenarios of COVID-19 in Europe. Comparison of the Epidemic Dynamics between High and Very High Vaccinated Countries. In Analysis and Prediction of COVID-19 for EU-EFTA-UK and Other Countries; Research Report; Universitat Politècnica de Catalunya: Barcelona, Spain, 2020; pp. 9–15. Available online: https://biocomsc.upc.edu/en/shared/20211022_report_299.pdf (accessed on 22 October 2021).
- Kohli, M.; Maschio, M.; Becker, D.; Weinstein, M.C. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine 2021, 39, 1157–1164. [Google Scholar] [CrossRef]
- Siedner, M.J.; Alba, C.; Fitzmaurice, K.P.; Gilbert, R.F.; Scott, J.A.; Shebl, F.M.; Ciaranello, A.; Reddy, K.P.; Freedberg, K.A. Cost-effectiveness of COVID-19 vaccination in low-and middle-income countries. medRxiv 2021. [Google Scholar] [CrossRef]
- Debrabant, K.; Grønbæk, L.; Kronborg, C. The Cost-Effectiveness of a COVID-19 Vaccine in a Danish Context; Discussion Papers on Business and Economics; University of Southern Denmark: Odense, Denmark, 2021. [Google Scholar]
- Wang, W.C.; Fann, J.C.Y.; Chang, R.E.; Jeng, Y.C.; Hsu, C.Y.; Chen, H.H.; Liu, J.T.; Yen, A.M.F. Economic Evaluation for Mass Vaccination against COVID-19. J. Formos. Med. Assoc. 2021, 120, S95–S109. [Google Scholar] [CrossRef]
- Shaker, M.; Abrams, E.M.; Greenhawt, M. A cost-effectiveness evaluation of hospitalizations, fatalities, and economic outcomes associated with universal versus anaphylaxis risk-stratified COVID-19 vaccination strategies. J. Allergy Clin. Immunol. 2021, 9, 2658–2668.e3. [Google Scholar] [CrossRef] [PubMed]
- Padula, W.V.; Malaviya, S.; Reid, N.M.; Cohen, B.G.; Chingcuanco, F.; Ballreich, J.; Tierce, J.; Alexander, G.C. Economic value of vaccines to address the COVID-19 pandemic: A US cost-effectiveness and budget impact analysis. J. Med. Econ. 2021, 24, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Volodymyrovych, T.Y.; Ivanovich, S.V.; Tetiana, K.; Yaroslavovych, T.B. Pharmaco Economics Analysis of COVID-19 Vaccines in Ukraine. J. Pharm. Res. Int. 2021, 33, 140–147. [Google Scholar] [CrossRef]
- Hagens, A.; İnkaya, A.Ç.; Yildirak, K.; Sancar, M.; van der Schans, J.; Acar Sancar, A.; Ünal, S.; Postma, M.; Yeğenoğlu, S. COVID-19 Vaccination Scenarios: A Cost-Effectiveness Analysis for Turkey. Vaccines 2021, 9, 399. [Google Scholar] [CrossRef]
- García-Altés, A. Systematic review of economic evaluation studies: Are vaccination programs efficient in Spain? Vaccine 2013, 31, 1656–1665. [Google Scholar] [CrossRef] [PubMed]
- CatSalut; Catalan Health Service. Any COVID: Despesa. Available online: https://catsalut.gencat.cat/ca/coneix-catsalut/any-covid/despesa/ (accessed on 8 November 2021).
- Català Sabaté, M.; Cardona Iglesias, P.J.; Prats Soler, C.; Alonso Muñoz, S.; Álvarez Lacalle, E.; Marchena Angos, M.; Conesa Ortega, D.; López Codina, D.; Analysis and Prediction of COVID-19 for EU-EFTA-UK and Other Countries. Report 236. Available online: http://hdl.handle.net/2117/346442 (accessed on 28 May 2021).
- Català, M.; Pino, D.; Marchena, M.; Palacios, P.; Urdiales, T.; Cardona, P.J.; Alonso, S.; López-Codina, D.; Prats, C.; Alvarez-Lacalle, E. Robust estimation of diagnostic rate and real incidence of COVID-19 for European policymakers. PLoS ONE 2021, 16, e0243701. [Google Scholar] [CrossRef]
- López Seguí, F.; Estrada Cuxart, O.; Mitjà i Villar, O.; Hernández Guillamet, G.; Prat Gil, N.; Maria Bonet, J.; Isnard Blanchar, M.; Moreno Millan, N.; Blanco, I.; Vilar Capella, M.; et al. A Cost-Benefit Analysis of the COVID-19 Asymptomatic Mass Testing Strategy in the North Metropolitan Area of Barcelona. Int. J. Environ. Res. Public Health 2021, 18, 7028. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and efficacy of single-dose Ad26. COV2. S vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Cabezas, C.; Coma, E.; Mora-Fernandez, N.; Li, X.; Martinez-Marcos, M.; Fina, F.; Fabregas, M.; Hermosilla, E.; Jover, A.; Contel, J.C.; et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with COVID-19 in nursing homes and healthcare workers in Catalonia: Prospective cohort study. BMJ 2021, 374, n1868. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: One dose of vaccine cuts risk of passing on infection by as much as 50%, research shows. BMJ 2021, 373, n1112. [Google Scholar] [CrossRef] [PubMed]
- Chodick, G.; Tene, L.; Patalon, T.; Gazit, S.; Tov, A.B.; Cohen, D.; Muhsen, K. Assessment of Effectiveness of 1 Dose of BNT162b2 Vaccine for SARS-CoV-2 Infection 13 to 24 Days After Immunization. JAMA Netw. Open 2021, 4, e2115985. [Google Scholar] [CrossRef] [PubMed]
- Ibarrondo, F.J.; Hofmann, C.; Fulcher, J.A.; Goodman-Meza, D.; Mu, W.; Hausner, M.A.; Ali, A.; Balamurugan, A.; Taus, E.; Elliott, J.; et al. Primary, Recall, and Decay Kinetics of SARS-CoV-2 Vaccine Antibody Responses. ACS Nano 2021, 15, 11180–11191. [Google Scholar] [CrossRef] [PubMed]
- Covid Data in Catalonia (Administrative Register) Dades Covid (Catalunya). Available online: www.dadescovid.cat (accessed on 22 November 2021).
- Catalan Ministry of Health (Departament de Salut). ORDER SLT/71/2020, of 2 June, Which Regulates the Billable Cases and Concepts and Approves the Public Prices Corresponding to the Services Provided by the Catalan Institute of Health. Available online: http://cido.diba.cat/legislacio/10263520/ordre-slt712020-de-2-de-juny-per-la-qual-es-regulen-els-suposits-i-conceptes-facturables-i-saproven-els-preus-publics-corresponents-als-serveis-que-presta-linstitut-catala-de-la-salut-departament-de-salut (accessed on 29 December 2021).
- Catalan Ministry of Health (Departament de Salut). ORDER SLT/63/2020, of March 8, Approving the Public Prices of the Catalan Health Service. Available online: http://cido.diba.cat/legislacio/10132337/ordre-slt632020-de-8-de-marc-per-la-qual-saproven-els-preus-publics-del-servei-catala-de-la-salut-departament-de-salut (accessed on 29 December 2021).
- Ministerio de Sanidad. Estrategia de Vacunación COVID en España. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/vacunaCovid19.htm (accessed on 21 December 2021).
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021. [Google Scholar] [CrossRef]
- Pifarré i Arolas, H.; Vidal-Alaball, J.; Gil, J.; López, F.; Nicodemo, C.; Saez, M. Missing Diagnoses during the COVID-19 Pandemic: A Year in Review. Int. J. Environ. Res. Public Health 2021, 18, 5335. [Google Scholar] [CrossRef] [PubMed]
- Lopez Segui, F.; Hernandez Guillamet, G.; Pifarré Arolas, H.; Marin-Gomez, F.X.; Ruiz Comellas, A.; Ramirez Morros, A.M.; Adroher Mas, C.; Vidal-Alaball, J. Characterization and Identification of Variations in Types of Primary Care Visits Before and During the COVID-19 Pandemic in Catalonia: Big Data Analysis Study. J. Med. Internet Res. 2021, 23, e29622. [Google Scholar] [CrossRef] [PubMed]
| Group | Population | Cases | Hosp. | ICU | Deaths |
|---|---|---|---|---|---|
| 0–9 years | 187,133 (10.0%) | 3695 (5.5%) | 26 (0.4%) | 2 (0.2%) | 0 (0.0%) |
| 10–19 years | 217,566 (11.6%) | 9346 (14.0%) | 44 (0.7%) | 4 (0.4%) | 0 (0.0%) |
| 20–29 years | 192,940 (10.3%) | 8850 (13.3%) | 134 (2.2%) | 7 (0.8%) | 1 (0.1%) |
| 30–39 years | 240,411 (12.8%) | 8493 (12.8%) | 310 (5.1%) | 38 (4.1%) | 5 (0.5%) |
| 40–49 years | 330,845 (17.6%) | 12,005 (18.0%) | 604 (9.9%) | 89 (9.7%) | 11 (1.2%) |
| 50–59 years | 268,237 (14.3%) | 10,137 (15.2%) | 967 (15.8%) | 188 (20.4%) | 35 (3.7%) |
| 60–69 years | 202,241 (10.8%) | 6072 (9.1%) | 1126 (18.4%) | 230 (25.0%) | 72 (7.6%) |
| 70–79 years | 145,686 (7.8%) | 3981 (6.0%) | 1246 (20.3%) | 260 (28.3%) | 173 (18.3%) |
| over 80 years | 93,861 (5.0%) | 4009 (6.0%) | 1674 (27.3%) | 102 (11.1%) | 647 (68.5%) |
| Perspective | Variable | Unit Cost (€) | N (S1) | N (S2) | N (S3) | €M (S1) | €M (S2) | €M (S3) | € (%) (S1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Social | Health System | ICU | 43,400/ discharge | 1.950 | 2.200 | 1.700 | 85 | 95 | 74 | 18.00% |
| Hospita-lizations | 6050/ discharge | 12.750 | 14.500 | 11.000 | 77 | 88 | 67 | 16.40% | ||
| PCR | 75 | 340.000 | 420.000 | 260.000 | 26 | 32 | 20 | 5.42% | ||
| RAT | 40 | 170.000 | 210.000 | 130.000 | 7 | 8 | 5 | 1.45% | ||
| Deaths | 2.92 QALY/death at €25,000/QALY | 3.450 | 4.300 | 2.600 | 252 | 314 | 190 | 53.56% | ||
| Cases with sequelae | 2.78 QALY/case at €25,000/QALY | 350 | 430 | 270 | 24 | 30 | 19 | 5.17% | ||
| € Total saved (millions) | 470 | 567 | 374 | 100% | ||||||
| Concept | Cost/Dose (€) | Total Costs (€M) | Cost (%) |
|---|---|---|---|
| HR + Facilities | 35.00 | 99.92 | 72.84% |
| Vaccines | 13.05 | 37.26 | 27.16% |
| Total | 48.05 | 137 | 100% |
| Scenario | B/C Ratio (Social Perspective) | B/C Ratio (Health System Perspective) | Benefit/Dose (Social Perspective) | Benefit/Dose (Health System Perspective) |
|---|---|---|---|---|
| 1 Base; Average effectiveness | 3.4 | 1.4 | 116.67 | 19.93 |
| 2 Low effectiveness | 2.7 | 1.2 | 82.81 | 9.75 |
| 3 High effectiveness | 4.1 | 1.6 | 150.52 | 30.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, F.; Català, M.; Prats, C.; Estrada, O.; Oliva, I.; Prat, N.; Isnard, M.; Vallès, R.; Vilar, M.; Clotet, B.; et al. A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines 2022, 10, 59. https://doi.org/10.3390/vaccines10010059
López F, Català M, Prats C, Estrada O, Oliva I, Prat N, Isnard M, Vallès R, Vilar M, Clotet B, et al. A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines. 2022; 10(1):59. https://doi.org/10.3390/vaccines10010059
Chicago/Turabian StyleLópez, Francesc, Martí Català, Clara Prats, Oriol Estrada, Irene Oliva, Núria Prat, Mar Isnard, Roser Vallès, Marc Vilar, Bonaventura Clotet, and et al. 2022. "A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia" Vaccines 10, no. 1: 59. https://doi.org/10.3390/vaccines10010059
APA StyleLópez, F., Català, M., Prats, C., Estrada, O., Oliva, I., Prat, N., Isnard, M., Vallès, R., Vilar, M., Clotet, B., Argimon, J. M., Aran, A., & Ara, J. (2022). A Cost–Benefit Analysis of COVID-19 Vaccination in Catalonia. Vaccines, 10(1), 59. https://doi.org/10.3390/vaccines10010059






