Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study
Abstract
:1. Introduction
2. Patients and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Statement
References
- Prevalence of Long COVID Symptoms and COVID-19 Complications—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/healthandlifeexpectancies/datasets/prevalenceoflongcovidsymptomsandcovid19complications (accessed on 16 December 2020).
- Groff, D.; Sun, A.; Ssentongo, A.E.; Ba, D.M.; Parsons, N.; Poudel, G.R.; Lekoubou, A.; Oh, J.S.; Ericson, J.E.; Ssentongo, P.; et al. Short-term and Long-term Rates of Postacute Sequelae of SARS-CoV-2 Infection: A Systematic Review. JAMA Netw. Open 2021, 4, e2128568. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Scherlinger, M.; Felten, R.; Gallais, F.; Nazon, C.; Chatelus, E.; Pijnenburg, L.; Mengin, A.; Gras, A.; Vidailhet, P.; Arnould-Michel, R.; et al. Refining “Long-COVID” by a Prospective Multimodal Evaluation of Patients with Long-Term Symptoms Attributed to SARS-CoV-2 Infection. Infect. Dis. Ther. 2021, 10, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.G.; Monte, A.A.; Littlefield, K.; Vest, A.; Palmer, B.E. Vaccination Hesitancy and Postacute Sequelae of SARS-CoV-2: Is It Time to Reconsider? Viral Immunol. 2021, 34, 666–668. [Google Scholar] [CrossRef] [PubMed]
- Desimmie, B.A.; Raru, Y.Y.; Awadh, H.M.; He, P.; Teka, S.; Willenburg, K.S. Insights into SARS-CoV-2 Persistence and Its Relevance. Viruses 2021, 13, 1025. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.T.; Milne, A.; Stadon, L.; Maskell, N.A.; Hamilton, F.W. Are vaccines safe in patients with Long COVID? A prospective observational study. medRxiv 2021. [Google Scholar] [CrossRef]
- HAS. Symptômes Prolongés Suite à une COVID-19 de L’adulte—Diagnostic et Prise en Charge. Haute Autorité de Santé. 2021. Available online: https://www.has-sante.fr/jcms/p_3237041/fr/symptomes-prolonges-suite-a-une-covid-19-de-l-adulte-diagnostic-et-prise-en-charge (accessed on 24 June 2021).
- Tran, V.T.; Riveros, C.; Clepier, B.; Desvarieux, M.; Collet, C.; Yordanov, Y.; Ravaud, P. Development and validation of the long covid symptom and impact tools, a set of patient-reported instruments constructed from patients’ lived experience. Clin. Infect. Dis. 2021. epub ahead of print. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.F.; Gouraud, C.; et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern. Med. 2021, e216454. [Google Scholar] [CrossRef]
- Machingaidze, S.; Wiysonge, C.S. Understanding COVID-19 vaccine hesitancy. Nat. Med. 2021, 27, 1338–1339. [Google Scholar] [CrossRef] [PubMed]
- Peghin, M.; Palese, A.; Venturini, M.; De Martino, M.; Gerussi, V.; Graziano, E.; Bontempo, G.; Marrella, F.; Tommasini, A.; Fabris, M.; et al. Post-COVID-19 symptoms 6 months after acute infection among hospitalized and non-hospitalized patients. Clin. Microbiol. Infect. 2021, 27, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Gray-Burrows, K.A.; Willis, T.A.; Foy, R.; Rathfelder, M.; Bland, P.; Chin, A.; Hodgson, S.; Ibegbuna, G.; Prestwich, G.; Samuel, K.; et al. Role of patient and public involvement in implementation research: A consensus study. BMJ Qual. Saf. 2018, 27, 858–864. [Google Scholar] [CrossRef] [Green Version]
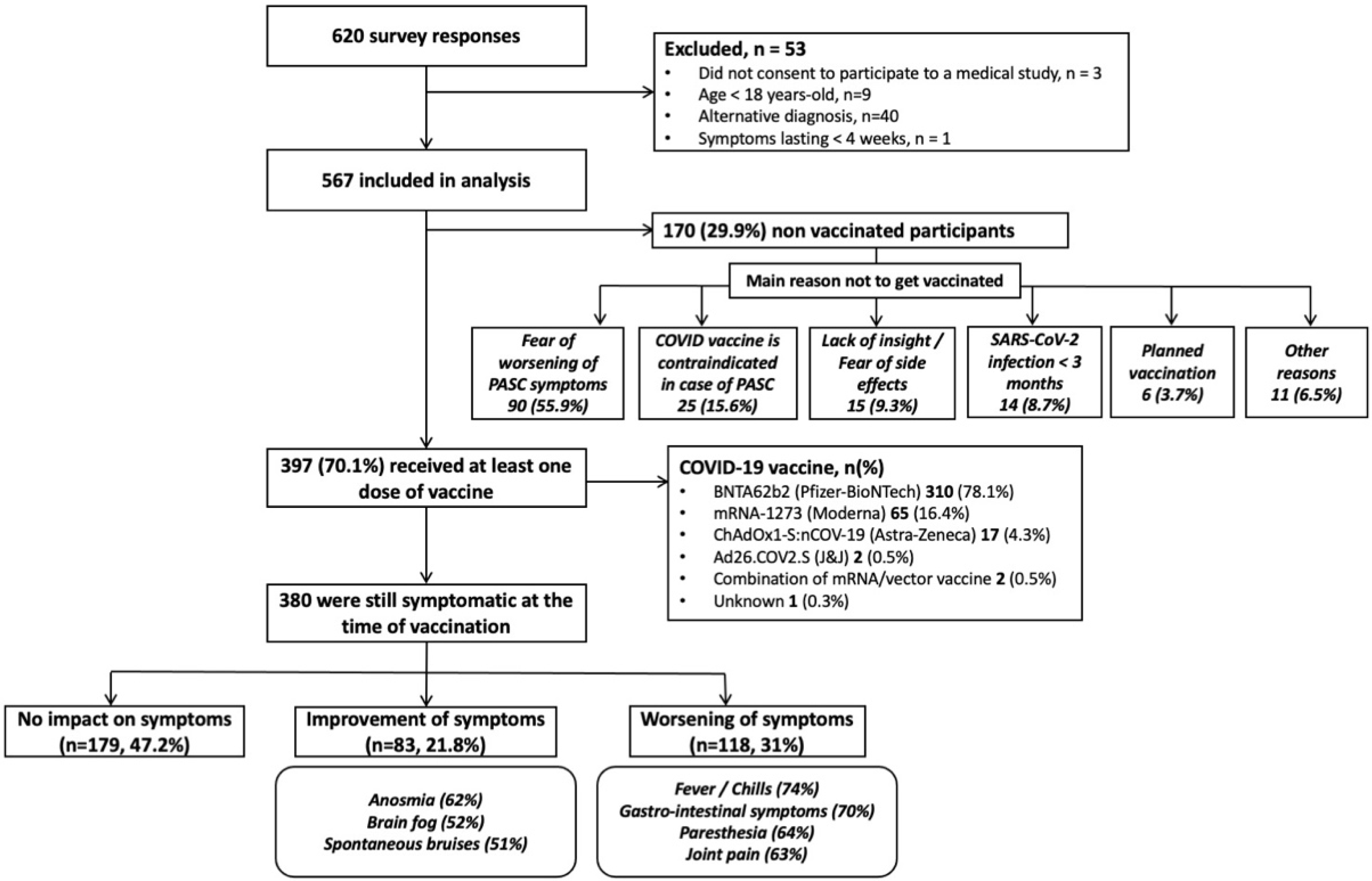
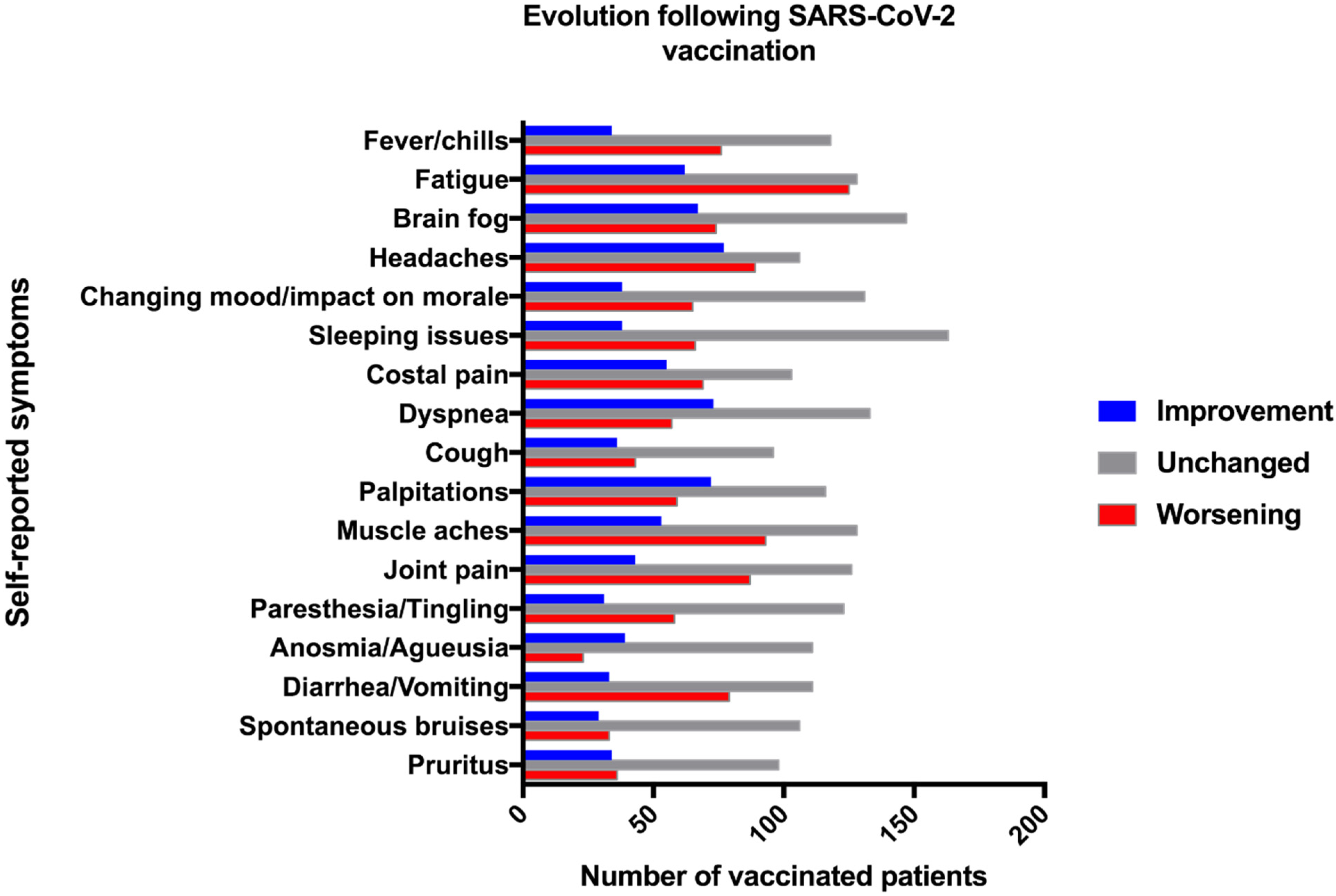
| Total PASC Population (n = 567) | Vaccinated Population (n = 397) | Non-Vaccinated Population (n = 170) | p-Value Vaccinated vs. Non-Vaccinated | |
|---|---|---|---|---|
| Female sex, % (n) | 83.4% (473) | 85.9% (327) | 82.4% (146) | ns |
| Age, median (IQR) | 44 (37–50) | 44 (37–50) | 42 (36–49) | ns |
COVID-19 severity
| 94.9% (538) | 95.3% (376) | 94.7% (162) | ns |
| 5.1 (25) | 4.7 (18) | 5 3 (7) | ||
| 0.7% (4) | 0.8% (3) | 0.6% (1) | ||
Confirmed COVID-19, % (n)
| 64.4% (365) | 63% (250) | 67.7% (115) | ns |
| 45% (255) | 43.1% (171) | 49.4% (84) | ||
| 22.8% (129) | 22.2% (88) | 24.1% (41) | ||
| 32.3% (183) | 30.2% (120) | 37.1% (63) | ||
| 8.6% (49) | 8.1% (32) | 10% (17) | ||
| Time since initial COVID-19, days, median (IQR) | 475 (261–506) | 483 (266–506) | 325 (180–507) | 0.0066 |
| Number of persisting symptoms, median (IQR) | 12 (9–15) | 12 (9–15) | 13 (10–15) | ns |
Professional activity
| 45% (255) | 47.4% (188) | 39.4% (67) | 0.016 |
| 18% (102) | 19.4% (77) | 14.7% (25) | ||
| 37% (210) | 33.3% (132) | 45.9% (78) | ||
Vaccination
| - | 357 (198–431) | - - | - |
| 64.2% (255) | ||||
| 35.8% (142) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scherlinger, M.; Pijnenburg, L.; Chatelus, E.; Arnaud, L.; Gottenberg, J.-E.; Sibilia, J.; Felten, R. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines 2022, 10, 46. https://doi.org/10.3390/vaccines10010046
Scherlinger M, Pijnenburg L, Chatelus E, Arnaud L, Gottenberg J-E, Sibilia J, Felten R. Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines. 2022; 10(1):46. https://doi.org/10.3390/vaccines10010046
Chicago/Turabian StyleScherlinger, Marc, Luc Pijnenburg, Emmanuel Chatelus, Laurent Arnaud, Jacques-Eric Gottenberg, Jean Sibilia, and Renaud Felten. 2022. "Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study" Vaccines 10, no. 1: 46. https://doi.org/10.3390/vaccines10010046
APA StyleScherlinger, M., Pijnenburg, L., Chatelus, E., Arnaud, L., Gottenberg, J.-E., Sibilia, J., & Felten, R. (2022). Effect of SARS-CoV-2 Vaccination on Symptoms from Post-Acute Sequelae of COVID-19: Results from the Nationwide VAXILONG Study. Vaccines, 10(1), 46. https://doi.org/10.3390/vaccines10010046







