Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review
Abstract
:1. Highlights
2. Introduction
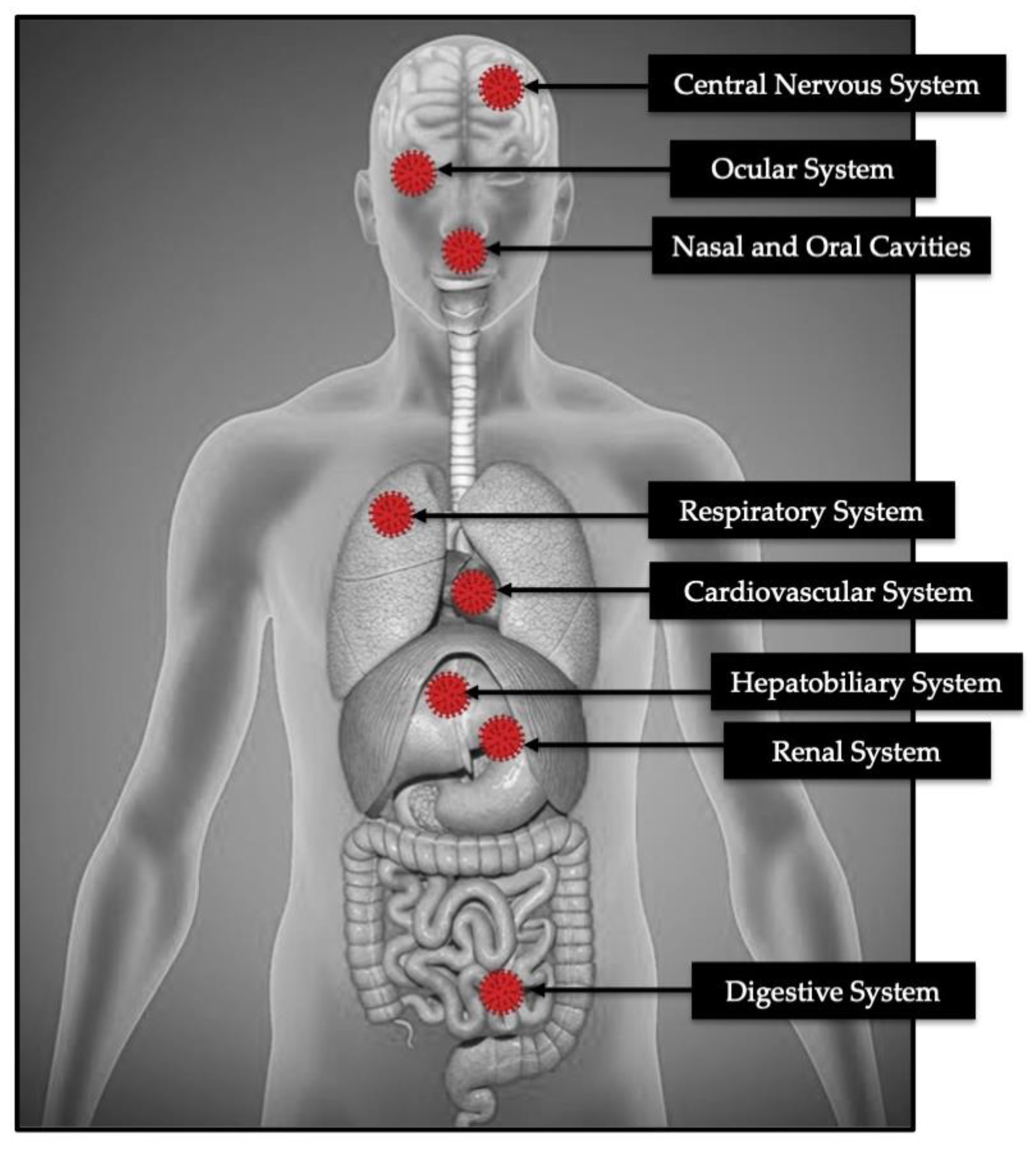
3. Pathophysiology and Etiopathogenesis
3.1. The Behaviour of the Virus
3.1.1. Type I Interferon (IFN-1) Signalling Pathway
3.1.2. Role of Mitochondrial Antiviral-Signalling Protein (MAVS) and IIR
3.1.3. Host–Viral Interaction
3.2. Cytokines Outburst in COVID-19
3.3. The Severity of COVID-19 in Co-Relation to Biochemistry Analysis
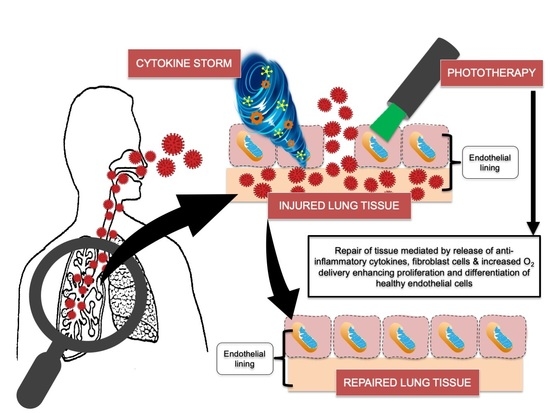
4. The Rational in Utilising Phototherapy in COVID-19 Management
4.1. Photobiomodulation (PBM) Therapy (PBMT)
4.1.1. Mechanisms of Action and Its Relation to COVID-19
ATP Versus COVID-19
NO Versus COVID-19
ROS
4.1.2. PBM Effects on Modulating the Immune Response
4.1.3. Effects of PBMT on Angiogenesis Versus COVID-19
4.1.4. Light Emitted Diodes (LEDs)
4.2. Photodynamic Therapy (PDT)
4.2.1. Mechanism of Action of PDT
4.2.2. Biological Response of Viral Infections to PDT
4.2.3. Impact of Various PS on Viruses
Methylene Blue (MB)
Indocyanine Green (ICG)
ALA and NANO-PS
4.3. Ultrashort Pulsed (USP) Laser as an Antiviral Agent
4.4. Ultraviolet (UV) Therapy
5. Potential Future Scope of Phototherapy in Augmenting the COVID-19 Vaccine Production
5.1. Utilisation of USP Laser Irradiation for Inactivation of SARS-CoV-2 to Optimise Vaccine Production
5.2. Potential Role of Lasers as COVID-19 Vaccine Adjuvants
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jin, Y.; Yang, H.; Ji, W.; Wu, W.; Chen, S.; Zhang, W.; Duan, G. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses 2020, 12, 372. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Liu, S.M.; Yu, X.H.; Tang, C.L.; Tang, C.K. Coronavirus disease 2019 (COVID-19): Current status and future perspectives. Int. J. Antimicrob. Agents 2020, 55, 105951. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R. Photobiomodulation and Antiviral Photodynamic Therapy as a Possible Novel Approach in COVID-19 Management. Photobiomodul. Photomed. Laser Surg. 2020, 38, 255–257. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Situation Update Worldwide, as of 15 September 2020. Available online: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases (accessed on 15 September 2020).
- Esmaeelinejad, M.; Bayat, M. Effect of low-level laser therapy on the release of interleukin-6 and basic fibroblast growth factor from cultured human skin fibroblasts in normal and high glucose mediums. J. Cosmet. Laser Ther. 2013, 15, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Usumez, A.; Cengiz, B.; Oztuzcu, S.; Demir, T.; Aras, M.H.; Gutknecht, N. Effects of laser irradiation at different wavelengths (660, 810, 980, and 1,064 nm) on mucositis in an animal model of wound healing. Lasers Med. Sci. 2014, 29, 1807–1813. [Google Scholar] [CrossRef]
- Kuffler, D.P. Photobiomodulation in promoting wound healing: A review. Regen. Med. 2016, 11, 107–122. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Johnson, M.I.; Iversen, V.; Aimbire, F.; Lopes-Martins, R.A. Low-level laser therapy in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed. Laser Surg. 2006, 24, 158–168. [Google Scholar] [CrossRef] [Green Version]
- Alayat, M.S.M.; Atya, A.M.; Ali, M.M.E.; Shousha, T.M. Correction to: Long-term effect of high-intensity laser therapy in the treatment of patients with chronic low back pain: A randomized blinded placebo-controlled trial. Lasers Med. Sci. 2020, 35, 297. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, A.P.; Paraguassú, G.M.; Silveira, N.T.; de Souza, J.; Cangussú, M.C.; dos Santos, J.N.; Pinheiro, A.L.B. Laser and LED phototherapies on angiogenesis. Lasers Med. Sci. 2013, 28, 981–987. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Nagata, K.; Tedford, C.E.; McCarthy, T.; Hamblin, M.R. Low-level laser therapy (LLLT) reduces oxidative stress in primary cortical neurons in vitro. J. Biophotonics. 2013, 6, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef] [PubMed]
- Kashanskaia, E.P.; Fedorov, A.A. Low-intensity laser radiation in the combined treatment of patients with chronic obstructive bronchitis. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2009, 2, 19–22. [Google Scholar]
- de Lima, F.M.; Villaverde, A.B.; Albertini, R.; Correa, J.C.; Carvalho, R.L.; Munin, E.; Araujo, T.; Silva, J.A.; Aimbire, F. Dual Effect of low-level laser therapy (LLLT) on the acute lung inflammation induced by intestinal ischemia and reperfusion: Action on anti- and pro-inflammatory cytokines. Lasers Surg. Med. 2011, 43, 410–420. [Google Scholar] [CrossRef]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.G.; Ferreira, A.P.; Côrtes, A.J.; Aarestrup, B.J.; Andrade, L.C.; Aarestrup, F.M. Low-level laser reduces the production of TNF-alpha, IFN-gamma, and IL-10 induced by OVA. Lasers Med. Sci. 2013, 28, 1519–1525. [Google Scholar] [CrossRef]
- Aimbire, F.; Lopes-Martins, R.A.; Castro-Faria-Neto, H.C.; Leonardo, P.S.; Iversen, V.V.; Lopes-Martins, R.A. Low-level laser therapy can reduce lipopolysaccharide-induced contractile force dysfunction and TNF-alpha levels in rat diaphragm muscle. Lasers Med. Sci. 2006, 21, 238–244. [Google Scholar] [CrossRef]
- Szymanska, J.; Goralczyk, K.; Klawe, J.J.; Lukowicz, M.; Michalska, M.; Goralczyk, B.; Zalewski, P.; Newton, J.L.; Gryko, L.; Zajac, A.; et al. Phototherapy with low-level laser influences the proliferation of endothelial cells and vascular endothelial growth factor and transforming growth factor-beta secretion. J. Physiol. Pharmacol. 2013, 64, 387–391. [Google Scholar] [PubMed]
- Agaiby, A.D.; Ghali, L.R.; Wilson, R.; Dyson, M. Laser modulation of angiogenic factor production by T-lymphocytes. Lasers Surg. Med. 2000, 26, 357–363. [Google Scholar]
- Basso, F.G.; Oliveira, C.F.; Kurachi, C.; Hebling, J.; Costa, C.A. Biostimulatory effect of low-level laser therapy on keratinocytes in vitro. Lasers Med. Sci. 2013, 28, 367–374. [Google Scholar] [CrossRef]
- Oliveira, M.C., Jr.; Greiffo, F.R.; Rigonato-Oliveira, N.C.; Custodio, R.W.; Silva, V.R.; Damaceno-Rodrigues, N.R.; Almeida, F.M.; Albertini, R.; Lopes-Martins, R.A.B.; de Oliveira, L.V.F. Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J. Photochem. Photobiol. B 2014, 134, 57–63. [Google Scholar] [CrossRef]
- St Denis, T.G.; Dai, T.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light: Antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Pathogen inactivation in blood products. Curr. Med. Chem. 2002, 9, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, S.; Brauns, T.; Gelfand, J.; Poznansky, M.C. Laser vaccine adjuvants. History, progress, and potential. Hum. Vaccin. Immunother. 2014, 10, 1892–1907. [Google Scholar] [CrossRef] [PubMed]
- Sallard, E.; Lescure, F.X.; Yazdanpanah, Y.; Mentre, F.; Peiffer-Smadja, N. Type 1 interferons as a potential treatment against COVID-19. Antivir. Res. 2020, 178, 104791. [Google Scholar] [CrossRef] [PubMed]
- de Wit, E.; van Doremalen, N.; Falzarano, D.; Munster, V.J. SARS and MERS: Recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016, 14, 523–534. [Google Scholar] [CrossRef]
- Lokugamage, K.G.; Hage, A.; de Vries, M.; Valero-Jimenez, A.M.; Schindewolf, C.; Dittmann, M.; Rajsbaum, R.; Menachery, V.D. SARS-CoV-2 sensitive to type I interferon pretreatment. BioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef]
- Dong, L.; Hu, S.; Gao, J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Lu, H. Drug treatment options for the 2019-new coronavirus (2019-nCoV). Biosci. Trends 2020, 14, 69–71. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, C.; Horner, S.M. MAVS coordination of antiviral innate immunity. J. Virol. 2015, 89, 6974–6977. [Google Scholar] [CrossRef] [Green Version]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-KappaB and IRF3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leung, T.; Hoffmann, A.; Baltimore, D. One nucleotide in a kappa B site can determine cofactor specificity for NF-kappa B dimers. Cell 2004, 118, 453–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwanaszko, M.; Kimmel, M. NF-κB and IRF pathways: Cross-regulation on target genes promoter level. BMC Genom. 2015, 16, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, I. Mitochondrial factors in the regulation of innate immunity. Microbes Infect. 2009, 11, 729–736. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, A.; Tiwari-Pandey, R.; Pandey, N.R. Mitochondria: The indispensable players in innate immunity and guardians of the inflammatory response. J. Cell Commun. Signal 2019, 13, 303–318. [Google Scholar] [CrossRef]
- Jacobs, J.L.; Coyne, C.B. Mechanisms of MAVS regulation at the mitochondrial membrane. J. Mol. Biol. 2013, 425, 5009–5019. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Horton, R. COVID-19- bewilderment and candour. Lancet 2020, 395, 1178. [Google Scholar] [CrossRef]
- Frieman, M.; Heise, M.; Baric, R. SARS coronavirus and innate immunity. Virus Res. 2008, 133, 101–112. [Google Scholar] [CrossRef]
- Inoue, Y.; Tanaka, N.; Tanaka, Y.; Inoue, S.; Morita, K.; Zhuang, M.; Hattori, T.; Sugamura, K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007, 81, 8722–8729. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.B.; Tallant, E.A.; Diz, D.I.; Gallagher, P.E. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef] [Green Version]
- Simmons, G.; Gosalia, D.N.; Rennekamp, A.J.; Reeves, J.D.; Diamond, S.L.; Bates, P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. USA 2005, 102, 11876–11881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef] [PubMed]
- Lovren, F.; Quan, A.; Teoh, H.; Wong, G.; Shukla, P.C.; Levitt, K.S.; Oudit, G.Y.; Al-Omran, M.; Stewart, D.J.; Slutsky, A.S.; et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1377–H1384. [Google Scholar] [CrossRef] [Green Version]
- Cao, W.; Li, T. COVID-19: Towards understanding of pathogenesis. Cell Res. 2020, 30, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar]
- Velavan, T.P.; Meyer, C.G. Mild versus severe COVID-19: Laboratory markers. Int. J. Infect. Dis. 2020, 95, 304–307. [Google Scholar] [CrossRef]
- Mangalmurti, N.; Hunter, C.A. Cytokine Storms: Understanding COVID-19. Immunity 2020, 53, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Zhou, L.; Hu, Z.; Zhang, S.; Yang, S.; Tao, Y. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020, 71, 762–768. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Wang, X.; Xu, W.; Hu, G.; Xia, S.; Sun, Z.; Liu, Z.; Xie, Y.; Zhang, R.; Jiang, S.; Lu, L. Retraction Note to: SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell Mol. Immunol. 2020, 17, 894. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothan, H.A.; Byrareddy, S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 10433. [Google Scholar] [CrossRef]
- Li, X.; Geng, M.; Peng, Y.; Meng, L.; Lu, S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm. Anal. 2020, 10, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.C.; Maddox, T.M.; Messerli, F.H. Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiol. 2020, 5, 745–747. [Google Scholar] [CrossRef] [Green Version]
- Loeffelholz, M.J.; Tang, Y.W. Laboratory diagnosis of emerging human coronavirus infections- the state of the art. Emerg. Microbes Infect. 2020, 9, 747–756. [Google Scholar] [CrossRef]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkruys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garretta, E.; Del Pozo, C.H.; Prosper, F.; et al. Inhibition of SARS- CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913. [Google Scholar] [CrossRef]
- Solmaz, H.; Ulgen, Y.; Gulsoy, M. Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med. Sci. 2017, 32, 903–910. [Google Scholar] [CrossRef]
- Lee, N.; Wigg, J.; Carroll, J.D. The use of low-level light therapy in the treatment of head and neck oedema. J. Lymphoedema 2013, 8, 35–42. [Google Scholar]
- Wüst, R.C.; Degens, H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2007, 2, 289–300. [Google Scholar] [PubMed]
- Jere, S.W.; Houreld, N.N.; Abrahamse, H. Photobiomodulation and the expression of genes related to the JAK/STAT signaling pathway in wounded and diabetic wounded cells. J. Photochem. Photobiol. B 2020, 204, 111791. [Google Scholar] [CrossRef]
- Mokoena, D.R.; Houreld, N.N.; Kumar, S.S.D.; Abrahamse, H. Photobiomodulation at 660 nm Stimulates Fibroblast Differentiation. Lasers Surg. Med. 2020, 52, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; De Gregorio, V.; Fusco, A.; Farina, E.; Baroni, A.; Espositio, V.; Contaldo, M.; Petruzzi, M.; Pannone, G.; Serpico, R. Inhibition of HSV-1 replication by laser diode-irradiation: Possible mechanism of action. Int. J. Immunopathol. Pharmacol. 2010, 23, 1167–1176. [Google Scholar] [CrossRef]
- Percival, S.L.; Francolini, I.; Donelli, G. Low-level laser therapy as an antimicrobial and antibiofilm technology and its relevance to wound healing. Future Microbiol. 2015, 10, 55–272. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. J. Photochem. Photobiol. B 2018, 94, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.C.H.; Huang, Y.Y.; Arany, P.R.; Hamblin, M.R. Role of reactive oxygen species in low level light therapy. Proc. SPIE 2009, 716502–716511. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnstock, G. Purines and sensory nerves. Handb. Exp. Pharmacol. 2009, 194, 333–392. [Google Scholar]
- Huang, Y.Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar] [CrossRef]
- Riteau, N.; Baron, L.; Villeret, B.; Guillou, N.; Savigny, F.; Ryffel, B.; Rassendren, F.; Le Bert, M.; Gombault, A.; Couillin, I. ATP release and purinergic signaling: A common pathway for particle-mediated inflammasome activation. Cell Death Dis. 2012, 3, e403. [Google Scholar] [CrossRef] [Green Version]
- Ferraresi, C.; Hamblin, M.R.; Parizotto, N.A. Low-level laser (light) therapy (LLLT) on muscle tissue: Performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012, 1, 267–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferraresi, C.; Kaippert, B.; Avci, P.; Huang, Y.Y.; de Sousa, M.V.; Bagnato, V.S.; Parizotto, N.A.; Hamblin, M.R. Low-level laser (light) therapy increases mitochondrial membrane potential and ATP synthesis in C2C12 myotubes with a peak response at 3-6 h. Photochem. Photobiol. 2015, 91, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Ferraresi., C.; Parizotto, N.A.; de Sousa, M.V.; Kaippert, B.; Huang, Y.Y.; Koso, T.; Bagnato, V.S.; Hamblin, M.R. Light- emitting diode therapy in exercise-trained mice increases muscle performance, cytochrome c oxidase activity, ATP and cell proliferation. J. Biophotonics 2015, 8, 740–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Reddy, D.D.; Nalawade, S.S.; Pal, S.; Gonzalez-Lima, F.; Liu, H. Impact of heat on metabolic and hemodynamic changes in transcranial infrared laser stimulation measured by broadband near-infrared spectroscopy. Neurophotonics 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Linares, S.N.; Beltrame, T.; Ferraresi, C.; Galdino, G.A.M.; Catai, M. Photobiomodulation effect on local hemoglobin concentration assessed by near-infrared spectroscopy in humans. Lasers Med. Sci. 2020, 35, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tian, F.; Soni, S.S.; Gonzalez- Lima, F.; Liu, H. Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci. Rep. 2016, 6, 30540. [Google Scholar] [CrossRef] [Green Version]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric oxide and geriatrics: Implications in diagnostics and treatment of the elderly. J. Geriatr. Cardiol. 2011, 8, 230–242. [Google Scholar] [CrossRef] [Green Version]
- Åkerström, S.; Gunalan, V.; Keng, C.T.; Tan, Y.J.; Mirazimi, A. Dual effect of nitric oxide on SARS-CoV replication: Viral RNA production and palmitoylation of the S protein are affected. Virology 2009, 395, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Berardi, M.; Li, W.; Farzan, M.; Dormitzer, P.R.; Harrison, S.C. Conformational states of the severe acute respiratory syndrome coronavirus spike protein ectodomain. J. Virol. 2006, 80, 6794–6800. [Google Scholar] [CrossRef] [Green Version]
- Akerstrom, S.; Mousavi-Jazi, M.; Klingstrom, J.; Leijon, M.; Lundkvist, A.; Mirazimi, A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol. 2005, 79, 1966–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keyaerts, E.; Vijgen, L.; Chen, L.; Maes, P.; Göran Hedenstierna, P.G.; Ranst, M.V. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004, 8, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitric Oxide Gas Inhalation for Severe Acute Respiratory Syndrome in COVID-19. (NOSARSCOVID). Available online: https://clinicaltrials.gov/ct2/show/NCT04290871 (accessed on 15 September 2020).
- Mitchell, U.H.; Mack, G.L. Low-level laser treatment with near-infrared light increases venous nitric oxide levels acutely: A single-blind, randomized clinical trial of efficacy. Am. J. Phys. Med. Rehabil. 2013, 92, 151–156. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaroli, A.; Ravera, S.; Baldini, F.; Benedicenti, S.; Panfoli, I.; Vergani, L. Photobiomodulation with 808-nm diode laser light promotes wound healing of human endothelial cells through increased reactive oxygen species production stimulating mitochondrial oxidative phosphorylation. Lasers Med. Sci. 2019, 34, 495–504. [Google Scholar] [CrossRef]
- Góralczyk, K.; Szymańska, J.; Lukowicz, M.; Drela, E.; Kotzbach, R.; Dubiel, M.; Michalska, M.; Góralczyk, B.; Zajac, A.; Rosc, D. Effect of LLLT on endothelial cells culture. Lasers Med. Sci. 2015, 30, 273–278. [Google Scholar] [CrossRef]
- Fujimaki, Y.; Shimoyama, T.; Liu, Q.; Umeda, T.; Nakaji, S.; Sugawara, K. Low-level laser irradiation attenuates production of reactive oxygen species by human neutrophils. J. Clin. Laser Med. Surg. 2003, 21, 165–170. [Google Scholar] [CrossRef]
- Chow, R.T.; Johnson, M.I.; Lopes-Martins, R.A.; Bjordal, J.M. Efficacy of low-level laser therapy in the management of neck pain: A systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet 2009, 374, 1897–1908. [Google Scholar] [CrossRef]
- Alves, C.; Moraes, G.A.C.; Brito, A.A.; Santos, G.T.; Rigonato-Oliveira, N.C.; Vitoretti, B.L.; Soares, S.S.; Matos, Y.; Rangel, M.A.; Aimbire, F.; et al. Low Level Laser therapy (LLL) modulates pulmonary immune response and expression of P2X7 purinergic receptor in experimental model of Chronic Obstructive Pulmonary Disorder (COPD). Eur. Respir. J. 2017, 50, PA4457. [Google Scholar] [CrossRef]
- Mehani, S.H.M. Immunomodulatory effects of two different physical therapy modalities in patients with chronic obstructive pulmonary disease. J. Phys. Ther. Sci. 2017, 29, 1527–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergio, L.; Thome, A.M.C.; Trajano, L.; Mencalha, A.L.; da Fonseca, A.S.; de Paoli, F. Photobiomodulation prevents DNA fragmentation of alveolar epithelial cells and alters the mRNA levels of caspase 3 and Bcl-2 genes in acute lung injury. Photochem. Photobiol. Sci. 2018, 17, 975–983. [Google Scholar] [CrossRef]
- du Cunha Moraes, G.; Vitoretti, L.B.; de Brito, A.A.; Alves, C.E.; de Oliveira, N.C.R.; Dias, A.D.S.; Matos, Y.S.T.; Oliveira, M.C., Jr.; Oliveira, L.V.F.; da Palma, R.K.; et al. Low-Level Laser Therapy Reduces Lung Inflammation in an Experimental Model of Chronic Obstructive Pulmonary Disease Involving P2X7 Receptor. Oxid. Med. Cell Longev. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Assis, L.; Moretti, A.I.; Abrahao, T.B.; Cury, V.; Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012, 44, 726–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hentschke, V.S.; Jaenisch, R.B.; Schmeing, L.A.; Cavinato, P.R.; Xavier, L.L.; Dal Lago, P. Low-level laser therapy improves the inflammatory profile of rats with heart failure. Lasers Med. Sci. 2013, 28, 1007–1016. [Google Scholar] [CrossRef]
- de Lima, F.M.; Aimbire, F.; Miranda, H.; Vieira, R.P.; de Oliveira, A.P.; Albertini, R. Low-level laser therapy attenuates the myeloperoxidase activity and inflammatory mediator generation in lung inflammation induced by gut ischemia and reperfusion: A dose-response study. J. Lasers Med. Sci. 2014, 5, 63–70. [Google Scholar] [PubMed]
- de Lima, F.M.; Albertini, R.; Dantas, Y.; Maia-Filho, A.L.; Santana, C.L.; Castro-Faria-Neto, H.C.; Franca, C.; Villaverde, A.B.; Aimbire, F. Low-level laser therapy restores the oxidative stress balance in acute lung injury induced by gut ischemia and reperfusion. Photochem. Photobiol. 2013, 89, 179–188. [Google Scholar] [CrossRef]
- da Silva, M.C.; Leal, M.P.; Brochetti, R.A.; Braga, T.; Vitoretti, L.B.; Camara, N.O.S.; Damazo, A.S.; de Oliveira, A.P.; Chavantes, M.C.; Franco, A. Low Level Laser Therapy Reduces the Development of Lung Inflammation Induced by Formaldehyde Exposure. PLoS ONE 2015, 10, e0142816. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.R.; Marcondes, P.; Silva, M.; Villaverde, A.B.; Castro-Faria-Neto, H.C.; Vieira, R.P.; Aimbire, F.; de Oliveira, A.P. Low-level laser therapy inhibits bronchoconstriction, Th2 inflammation and airway remodeling in allergic asthma. Respir. Physiol. Neurobiol. 2014, 194, 37–48. [Google Scholar] [CrossRef]
- Peron, J.P.S.; de Brito, A.A.; Pelatti, M.; Brandao, W.N.; Vitoretti, L.B.; Greiffo, F.R.; de Silveira, E.C.; Oliveira, M.C., Jr.; Maluf, M.; Evagelista, L.; et al. Human tubal derived mesenchymal stromal cells associated with low level laser therapy significantly reduces cigarette smoke-induced COPD in C57 BL/ 6 mice. PLoS ONE 2015, 10, e0136942. [Google Scholar] [CrossRef] [Green Version]
- Mura, M.; Andrade, C.F.; Han, B.; Seth, R.; Zhang, Y.; Bai, X.-H.; Waddel, T.K.; Hwang, D.; Keshavjee, S.; Liu, M. Intestinal ischemia- reperfusion-induced acute lung injury and oncotic cell death in multiple organs. Shock 2007, 28, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Cury, V.; Moretti, A.I.S.; Assis, L.; Bossinia, P.; Crusca, J.S.; Neto, C.B.; Fangel, R.; de Souza, H.P.; Hamblin, M.R.; Parizotto, N.A. Low-level laser therapy increases angiogenesis in a model of ischemic skin flap in rats mediated by VEGF, HIF-1α and MMP-2. Photochem. Photobiol. B 2014, 125, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, Y.L.; Chou, L.W.; Chang, P.L.; Yang, C.C.; Kao, M.J.; Hong, C.Z. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: Possible involvements in hypoxia-inducible factor 1α (HIF-1α). J. Comp. Neurol. 2012, 520, 2903–2916. [Google Scholar] [CrossRef]
- Thais-Meneguzzo, D.; Soares-Ferreira, L.; de Carvalho, M.E.; Fukuda-Nakashima, C. Intravascular laser irradiation of blood. In Low-Level Light Therapy: Photobiomodulation; SPIE Press: Bellingham, WA, USA, 2018; pp. 319–330. [Google Scholar]
- Szymczyszyn, A.; Doroszko, A.; Szahidewicz-Krupska, E.; Rola, P.; Gutherc, R.; Jasiczek, J.; Mazur, G.; Derkacz, A. Effect of the transdermal low-level laser therapy on endothelial function. Lasers Med. Sci. 2016, 31, 1301–1307. [Google Scholar] [CrossRef]
- Muili, K.A.; Gopalakrishnan, S.; Meyer, S.L.; Eells, J.T.; Lyons, J.A. Amelioration of experimental autoimmune encephalomyelitis in C57BL/6 mice by photobiomodulation induced by 670 nm light. PLoS ONE 2012, 7, e30655. [Google Scholar] [CrossRef] [Green Version]
- Oron, A.; Oron, U. Low-level laser therapy to the bone marrow ameliorates neurodegenerative disease progression in a mouse model of Alzheimer’s disease: A minireview. Photomed. Laser Surg. 2016, 34, 627–630. [Google Scholar] [CrossRef]
- Domínguez, A.; Velásquez, S.A.; David, M.A. Can transdermal photobiomodulation help us at the time of COVID-19? Photobiomodul. Photomed. Laser Surg. 2020, 38, 1–2. [Google Scholar] [CrossRef]
- Fisher-Wellman, K.; Bell, H.K.; Bloomer, R.J. Oxidative stress and antioxidant defense mechanisms linked to exercise during cardiopulmonary and metabolic disorders. Oxid. Med. Cell Longev. 2009, 2, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Milic, V.D.; Stankov, K.; Injac, R.; Djordjevic, A.; Srdjenovic, B.; Govedarica, B.; Radic, N.; Simic, V.D.; Strukelj, B. Activity of antioxidative enzymes in erythrocytes after a single dose administration of doxorubicin in rats pretreated with fullerenol C(60)(OH)(24). Toxicol. Mech. Methods 2009, 19, 24–28. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, J.F.; Shen, Y.; Wang, W.; Wei, Y.J.; Hu, S. Low level laser irradiation precondition to create friendly milieu of infarcted myocardium and enhance early survival of transplanted bone marrow cells. J. Cell Mol. Med. 2010, 14, 1975–1987. [Google Scholar] [CrossRef] [Green Version]
- Helrigle, C.; de Carvalho, P.D.; Casalechi, H.L.; Leal-Junior, E.C.P.; Fernandes, G.H.C.; Helrigel, P.A.; Rabelo, R.L.; Alexio-Junior, I.O.; Aimbire, F.; Albertini, R. Effects of low-intensity non-coherent light therapy on the inflammatory process in the calcaneal tendon of ovariectomized rats. Lasers Med. Sci. 2016, 31, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kuboyama, N.; Ohta, M.; Sato, Y.; Abiko, Y. Anti-inflammatory activities of light emitting diode irradiation on collagen-induced arthritis in mice (a secondary publication). Laser Ther. 2014, 23, 191–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, H.; Lim, W.; Kim, I.; Kim, J.; Ko, Y.; Kwon, H.; Kim, S.; Kabir, K.M.; Li, X.; Kim, O.; et al. Inflammatory cytokines are suppressed by light-emitting diode irradiation of P. gingivalis LPS-treated human gingival fibroblasts: Inflammatory cytokine changes by LED irradiation. Lasers Med. Sci. 2012, 27, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Mamalis, A.; Jagdeo, J. Light-emitting diode-generated red light inhibits keloid fibroblast proliferation. Dermatol. Surg. 2015, 41, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Brochetti, R.A.; Leal, M.P.; Rodrigues, R.; da Palma, R.K.; de Oliveira, L.V.F.; Horliana, A.C.; Damazo, A.S.; de Oliveira, A.P.L.; Vieira, R.P.; Franco, A.L. Photobiomodulation therapy improves both inflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med. Sci. 2017, 32, 1825–1834. [Google Scholar] [CrossRef]
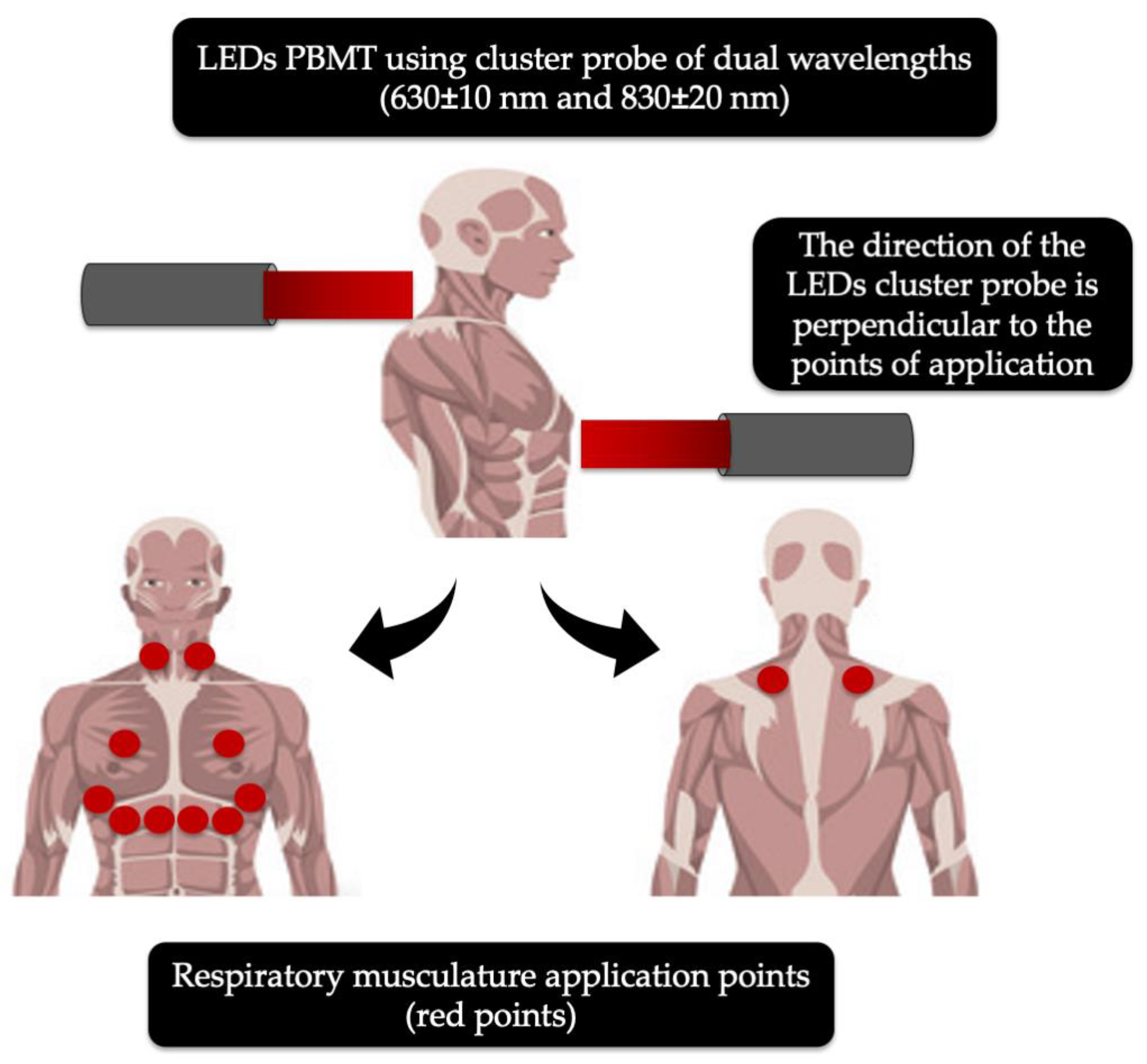
- de Souza, G.H.M.; Ferraresi, C.; Moreno, M.A.; Pessoa, B.V.; Damiani, A.P.M.; Filho, V.G.; Dos Santos, G.V.; Zamuner, A.R. Acute effects of photobiomodulation therapy applied to respiratory muscles of chronic obstructive pulmonary disease patients: A double-blind, randomized, placebo-controlled crossover trial. Lasers Med. Sci. 2020, 35, 1055–1063. [Google Scholar] [CrossRef]
- Hamblin, M.R. The role of nitric oxide in low level light therapy. Proc. SPIE 2008, 6846, 1–14. [Google Scholar] [CrossRef]
- Uozumi, Y.; Nawashiro, H.; Sato, S.; Kawauchi, S.; Shima, K.; Kikuchi, M. Targeted increase in cerebral blood flow by transcranial near-infrared laser irradiation. Lasers Surg. Med. 2010, 42, 566–576. [Google Scholar] [CrossRef]
- Kimizuka, Y.; Callahan, J.J.; Huang, Z.; Morse, K.; Katagiri, W.; Shigeta, A.; Bronson, R.; Takeuchi, S.; Shimaoka, Y.; Chan, M.P.; et al. Semiconductor diode laser device adjuvanting intradermal vaccine. Vaccine 2017, 35, 2404–2412. [Google Scholar] [CrossRef] [Green Version]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef] [Green Version]
- Korbelik, M.; Parkins, C.S.; Shibuya, H.; Cecic, I.; Stratford, M.R.; Chaplin, D.J. Nitric oxide production by tumour tissue: Impact on the response to photodynamic therapy. Br. J. Cancer 2000, 82, 1835–1843. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.; Berhanu, G.; Desalegn, C.; Kandi, V. Sever Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2): An update. Cureus 2020, 12, e7423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darnell, M.E.; Subbarao, K.; Feinstone, S.M.; Taylor, D.R. Inactivation of the coronavirus that induces severe acute respiratory syndrome, SARS-CoV. J. Virol. Methods 2004, 121, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Teles, L.; Moreira, B.; Pacheco, M.; Lourenco, L.M.; Neves, M.G.; Tome, J.P.; Faustino, M.A.; Almeida, A. An effective and potentially safe blood disinfection protocol using tetrapyrrolic photosensitizers. Future Med. Chem. 2017, 9, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.J. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus. Med. Rev. 2002, 16, 61–66. [Google Scholar] [CrossRef]
- Darnell, M.E.; Taylor, D.R. Evaluation of inactivation methods for severe acute respiratory syndrome coronavirus in noncellular blood products. Transfusion 2006, 46, 1770–1777. [Google Scholar] [CrossRef] [Green Version]
- Leclercq, I.; Batéjat, C.; Burguière, A.M.; Manuguerra, J.C. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir. Viruses 2014, 8, 585–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Muller, T.H.; Seltsam, A. Inactivation of Ebola virus and Middle East respiratory syndrome coronavirus in platelet concentrates and plasma by ultraviolet C light and methylene blue plus visible light, respectively. Transfusion 2018, 58, 2202–2207. [Google Scholar] [CrossRef] [Green Version]
- Eickmann, M.; Gravemann, U.; Handke, W.; Tolksdorf, F.; Reichenberg, S.; Müller, T.H.; Seltsam, A. Inactivation of three emerging viruses-severe acute respiratory syndrome coronavirus, Crimean-Congo haemorrhagic fever virus and Nipah virus-in platelet concentrates by ultraviolet C light and in plasma by methylene blue plus visible light. Vox. Sang. 2020, 115, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exchange Transfusion Versus Plasma From Convalescent Patients With Methylene Blue in Patients With COVID-19 (COVID-19). Available online: https://clinicaltrials.gov/ct2/show/NCT04376788 (accessed on 8 September 2020).
- Jin, C.; Yu, B.; Zhang, J.; Wu, H.; Zhou, H.; Liu, F.; Lu, X.; Cheng, L.; Jiang, M.; Wu, N. Methylene Blue Photochemical Treatment as a Reliable SARS-CoV-2 Plasma Virus Inactivation Method for Blood Safety and Convalescent Plasma Therapy for the COVID-19 Outbreak. Research Square. 2020. [Pre-print]. Available online: https://www.researchsquare.com/article/rs-17718/v1 (accessed on 15 September 2020). [CrossRef] [Green Version]
- Boni, L.; David, G.; Mangano, A.; Dionigi, G.; Rausei, S.; Spampatti, S.; Cassinotti, E.; Fingerhut, A. Clinical applications of indocyanine green (ICG) enhanced fluorescence in laparoscopic surgery. Surg. Endosc. 2015, 29, 2046–2055. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.; Li, Y.; Zou, Z.; Qiao, W.; Yao, X.; Su, Y.; Guo, H. Inactivation of bovine immunodeficiency virus by photodynamic therapy with HMME. Chin. Opt. Lett. 2008, 6, 944–946. [Google Scholar]
- Choi, M.C.; Jung, S.G.; Park, H.; Lee, S.Y.; Lee, C.; Hwang, Y.Y.; Kim, S.J. Photodynamic Therapy for Management of Cervical Intraepithelial Neoplasia II and III in Young Patients and Obstetric Outcomes. Lasers Surg. Med. 2013, 45, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Ichimura, H.; Yamaguchi, S.; Kojima, A.; Tanaka, T.; Niiya, K.; Takemori, M.; Hasegawa, K.; Nishimura, R. Eradication and reinfection of human papillomavirus after photodynamic therapy for cervical intraepithelial neoplasia. Int. J. Clin. Oncol. 2003, 8, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Li, Y.; Zheng, Y.; Ye, X.; Zheng, L.; Li, C.; Xue, Z. Photoinactivation of cell-free human immunodeficiency virus by hematoporphyrin monomethyl ether. Lasers Med. Sci. 2012, 27, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, I.; Douaisi, M.P.; Mondal, D.; Kane, R.S. Light-activated nanotube–porphyrin conjugates as effective antiviral agents. Nanotechnology 2012, 23, 105101. [Google Scholar] [CrossRef] [PubMed]
- Tsen, S.W.D.; Tsen, Y.S.D.; Tsen, K.T.; Wu, T.C. Selective inactivation of viruses with femtoseconds laser pulses and its potential use for in vitro therapy. J. Healthc. Eng. 2010, 1, 185–196. [Google Scholar] [CrossRef] [Green Version]
- Tsen, K.T.; Tsen, S.W.D.; Fu, Q.; Lindsay, S.M.; Li, Z.; Cope, S.; Vaiana, S.; Kiang, J.G. Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: Insight into the possible inactivation mechanisms. J. Biomed. Opt. 2011, 16, 078003. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.W.D.; Chang, C.L.; Hung, C.F.; Wu, T.C.; Kiang, J.G. Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process. J. Biomed. Opt. 2007, 12, 064030. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.W.D.; Chang, C.L.; Hung, C.F.; Wu, T.C.; Kiang, J.G. Inactivation of viruses with a very low power visible femtosecond laser. J. Phys. Condens. Matter 2007, 19, 322102. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.W.D.; Chang, C.L.; Hung, C.F.; Wu, T.C.; Kiang, J.G. Inactivation of viruses by coherent excitations with a low power visible femtosecond laser. Virol J. 2007, 4, 50. [Google Scholar] [CrossRef] [Green Version]
- Tsen, K.T.; Tsen, S.W.D.; Sankey, O.F.; Kiang, J.G. Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses. J. Phys. Condens. Matter 2007, 19, 472201. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.W.D.; Chang, C.L.; Hung, C.F.; Wu, T.C.; Kiang, J.G. Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses. J. Phys. Condens. Matter 2008, 20, 252205. [Google Scholar] [CrossRef]
- Tsen, K.T.; Tsen, S.W.D.; Fu, Q.; Lindsay, S.M.; Kibler, K.; Jacobs, B.; Wu, T.C.; Karanam, B.; Jagu, S.; Roden, R.B.; et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. J. Biomed. Opt. 2009, 14, 064042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsen, S.W.D.; Wu, T.C.; Kiang, J.G.; Tsen, K.T. Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation. J. Biomed. Sci. 2012, 19, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Hu, X.; Hamblin, M.R. Ultraviolet blood irradiation: Is it time to remember “the cure that time forgot”? J. Photochem. Photobiol. B 2016, 157, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.M.; Hassan, A.M.; Tolah, A.M.; Alsaadi, M.A.; Abunada, Q.; Damanhouri, G.A.; El-Kafrawy, S.A.; Picard-Maureau, M.; Azhar, E.I.; Hindawi, S.I. Amotosalen and ultraviolet A light efficiently inactivate MERS-coronavirus in human platelet concentrates. Transfus. Med. 2019, 29, 434–441. [Google Scholar] [CrossRef] [Green Version]
- Thanh, L.T.; Andreadakis, Z.; Kumar, A.; Roman, R.G.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef]
- Robertson, J.S.; Nicolson, C.; Harvey, R.; Johnson, R.; Major, D.; Guilfoyle, K.; Roseby, S.; Newman, R.; Collin, R.; Wallis, C.; et al. The development of vaccine viruses against pandemic A(H1N1) influenza. Vaccine 2011, 29, 1836–1843. [Google Scholar] [CrossRef]
- Tsen, S.W.D.; Donti, N.; La, V.; Hsieh, W.H.; Li, Y.D.; Knoff, J.; Chen, A.; Wu, T.C.; Hung, C.F.; Achilefu, S.; et al. Chemical-free inactivated whole influenza virus vaccine prepared by ultrashort pulsed laser treatment. J. Biomed. Opt. 2015, 20, 051008. [Google Scholar] [CrossRef]
- Herrera-Rodriguez, J.; Signorazzi, A.; Holtrop, M.; de Vries-Idema, J.; Huckriede, A. Inactivated or damaged? Comparing the effect of inactivation methods on influenza virions to optimize vaccine production. Vaccine 2019, 37, 1630–1637. [Google Scholar] [CrossRef]
- Tsen, S.W.D.; Chapa, T.; Beatty, W.; Xu, B.; Tsen, K.T.; Achilefu, S. Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus. Antivir. Res. 2014, 110, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- She, Y.M.; Cheng, K.; Farnsworth, A.; Li, X.; Cyr, T.D. Surface modifications of influenza proteins upon virus inactivation by β-propiolactone. Proteomics 2013, 13, 3537–3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsen, S.W.D.; Kingsley, D.H.; Poweleit, C.; Achilefu, S.; Soroka, S.D.; Wu, T.C.; Tsen, K.T. Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser. Virol. J. 2014, 11, 20. [Google Scholar] [CrossRef]
- Chen, X.; Kim, P.; Farinelli, B.; Doukas, A.; Yun, S.H.; Gelfand, J.A.; Anderson, R.R.; Wu, M.X. A Novel Laser Vaccine Adjuvant Increases the Motility of Antigen Presenting Cells. PLoS ONE 2010, 5, e13776. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, J.C.; Rodríguez, E.G. Vaccine adjuvants revisited. Vaccine 2007, 25, 3752–3762. [Google Scholar] [CrossRef]
- Gherardi, R.K.; Coquet, M.; Cherin, P.; Belec, L.; Moretto, P.; Dreyfus, P.A.; Pellisier, J.F.; Chariot, P.; Authier, F.J. Macrophagic myofasciitis lesions assess long-term persistence of vaccine-derived aluminium hydroxide in muscle. Brain 2001, 124, 1821–1831. [Google Scholar] [CrossRef]
- Wang, Z.B.; Xu, J. Better Adjuvants for Better Vaccines: Progress in Adjuvant Delivery Systems, Modifications, and Adjuvant-Antigen Codelivery. Vaccines 2020, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- HogenEsch, H.; O’Hagan, D.T.; Fox, C.B. Optimizing the utilization of aluminum adjuvants in vaccines: You might just get what you want. NPJ Vaccines 2018, 3, 51. [Google Scholar] [CrossRef]
- Reed, S.G.; Bertholet, S.; Coler, R.N.; Friede, M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009, 30, 23–32. [Google Scholar] [CrossRef]
- Lindblad, E.B. Aluminium compounds for use in vaccines. Immunol. Cell Biol. 2004, 82, 497–505. [Google Scholar] [CrossRef]
- Asa, P.B.; Wilson, R.B.; Garry, R.F. Antibodies to squalene in recipients of anthrax vaccine. Exp. Mol. Pathol. 2002, 73, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Kuroda, Y.; Yoshida, H.; Behney, K.M.; Mizutani, A.; Akaogi, J.; Nacionales, D.C.; Lorenson, T.D.; Rosenbauer, R.J.; Reeves, W.H. Induction of lupus autoantibodies by adjuvants. J. Autoimmun. 2003, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanna, R.; Dalvi, S.; Sălăgean, T.; Bordea, I.R.; Benedicenti, S. Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review. Antioxidants 2020, 9, 875. https://doi.org/10.3390/antiox9090875
Hanna R, Dalvi S, Sălăgean T, Bordea IR, Benedicenti S. Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review. Antioxidants. 2020; 9(9):875. https://doi.org/10.3390/antiox9090875
Chicago/Turabian StyleHanna, Reem, Snehal Dalvi, Tudor Sălăgean, Ioana Roxana Bordea, and Stefano Benedicenti. 2020. "Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review" Antioxidants 9, no. 9: 875. https://doi.org/10.3390/antiox9090875
APA StyleHanna, R., Dalvi, S., Sălăgean, T., Bordea, I. R., & Benedicenti, S. (2020). Phototherapy as a Rational Antioxidant Treatment Modality in COVID-19 Management; New Concept and Strategic Approach: Critical Review. Antioxidants, 9(9), 875. https://doi.org/10.3390/antiox9090875