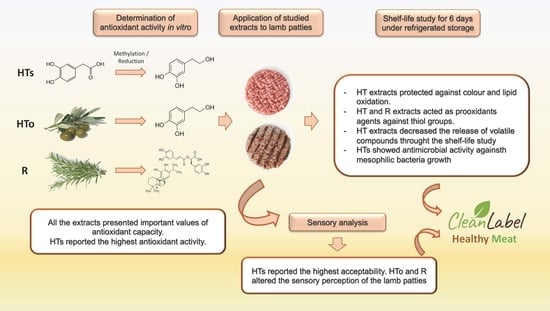
Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural and Synthetic Antioxidants
2.2. Total Phenolic Content (TPC) and Antioxidant Capacity
2.3. Lamb Burger Preparation
2.4. Proximal Composition and Mineral Bioavailability
2.5. Shelf-Life Study
2.5.1. Microbiological Analysis
2.5.2. Volatile Compounds by GC-MC
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results
3.1. Characterisation of Preservative Extracts
3.2. Proximate Composition and Bioavailability of the Mineral Fraction
3.3. Shelf-Life Study
3.4. Volatile Compounds (GS-MS)
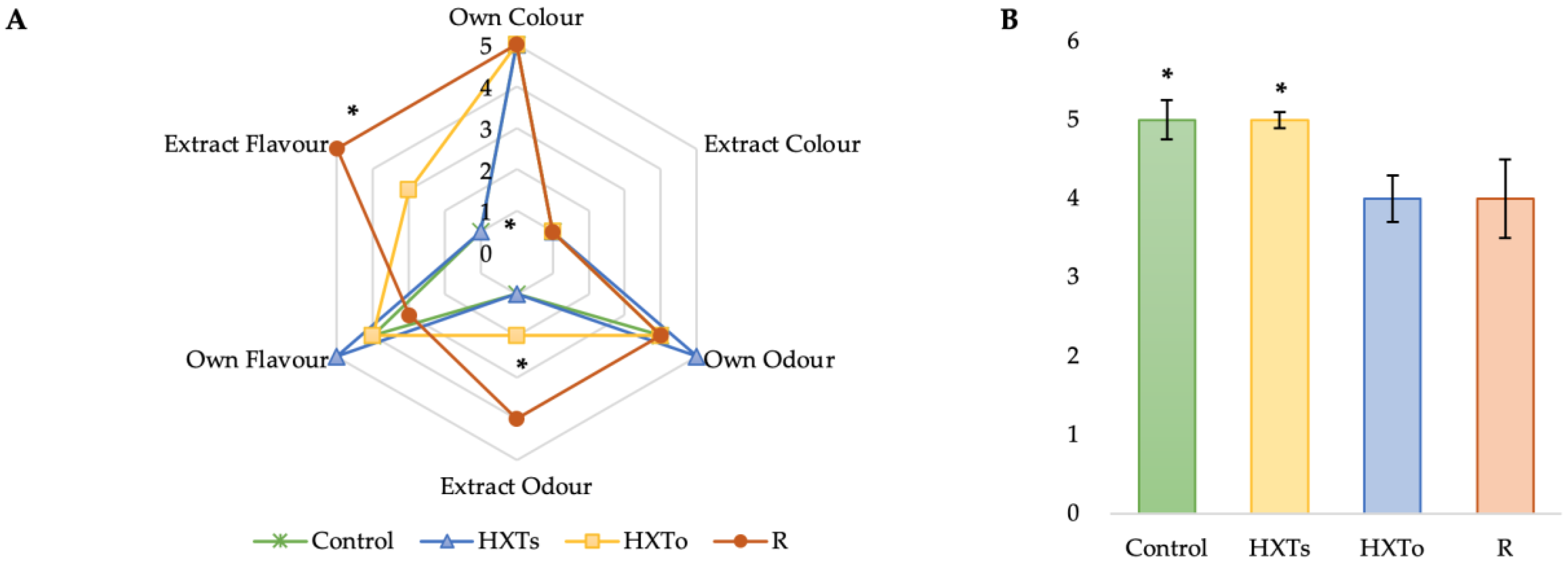
3.5. Sensory Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf life extension of lamb meat using thyme or oregano essential oils and modified atmosphere packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Fernández, A. Effect of ageing time on suckling lamb meat quality resulting from different carcass chilling regimes. Meat Sci. 2014, 96, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Osés, S.; Diez, A.M.; Melero, B.; Luning, P.A.; Jaime, I.; Rovira, J. Characterization by culture-dependent and culture-independent methods of the bacterial population of suckling-lamb packaged in different atmospheres. Food Microbiol. 2013, 36, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.W. Adverse Reactions to the Antioxidants Butylated Hydroxyanisole and Butylated Hydroxytoluene. In Food Allergy; Wiley: New York, NY, USA, 2014; pp. 393–401. [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Yangui, T.; Dhouib, A.; Rhouma, A.; Sayadi, S. Potential of hydroxytyrosol-rich composition from olive mill wastewater as a natural disinfectant and its effect on seeds vigour response. Food Chem. 2009, 117, 1–8. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Ros, G.; Nieto, G. Hydroxytyrosol: Health Benefits and Use as Functional Ingredient in Meat. Medicines 2018, 5, 13. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Cabrerizo, S.; De La Cruz, J.P.; López-Villodres, J.A.; Muñoz-Marin, J.; Guerrero, A.; Reyes, J.J.; Labajos, M.T.; Gonzalez-Correa, J.A. Role of the inhibition of oxidative stress and inflammatory mediators in the neuroprotective effects of hydroxytyrosol in rat brain slices subjected to hypoxia reoxygenation. J. Nutr. Biochem. 2013, 24, 2152–2157. [Google Scholar] [CrossRef]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2007, 226, 653–659. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez-Zamora, L.; Castillo, J.; Ros, G. Hydroxytyrosol extracts, olive oil and walnuts as functional components in chicken sausages. J. Sci. Food Agric. 2017, 97, 3761–3771. [Google Scholar] [CrossRef]
- Espín, J.C.; Soler-Rivas, C.; Cantos, E.; Tomás-Barberán, F.A.; Wichers, H.J. Synthesis of the antioxidant hydroxytyrosol using tyrosinase as biocatalyst. J. Agric. Food Chem. 2001, 49, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Bañon, S.; Méndez, L.; Almela, E. Effects of dietary rosemary extract on lamb spoilage under retail display conditions. Meat Sci. 2012, 90, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G. Incorporation of by-products of rosemary and thyme in the diet of ewes: Effect on the fatty acid profile of lamb. Eur. Food Res. Technol. 2013, 236, 379–389. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Jordán, M.; Bañon, S. Shelf life of meat from lambs given essential oil-free rosemary extract containing carnosic acid plus carnosol at 200 or 400 mg kg−1. Meat Sci. 2014, 96, 1452–1459. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañon, S.; Casanova, J.O. Use of dietary rosemary diterpenes to extend the preservation of sulphited-lamb products. Small Rumin. Res. 2015, 123, 269–277. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañon, S. Use of dietary rosemary diterpenes to inhibit rancid volatiles in lamb meat packed under protective atmosphere. Animal 2016, 10, 1391–1401. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A.J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Boil. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.A.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL)) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; Association of Official Analyticial Chemistry: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Effect of natural extracts obtained from food industry by-products on nutritional quality and shelf life of chicken nuggets enriched with organic Zn and Se provided in broiler diet. Poult. Sci. 2020, 99, 1491–1501. [Google Scholar] [CrossRef]
- Wang, L.L.; Xiong, Y.L. Inhibition of lipid oxidation in cooked beef patties by hydrolyzed potato protein is related to its reducing and radical scavenging ability. J. Agric. Food Chem. 2005, 53, 9186–9192. [Google Scholar] [CrossRef]
- Martínez, L.; Bastida, P.; Castillo, J.; Ros, G.; Nieto, G. Green alternatives to synthetic antioxidants, antimicrobials, nitrates, and nitrites in clean label Spanish Chorizo. Antioxidants 2019, 8, 184. [Google Scholar] [CrossRef]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Gikas, E. The LC–MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B 2017, 1041, 45–59. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and antimicrobial activity of rosemary, pomegranate and olive extracts in fish patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef]
- Nieto, G.; Martínez-Zamora, L.; Castillo, J.; Ros, G.; Nieto, G. Effect of hydroxytyrosol, walnut and olive oil on nutritional profile of Low-Fat Chicken Frankfurters. Eur. J. Lipid Sci. Technol. 2017, 119, 1600518. [Google Scholar] [CrossRef]
- Tai, J.; Cheung, S.; Wu, M.; Hasman, D. Antiproliferation effect of Rosemary (Rosmarinus officinalis) on human ovarian cancer cells in vitro. Phytomedicine 2012, 19, 436–443. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Wang, D.; Williams, B.A.; Ferruzzi, M.; D’Arcy, B.R. Microbial metabolites, but not other phenolics derived from grape seed phenolic extract, are transported through differentiated Caco-2 cell monolayers. Food Chem. 2013, 138, 1564–1573. [Google Scholar] [CrossRef]
- Martínez, L.; Ros, G.; Nieto, G. Fe, Zn and Se bioavailability in chicken meat emulsions enriched with minerals, hydroxytyrosol and extra virgin olive oil as measured by Caco-2 cell model. Nutrients 2018, 10, 969. [Google Scholar] [CrossRef]
- Santos-López, J.A.; Garcimartín, A.; Merino, P.; López-Oliva, M.E.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Effects of Silicon vs. Hydroxytyrosol-Enriched Restructured Pork on Liver Oxidation Status of Aged Rats Fed High-Saturated/High-Cholesterol Diets. PLoS ONE 2016, 11, e0147469. [Google Scholar] [CrossRef]
- Garcimartín, A.; Santos-López, J.A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Silicon-Enriched Restructured Pork Affects the Lipoprotein Profile, VLDL Oxidation, and LDL Receptor Gene Expression in Aged Rats Fed an Atherogenic Diet. J. Nutr. 2015, 145, 2039–2045. [Google Scholar] [CrossRef]
- Nieto, G.; Banon, S.; Garrido, M.D.; Nieto, G. Administration of distillate thyme leaves into the diet of Segureña ewes: Effect on lamb meat quality. Animal 2012, 6, 2048–2056. [Google Scholar] [CrossRef]
- Nieto, G.; Díaz, P.; Bañon, S.; Garrido, M.D. Effect on lamb meat quality of including thyme (Thymus zygis ssp. gracilis) leaves in ewes’ diet. Meat Sci. 2010, 85, 82–88. [Google Scholar] [CrossRef]
- Andrés, A.I.; Petrón, M.; Adámez, J.; López, M.; Timón, M. Food by-products as potential antioxidant and antimicrobial additives in chill stored raw lamb patties. Meat Sci. 2017, 129, 62–70. [Google Scholar] [CrossRef]
- Carballo, D.; Caro, I.; Andres, S.; Giráldez, F.; Mateo, J. Assessment of the antioxidant effect of astaxanthin in fresh, frozen and cooked lamb patties. Food Res. Int. 2018, 111, 342–350. [Google Scholar] [CrossRef]
- Muíño, I.; Díaz, M.T.; Apeleo, E.; Pérez-Santaescolástica, C.; Rivas-Cañedo, A.; Pérez, C.; Cañeque, V.; Lauzurica, S.; De La Fuente, J. Valorisation of an extract from olive oil waste as a natural antioxidant for reducing meat waste resulting from oxidative processes. J. Clean. Prod. 2017, 140, 924–932. [Google Scholar] [CrossRef]
- Enser, M.; Hallett, K.; Hewitt, B.; Fursey, G.; Wood, J. Fatty acid content and composition of english beef, lamb and pork at retail. Meat Sci. 1996, 42, 443–456. [Google Scholar] [CrossRef]
- Fishera, A.V.; Ensera, M.; Richardsona, R.I.; Wooda, J.D.; Nutea, G.R.; Kurta, E.; Sinclairb, L.A.; Wilkinson, R.G. Fatty acid composition and eating quality of lamb types derived from four diverse breed× production systems. Meat Sci. 2000, 55, 141–147. [Google Scholar] [CrossRef]
- Bravo-Lamas, L.; Barron, L.; Kramer, J.K.; Etaio, I.; Aldai, N. Characterization of the fatty acid composition of lamb commercially available in northern Spain: Emphasis on the trans-18:1 and CLA content and profile. Meat Sci. 2016, 117, 108–116. [Google Scholar] [CrossRef]
- Deiana, M.; Corona, G.; Incani, A.; Loru, D.; Rosa, A.; Atzeri, A.; Melis, M.P.; Dessì, M.A. Protective effect of simple phenols from extra virgin olive oil against lipid peroxidation in intestinal Caco-2 cells. Food Chem. Toxicol. 2010, 48, 3008–3016. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Abou-Arab, A.A.; Abu Salem, F.M. Antioxidant and antimicrobial effect of some natural plant extracts added to lamb patties during storage. Grasas Aceites 2011, 62, 139–148. [Google Scholar] [CrossRef][Green Version]
- Nieto, G.; Jongberg, S.; Andersen, M.L.; Skibsted, L.H.; Nieto, G. Thiol oxidation and protein cross-link formation during chill storage of pork patties added essential oil of oregano, rosemary, or garlic. Meat Sci. 2013, 95, 177–184. [Google Scholar] [CrossRef]
- Delles, R.M.; Xiong, Y.L.; True, A.D. Mild Protein Oxidation Enhanced Hydration and Myofibril Swelling Capacity of Fresh Ground Pork Muscle Packaged in High Oxygen Atmosphere. J. Food Sci. 2011, 76, C760–C767. [Google Scholar] [CrossRef]
- Delles, R.M.; Xiong, Y.L. The effect of protein oxidation on hydration and water-binding in pork packaged in an oxygen-enriched atmosphere. Meat Sci. 2014, 97, 181–188. [Google Scholar] [CrossRef]
- Jongberg, S.; Tørngren, M.A.; Gunvig, A.; Skibsted, L.H.; Lund, M.N. Effect of green tea or rosemary extract on protein oxidation in Bologna type sausages prepared from oxidatively stressed pork. Meat Sci. 2013, 93, 538–546. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Jongberg, S.; Ros, G.; Skibsted, L.H.; Nieto, G. Plant derived ingredients rich in nitrates or phenolics for protection of pork against protein oxidation. Food Res. Int. 2020, 129, 108789. [Google Scholar] [CrossRef]
- Tafesh, A.; Najami, N.; Jadoun, J.; Halahlih, F.; Riepl, H.; Azaizeh, H. Synergistic Antibacterial Effects of Polyphenolic Compounds from Olive Mill Wastewater. Evid. Based Complement. Altern. Med. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Alkass, J.E.; Baker, I.A.; Saleh, H.H. Reduction of Oxidative Rancidity and Microbial Activities of the Karadi Lamb Patties in Freezing Storage Using Natural Antioxidant Extracts of Rosemary and Ginger. Int. J. Agric. Food Res. 2013, 2. [Google Scholar] [CrossRef]
| Ingredients | Control | HXTs | HXTo | R |
|---|---|---|---|---|
| Lamb meat (g) | 2560 | 2560 | 2560 | 2560 |
| Water (mL) | 640 | 640 | 640 | 640 |
| Commercial mix® (g/kg) | 69 | |||
| Preservative extracts (ppm) | ||||
| • HXTS | 200 | |||
| • HXTO | 200 | |||
| • R | 200 |
| Sample | Total Phenolic Content | Antioxidant Activity | |||
|---|---|---|---|---|---|
| ABTS | DPPH | ORAC | FRAP | ||
| HXTs | 93.9 ± 54 a | 93.3 ± 5.3 a | 88.9 ± 3.9 | 70,542 ± 299.6 a | 64,961 ± 1239.4 a |
| HXTo | 41.6 ± 81 b | 82.2 ± 4.5 b | 81.9 ± 1.3 | 40,993 ± 285.7 b | 60,457 ± 1439.4 b |
| R | 36.5 ± 26 c | 80.1 ± 5.1 b | 81.3 ± 5.0 | 13,929 ± 393.4 c | 17,790 ± 839.4 c |
| Proximate Composition (Average Percentage ± SD) | Mineral Bioavailability (mg/100 g) | |||||
|---|---|---|---|---|---|---|
| Samples | Moisture | Ash | Protein | Lipid | Fe | Si |
| Control | 72.82 ± 0.26 | 2.01 ± 0.09 | 14.28 ± 0.81 | 19.88 ± 0.73 | 1.13 ± 0.00 c | 23.53 ± 0.03 d |
| HXTs | 72.60 ± 0.91 | 1.93 ± 0.15 | 14.00 ± 0.19 | 19.19 ± 1.07 | 1.85 ± 0.16 a | 67.19 ± 0.08 a |
| HXTo | 71.33 ± 1.20 | 1.72 ± 0.07 | 14.43 ± 0.58 | 20.37 ± 0.30 | 1.75 ± 0.04 a | 62.53 ± 0.10 b |
| R | 73.04 ± 2.49 | 1.70 ± 0.19 | 15.24 ± 0.19 | 22.42 ± 2.09 | 1.42 ± 0.01 b | 48.95 ± 0.05 c |
| Days of Storage | ||||
|---|---|---|---|---|
| Samples | 0 | 1 | 3 | 6 |
| pH | ||||
| Control | 6.12 ± 0.04 | 5.86 ± 0.00 | 5.79 ± 0.01 | 5.76 ± 0.01 |
| HXTs | 6.10 ± 0.00 | 5.38 ± 0.01 | 5.35 ± 0.01 | 5.42 ± 0.01 |
| HXTo | 6.05 ± 0.01 | 5.06 ± 0.01 | 4.97 ± 0.01 | 5.07 ± 0.01 |
| R | 5.84 ± 0.00 | 5.30 ± 0.00 | 5.22 ± 0.00 | 5.31 ± 0.01 |
| L* | ||||
| Control | 55.36 ± 1.30 | 54.73 ± 1.61 | 53.75 ± 2.46 | 55.88 ± 2.47 |
| HXTs | 53.53 ± 1.74 | 55.57 ± 2.65 | 56.96 ± 1.53 | 56.27 ± 1.58 |
| HXTo | 54.36 ± 1.54 | 55.12 ± 1.02 | 57.11 ± 1.52 | 57.08 ± 0.63 |
| R | 52.63 ± 1.55 | 54.88 ± 1.01 | 56.18 ± 1.79 | 54.19 ± 1.16 |
| a* | ||||
| Control | 20.17 ± 0.27 a,x | 21.14 ± 0.51 a,x | 14.66 ± 1.03 a,y | 11.11 ± 0.27 z |
| HXTs | 17.89 ± 0.16 b,x | 16.33 ± 0.43 b,y | 11.81 ± 0.28 b,z | 11.52 ± 0.18 z |
| HXTo | 17.69 ± 0.30 b,x | 16.44 ± 0.42 b,y | 11.45 ± 1.31 b,z | 11.83 ± 0.47 z |
| R | 17.32 ± 0.50 b,x | 16.27 ± 1.37 b,x | 12.16 ± 0.16 b,y | 11.21 ± 0.41 z |
| b* | ||||
| Control | 14.73 ± 0.08 a,x | 14.61 ± 0.29 a,x | 10.67 ± 0.89 b,y | 8.52 ± 0.19 c,z |
| HXTs | 12.42 ± 0.24 b,x | 11.82 ± 0.13 c,y | 11.27 ± 0.21 b,y | 10.57 ± 0.16 b,z |
| HXTo | 12.38 ± 0.19 b | 11.57 ± 0.17 c | 12.05 ± 0.79 a | 11.25 ± 0.16 a |
| R | 12.41 ± 0.31 b,y | 12.16 ± 0.20 b,y | 12.07 ± 0.24 a,y | 10.26 ± 0.32 b,z |
| Lipid Oxidation: TBARs (mg MDA/kg) | ||||
| Control | 1.36 ± 0.22 a,y | 1.71 ± 0.26 y | 1.37 ± 0.09 b,y | 1.00 ± 0.15 b,z |
| HXTs | 0.70 ± 0.10 b,z | 1.42 ± 0.25 x | 1.12 ± 0.12 c,y | 0.65 ± 0.06 c,z |
| HXTo | 0.84 ± 0.03 b,z | 1.62 ± 0.11 x | 1.56 ± 0.14 a,x | 1.19 ± 0.04 a,y |
| R | 0.78 ± 0.08 b,z | 1.44 ± 0.05 x | 1.15 ± 0.08 c,y | 1.13 ± 0.10 a,y |
| Protein Oxidation: Thiol Loss (nmol/mg protein) | ||||
| Control | 29.04 ± 1.15 a,x | 21.78 ± 3.00 a,y | 6.48 ± 0.22 a,z | 5.75 ± 0.28 a,z |
| HXTs | 15.16 ± 1.26 b,x | 14.13 ± 1.87 b,x | 3.76 ± 0.17 b,y | 2.12 ± 0.06 b,z |
| HXTo | 18.69 ± 1.98 b,y | 16.26 ± 2.03 b,y | 2.54 ± 0.04 c,z | 2.25 ± 0.14 b,z |
| R | 17.54 ± 2.54 b,y | 16.33 ± 1.25 b,y | 2.54 ± 0.01 c,z | 2.01 ± 0.08 b,z |
| Days of Storage | |||||
|---|---|---|---|---|---|
| Microorganism | Samples | 0 | 1 | 3 | 6 |
| TVC | Control | 6350 ± 40 a,z | 25,000 ± 1200 c,y | 140,000 ± 25000 a,x | 220,000 ± 4250 a,w |
| HXTs | 3600 ± 50 c,z | 53,000 ± 650 a,y | 59,000 ± 4000 c,x,y | 80,000 ± 1150 d,w | |
| HXTo | 1550 ± 46 d,z | 55,000 ± 1500 a,y | 80,000 ± 5000 b,x | 180,000 ± 8000 b,w | |
| R | 4750 ± 34 b,z | 40,000 ± 700 b,y | 50,000 ± 2500 d,x,y | 107,000 ± 9500 c,w | |
| TCC | Control | 895 ± 70 b,z | 950 ± 80 c,y,z | 1280 ± 210 c,x,y | 2000 ± 120 c,w |
| HXTs | 3490 ± 67 a,z | 3000 ± 90 a,z | 3400 ± 200 b,z | 4000 ± 320 b,y | |
| HXTo | 3280 ± 110 a | 3930 ± 50 c | 3800 ± 380 b | 4900 ± 860 b | |
| R | 515 ± 48 c, z | 1460 ± 115 b,y | 8000 ± 190 a,x | 9300 ± 250 a,w | |
| E. coli | <10 | ||||
| Sample | Day 0 | Day 6 | Day 0 | Day 6 | ||
|---|---|---|---|---|---|---|
| 3-Methyl-1-butanol | Control | 3.10 ± 0.32 | 6.60 ± 0.01 a | 3-Methyl-1-butanal | 0.45 ± 0.07 | 1.78 ± 0.02 a |
| HXTs | 0.22 ± 0.03 | 2.16 ± 0.03 b | 0.12 ± 0.00 | 0.44 ± 0.03 d | ||
| HXTo | 1.01 ± 0.05 | 1.42 ± 0.05 c | 0.25 ± 0.02 | 0.60 ± 0.05 c | ||
| R | 0.45 ± 0.01 | 2.92 ± 0.04 b | 0.66 ± 0.04 | 1.35 ± 0.12 b | ||
| Hexanal | Control | 0.03 ± 0.00 | 1.99 ± 0.02 b | 2-Bornanone | 0.01 ± 0.00 | 0.38 ± 0.02 |
| HXTs | 0.02 ± 0.05 | 0.63 ± 0.02 c | nd | nd | ||
| HXTo | 0.03 ± 0.01 | 0.59 ± 0.05 c | nd | nd | ||
| R | 0.03 ± 0.01 | 5.88 ± 0.03 a | nd | nd | ||
| 2,3-Butanediol | Control | 0.16 ± 0.01 | 10.56 ± 0.02 a | Nonanal | 0.56 ± 0.00 | 1.70 ± 0.10 a |
| HXTs | 0.29 ± 0.01 | 3.33 ± 0.03 d | 0.03 ± 0.00 | 0.10 ± 0.00 c | ||
| HXTo | 1.09 ± 0.01 | 6.60 ± 0.05 c | 0.11 ± 0.02 | 0.31 ± 0.03 b | ||
| R | 0.39 ± 0.01 | 7.35 ± 0.01 b | 0.06 ± 0.01 | 0.43 ± 0.01 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Zamora, L.; Ros, G.; Nieto, G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants 2020, 9, 851. https://doi.org/10.3390/antiox9090851
Martínez-Zamora L, Ros G, Nieto G. Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants. 2020; 9(9):851. https://doi.org/10.3390/antiox9090851
Chicago/Turabian StyleMartínez-Zamora, Lorena, Gaspar Ros, and Gema Nieto. 2020. "Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers" Antioxidants 9, no. 9: 851. https://doi.org/10.3390/antiox9090851
APA StyleMartínez-Zamora, L., Ros, G., & Nieto, G. (2020). Synthetic vs. Natural Hydroxytyrosol for Clean Label Lamb Burgers. Antioxidants, 9(9), 851. https://doi.org/10.3390/antiox9090851