Abstract
As a facultative intracellular pathogen, Salmonella Enteritidis must develop an effective oxidative stress response to survive exposure to reactive oxygen species within the host. To study this defense mechanism, we carried out a series of oxidative stress assays in parallel with a comparative transcriptome analyses using a next generation sequencing approach. It was shown that the expression of 45% of the genome was significantly altered upon exposure to H2O2. Quantitatively the most significant (≥100 fold) gene expression alterations were observed among genes encoding the sulfur utilization factor of Fe-S cluster formation and iron homeostasis. Our data point out the multifaceted nature of the oxidative stress response. It includes not only numerous mechanisms of DNA and protein repair and redox homeostasis, but also the key genes associated with osmotic stress, multidrug efflux, stringent stress, decrease influx of small molecules, manganese and phosphate starvation stress responses. Importantly, this study revealed that oxidatively stressed S. Enteritidis cells simultaneously repressed key motility encoding genes and induced a wide range of adhesin- and salmonellae-essential virulence-encoding genes, that are critical for the biofilm formation and intracellular survival, respectively. This finding indicates a potential intrinsic link between oxidative stress and pathogenicity in non-typhoidal Salmonella that needs to be empirically evaluated.
1. Introduction
Non-typhoidal Salmonella is a leading cause of foodborne gastroenteritis on the global scale. On the global scale, this foodborne pathogen is responsible for 80 million cases of gastroenteritis annually [1]. The situation in the USA is very similar to the global epidemiological picture of salmonellosis caused by non-typhoidal Salmonella. It has been estimated that from 2000 to 2008, non-typhoidal Salmonella serovars accounted for 1.2 million laboratory-confirmed illnesses, 19,000 hospitalizations, and 380 deaths each year in the USA [2]. The high incidence rate is made more significant by the fact that there have been no signs of decline in the incidence of salmonellosis over the past 15 years in the US. Intriguingly, infections caused by other five major food-borne pathogens (i.e., Escherichia coli O157:H7, Campylobacter spp., Listeria monocytogenes, Yersinia enterocolitica, and Shigella spp.) declined substantially over the same time [2].
Infections of humans with non-typhoidal Salmonella serovars occur mostly via the human food chain [3,4]. The important human food chain transmission route of non-typhoidal Salmonella is associated with ready-to-eat products such as vegetables [5,6], leafy green horticulture products [7,8], and fruits [9,10]. Traditionally, aqueous chlorine and ozone solutions are widely used in the food industry for sanitization of food-processing surfaces as well as vegetables and fruits prior to their distribution to the market [11,12]. Chlorine and ozone, potent oxidizing agents, impose an oxidative stress on cells, damaging nucleic acids, both DNA and RNA, proteins, lipids, and further causing membrane disorganization and genome breaks which result in cellular death. To survive oxidative stress assault in the food matrix and food processing facilities and successfully invade the host and survive intracellularly, these pathogens must acquire a robust oxidative stress protective mechanism.
A number of studies investigated the roles of transcription factors [13], RNA repair systems [14], sigma factors [15], and novel stress response proteins [16,17] in the oxidative stress response of non-typhoidal Salmonella. Although these studies gave us extremely valuable information on the functions of specific oxidative response mechanisms employed by these pathogens, they could not provide insights into the global impact of oxidative stress on the non-typhoidal Salmonella physiology. Recently, Fu et al. [18] by employing global comparative proteomics, identified 116 proteins in the proteome of Salmonella Typhimurium, treated with H2O2 that were significantly altered compared to the control. Among the altered proteome, iron acquisition systems were most significantly induced. Also, several phage-coding proteins with DNA repair capabilities were induced, whereas the apparatus of the Salmonella type III secretion system was repressed.
To gain insight into the expression dynamics of genes associated with distinct biological functions during an oxidative stress, expression of ten genes, including iroN, sitA, rpoS, rpoH, ycfR, dps, nrdM, pocR, and ompF, were tested after 30 min of 1 mM, 2 mM, and 4 mM H2O2 treatments. The 30 min of H2O2 treatment was selected as Salmonella enterica subspecies enterica serovar Enteritidis, hereinafter Salmonella Enteritidis cultures were already adapted at that point to different H2O2 treatments, showing only a slight decline in the viability. Finally, to determine the global impact of oxidative stress on the non-typhoidal Salmonella physiology, we carried out comparative transcriptome analyses using the next generation sequencing approach.
2. Materials and Methods
2.1. Bacterial Culture
Salmonella Enteritidis ATCC 13076 (American Type Culture Collection, Manassas, VA, USA) was used as the model organism. For each experiment, fresh culture was prepared by streaking frozen culture onto tryptic soy agar (EMD Chemicals Inc., Darmstadt, Germany), followed by incubation at 37 °C for 24 h. Three well-isolated colonies were used for subsequent inoculations.
2.2. Oxidative Stress Killing Assay
This assay was carried out using overnight cultures of the wild type S. Enteritidis strain grown in LB at 37 °C with continuous shaking at 180 rpm. These overnight cultures were diluted 1/100 in LB followed by growth to 0.4 using an optical density at 600 nm. The wild type cultures were exposed at their mid-exponential growth phase (0.4) to three incrementally increasing concentrations of H2O2, including: 1 mM, 2 mM, and 4 mM. Treatment was carried out for 90 min under the same incubation conditions. Wild type culture with no oxidative treatment was included as a control. Colony forming units (CFU) counts were carried out by 10-fold dilutions using sterile saline at time zero (before H2O2 exposure) and then at every 15 min of treatment. Aliquots of 0.1 mL were plated in triplicate followed by incubation at 37 °C for 24 h.
2.3. Incremental Oxidative Stress Gene Expression Assay
Cultures of S. Enteritidis were prepared as described above and after 30 min of oxidative treatment samples were taken and processed. Total RNA was isolated as described below followed by cDNA synthesis using Superscript™ III reverse transcriptase (RT) (Invitrogen by Life Technologies, Carlsbad, CA, USA). Quantitative PCR was carried out on a MiniOpticonTM Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) with iQTM SYBR Green Supermix kit (Bio-Rad Laboratories) as described previously [19]. Genes rtcR and yjjA, encoding for transcriptional regulatory protein RtcR and DUF2501 domain-containing protein, were selected as the internal reference genes to normalize the expression of the tested genes. Primers were designed to target expression of the following genes: rpoS, rpoH, iroN, sitA, dps, nrdM, trxC, ycfR, ompF, and pocR (Table 1). These ten genes were selected based on their distinct gene ontologies (GO) associated with the oxidative stress response. Selected the GO include, iron homeostasis, alternative sigma factors, multiple stress response, DNA protection, antioxidants, DNA replication, uptake of small solutes and anabolic processes. Melting curves were analyzed to check the product specificity. The level of gene expression for each target gene was compared with the internal reference control gene and the fold changes in expression were calculated by the comparative CT method [20].

Table 1.
Primers used in this study for the oxidative stress response gene expression assay and validation of RNA-seq data.
2.4. Preparation of Samples for Transcriptomics
Untreated and 30-min H2O2 (3 mM) treated cultures (0.4 at OD600) of S. Typhimurium were centrifuged at 4000 rpm for 5 min. Supernatants were discard and the cell pellets were washed two times followed by RNA extraction using the RNeasy Mini kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s instructions.
2.5. Global Transcriptomic Analysis
Sample quality preparation was done using capillary electrophoresis (Agilent Bio Analyzer 2100, Santa Clara, CA, USA) as previously described [17]. Only samples that had RNA integrity number 8 or greater were considered for further analysis. Illumina sequencing libraries were prepared using Illumina’s TruSeq Stranded Total RNA Library Prep Human/Mouse/Rat Sample Preparation Kit (Cat. # 20020597) at the University of Minnesota Genomics Center (Saint Paul, MN, USA). For rRNA depletion, 500 ng of total amount of RNA was used in combination with Ribozero capture probes. After this step, the mRNA was fragmented followed by reverse transcription into complementary DNA (cDNA). The resulting cDNA fragments were coded with indexed adaptors followed by amplification using 15 PCR cycles. Again capillary electrophoresis was used to validate library size distribution, while the library quantification was carried out using fluorimetry. Libraries normalization was carried out followed by their hybridization to a single read flow cell. Once hybridization completed, the flow cell was loaded on the HiSeq 2500 and sequenced using Illumina’s SBS chemistry. The primary analysis and index de-multiplexing were performed as previously described [17]. Read mapping was carried out using publicly available genome of S. Enteritidis P125109 strain. Quantification of gene expression was completed using Feature Counts. Significantly differentially expressed genes were identified using the edgeR feature in CLCGWB (Qiagen, Valencia, CA, USA) based on a minimum 2 times absolute fold change difference and p < 0.05 false discovery rate (FDR).
2.6. Validation of RNA-Seq Data by Real-Time PCR
Cultures of S. Enteritidis were prepared as described above and after 30 min of oxidative treatment samples were taken and processed. Total RNA was isolated as described above. Quantitative PCR assay was carried out as described above. Genes rtcR and yjjA were selected as the internal reference genes to normalize the expression of the tested genes. Primers were designed to target expression of the following genes: dinP, dnaJ, eutC, mdlA, osmY, pagO, spaR, malK, and nrdD (Table 1).
2.7. Experimental Replication and Gene Ontology Analysis
The data of all experiments represent the average of three biological replications. The cell viability during the oxidative treatments were analyzed by CoStat version 6.4 software (Co-Hort Software, Monterey, CA, USA) using the homogeneity of linear regression slopes approach. The gene ontology (GO) analysis was carried out using the Database for Annotation, Visualization and Integrated Discovery (DAVID) database [21] as well as the National Center for Biotechnology Information (NCBI).
2.8. RN-Seq Accession Numbers
RNA sequencing data for all three biological replications and two different treatments were deposited in the NCBI under accession number GSE 155479.
3. Results
3.1. Effect ofIincreasing H2O2 Concentrations on the Viability of S. Enteritidis
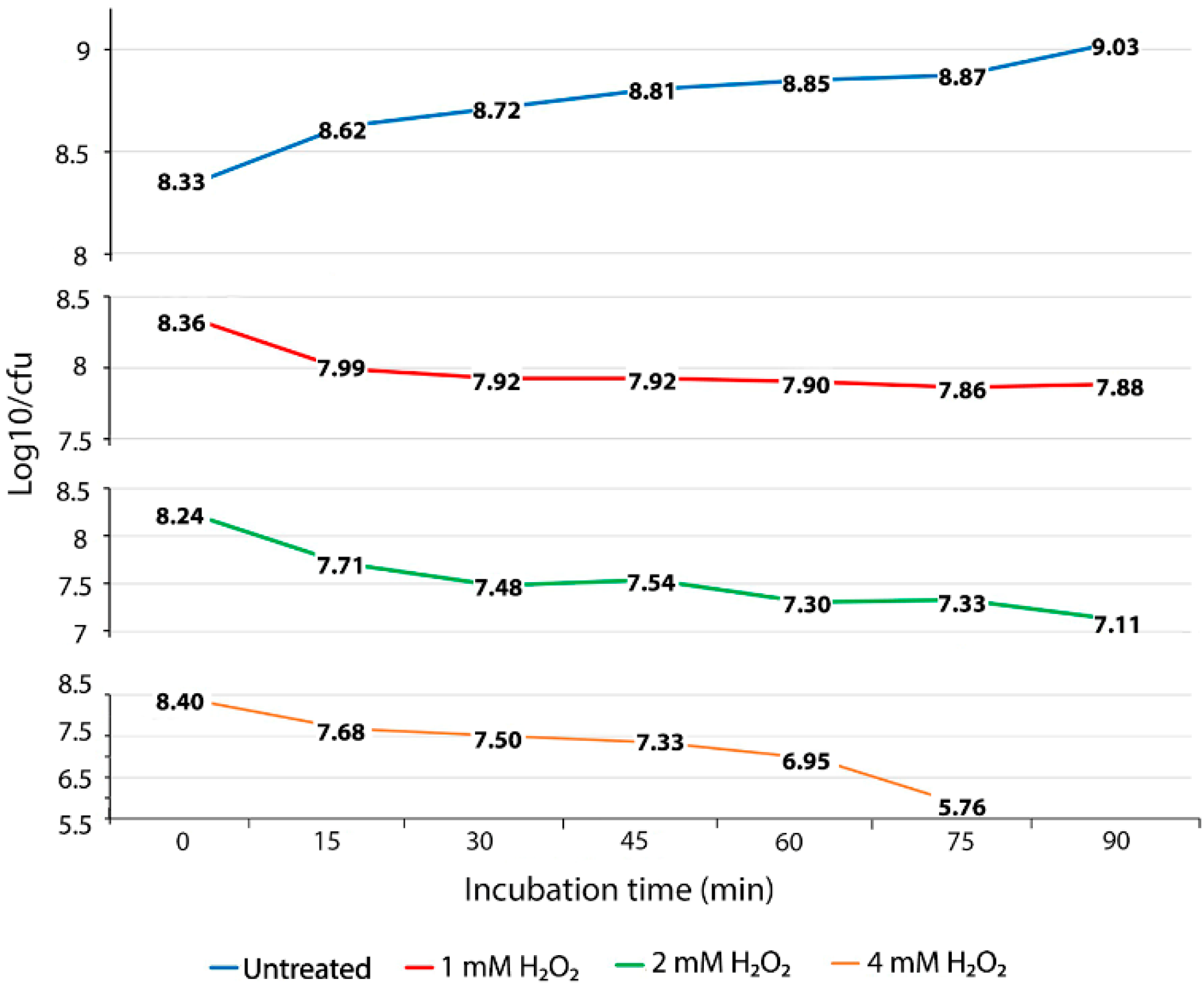
To evaluate the ability of S. Enteritidis to survive exposure to oxidative stress, we performed oxidative stress killing assays with increasing concentrations of H2O2 over a short time. The concentration of 1 mM of H2O2 caused a death rate of 0.37 log10 CFU for the first 15 min of exposure (Figure 1 and Figure S1). After this initial lethal period, the culture of S. Enteritidis exposed to the same concentration of H2O2 showed a slight decline in the number of viable cells, resulting in only 0.1 log10 CFU of reduction during the entire experiment (Figure 1). The exposure of S. Enteritidis to 1 mM of H2O2 resulted in two distinct survival phases, an initial and brief lethal phase followed by a long bacteriostatic phase (Figure 1 and Figure S1). Similarly to 1 mM, the concentration of 2 mM of H2O2 caused an initial lethal phase resulting in 0.53 log10 CFU decline (Figure 1). However, the survival pattern associated with the 2 mM H2O2 treatment was bactericidal not bacteriostatic (Figure 1). After an initial lethal period for the first 15 min of H2O2 exposure, the 2 mM H2O2 treatment continued to cause cell death, albeit at a slower rate compared to that of the initial 15-min period. This bactericidal phase was characterized by repetitive fluctuations of fast die-off periods (e.g., 0.23, 0.24 and 0.22 log10 CFU declines at the 30-min, 60-min, and 90-min measurements, respectively) and slight growth periods (e.g., 0.06 log10 CFU increase at the 45-min measurement and 0.03 log10 CFU increase at the 75-min measurement) (Figure 1). The exposure of S. Enteritidis to the greatest concentration of H2O2, 4 mM, caused again an initial lethal phase, characterized by a fast die-off rate, 0.72 log10 CFU decline over the first 15 min of treatment (Figure 1). Interestingly, this initial fast die-off period was followed by moderate die-off periods (e.g., 0.18 and 0.17 log10 CFU decline) at the next two measurements (Figure 1). After this moderate die-off period, the culture of S. Enteritidis entered progressively fast mortality rates resulting in a 0.38 log10 CFU decline at the 60-min measurement and 1.19 log10 CFU decline at the 75-min measurement (Figure 1). At the last, 90-min, measurement, the viable cell count could not be detected at 103 CFU per 1 mL, indicating a continuation of this progressive and fast mortality rate. The oxidative treatments with 1 mM, 2 mM, and 4 mM of H2O2 caused significantly higher (p < 0.01) mortality rates during the exponential growth phase of S. Enteritidis compared with no treatment of this pathogen under the same growth conditions. The average mortality rates of S. Enteritidis for every 15 min of H2O2 treatment were 0.08 log10 CFU decline for 1 mM, followed by 0.188 log10 CFU decline for 2 mM treatment and 0.468 log10 CFU decline for 4 mM. Although all three H2O2 treatments caused significantly higher mortality rates compared to that of no treatment, the survival patterns of H2O2 treated S. Enteritidis were different (Figure 1).
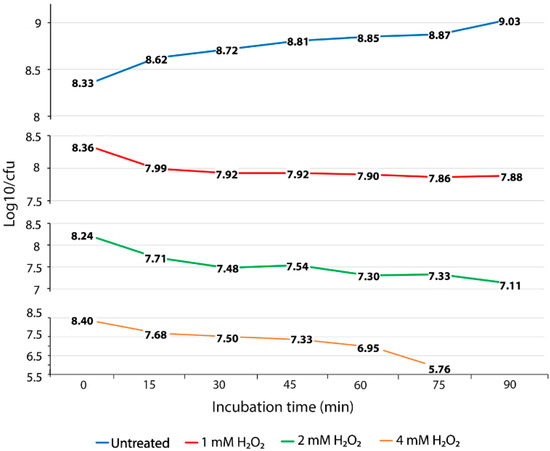
Figure 1.
Oxidative stress killing assay. Mortality of S. Enteritidis, expressed by reduced log10/CFU for every 15 min of the H2O2 treatment. The data correspond to the mean value of three biological replications. Each measurement value was composed of nine replications (each biological replication was made up of three technical replications).
3.2. Expression Levels of the Genes Associated with Oxidative Stress and Anabolic Processes during Incremental Increase of H2O2 Concentration
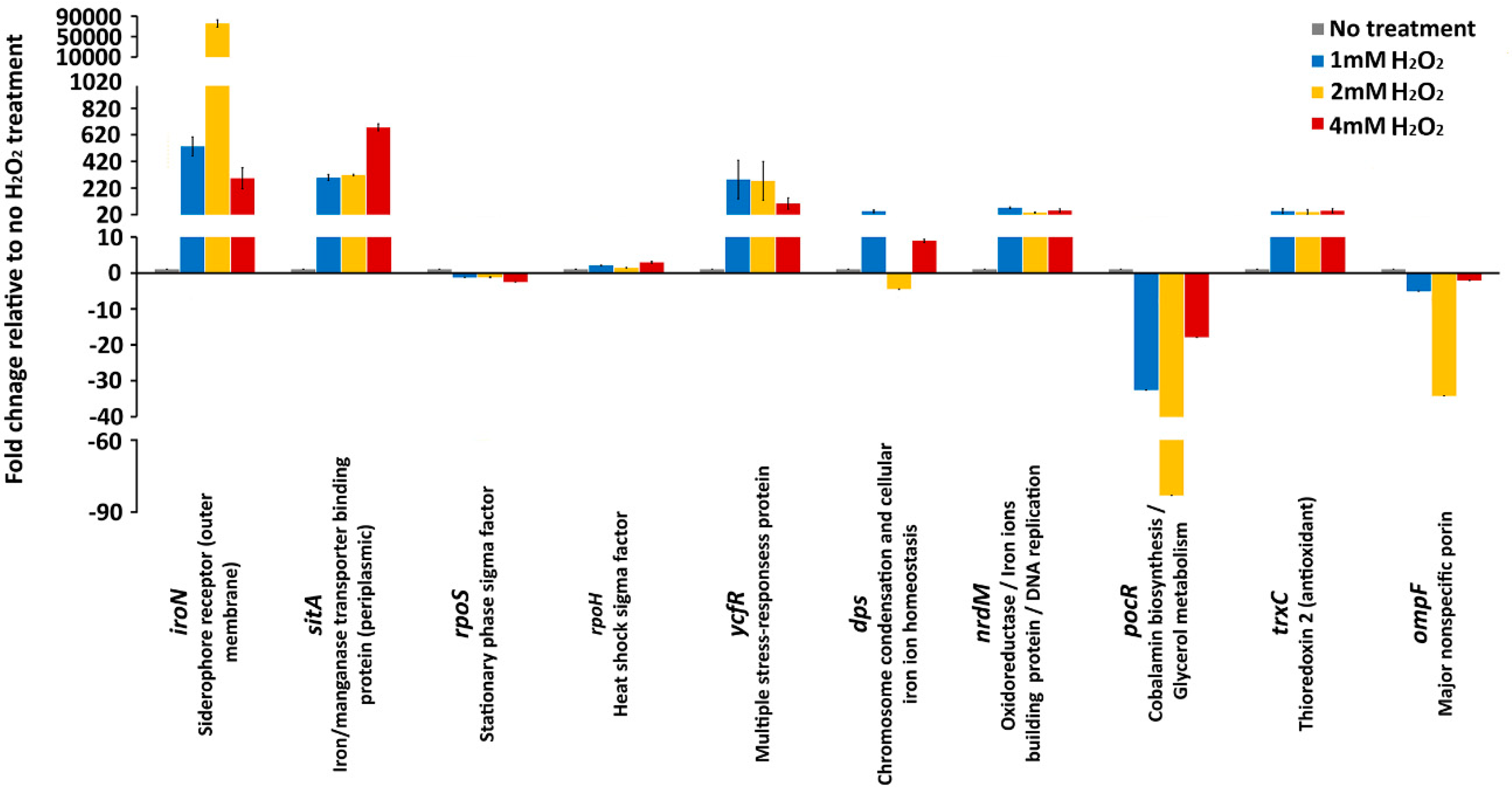
To determine the relationship between a death rate of S. Enteritidis and expression of genes that are associated with the most important oxidative stress adaptive processes, we carried out a qRT-PCR assay. The gene expression assay was carried out after 30 min of H2O2 treatment when Salmonella cells have already been adapted to the oxidative stressor (Figure 1). The targeted genes were selected from the following stress response and anabolism associated processes, iron homeostasis (iroN, sitA), alternative sigma factors (rpoS, rpoH), multiple stress responses (ycfR), DNA protection (dps), antioxidants (trxC), DNA replication (nrdM), uptake of small solutes (ompF) and anabolic process (pocR).
No significant changes in expression of rpoS were observed during 1 mM and 2 mM H2O2 treatment, whereas 4 mM H2O2 treatment caused a significant (2.5-fold) downregulation of the rpoS gene (Figure 2). Similarly to the rpoS gene, the oxidative treatments of S. Enteritidis caused minor alterations in the expression of the gene that encodes another stress response sigma factor, RpoH (Figure 2). The 1 mM and 4 mM treatments caused 2-fold and 2.9-fold upregulation of the rpoH gene, while 2 mM treatment caused no significant (1.4-fold) upregulation of the same gene.
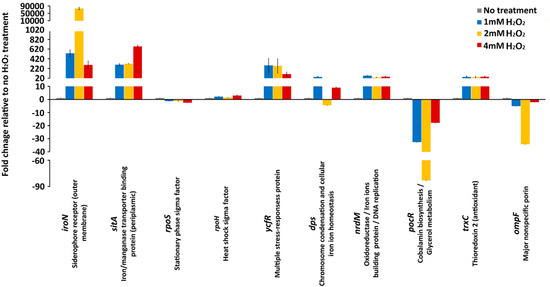
Figure 2.
Gene expression. mRNA expression levels of iroN, sitA (iron receptor), rpoS, rpoH (alternative sigma factors), ycfR, dps (stress response), nrdM, trxC (antioxidant), ompF (nonselective uptake), and pocR (anabolism) during no H2O2 treatment, 1 mM, 2 mM, and 4 mM H2O2 treatments. Values on the y-axis are relative expression levels (fold change) normalized against the level in the no H2O2 treatment group. The data correspond to the mean values of three biological replications. Error bars correspond to the standard deviation.
In sharp contrast to the genes that encode stress response sigma factors, the genes associated with iron homeostasis, in particular genes that encode the iron receptors, IroN (out membrane receptor) and SitA (periplasmic receptor) showed the greatest gene expression alterations (Figure 2). Most notably, the expression of iroN exhibited a profound upregulation with the 1 mM H2O2 treatment (532-fold) and with 2 mM H2O2 treatment, the upregulation of this gene was extraordinarily high (75664-fold) compared to that of the H2O2 untreated control. Interestingly, with the greatest concentration of oxidative stressor, 4 mM, the upregulation of the iroN gene was lower (292-fold) than that with 1 mM (532 fold) and 2 mM (75664 fold) treatment (Figure 2). Similarly, other genes involved in the iron homeostasis, sitA, showed large upregulation upon the oxidative stress treatment with 1 mM (298-fold), 2mM (316-fold) and 4 mM (675-fold) H2O2 treatment. Although both genes encode for iron receptors, their expression (stress response) patterns were different. The upregulation of the sitA gene was proportional to incremental increases of H2O2 concentration, whereas the upregulation of the iroN gene did not follow the level of H2O2 concentration (Figure 2).
The ycfR gene, which encodes a multiple stress response protein YcfR, was upregulated throughout the H2O2 treatments. The upregulation of the ycfR gene ranged from 283-fold and 273-fold to 103-fold in the Salmonella cultures exposed to 1 mM, 2 mM, and 4 mM H2O2, respectively (Figure 2). Similarly to the iroN gene, the greatest concentration of H2O2, 4 mM, caused the lowest upregulation of the ycfR gene, further indicating to some extent the similar patterns of the responses of these two oxidative stress response associated genes. However, the ycfR gene did not show an extremely high upregulation during the 2 mM H2O2 treatment, as the iroN gene showed (Figure 2). Another stress response gene, dps, had an unusual expression profile. During treatment with 1 mM H2O2 this gene was upregulated 44-fold, then during the 2 mM treatment the dps gene was downregulated 4-fold, and again upregulated 8.9-fold with 4 mM H2O2 treatment (Figure 2). This unusual expression profile of the dps gene, which encodes a protein that protects DNA from reactive oxygen species (ROS), indicates a complex interaction of the oxidative stress response associated genes.
The genes associated with DNA replication and oxidoreductase processes, nrdM and trxC, had similar genes expressions profiles (Figure 2). Both genes exhibited 35- to 70-fold upregulation during the oxidative treatments (Figure 2). The ompF gene, which encodes for a major nonspecific OmpF porin, and the pocR gene, which is associated with anabolic processes, exhibited downregulation during the oxidative treatments (Figure 2). Interestingly, the downregulation gene expression patterns for both the ompF and pocR genes were the same. The greatest gene alterations (e.g., downregulation) were observed during 2 mM H2O2 treatment, whereas the 4 mM H2O2 treatment caused the lowest level of the gene expression alteration for both genes (Figure 2).
3.3. Global Transcriptome Response of S. Enteritidis to Oxidative Stress.
After the determination of the stress response patterns of a limited number of genes during treatment with different concentrations of H2O2, we carried out the global transcriptome analyses using a single concentration of H2O2. In total, 2051 genes were significantly differently expressed (SDE) in S. Enteritidis during the 3 mM H2O2 treatment compared to the reference transcriptome of the same S. Enteritidis strain with no H2O2 treatment. Of the 2051 SDE genes, 1111 were induced and 941 genes were repressed, showing at least a 2-fold alteration in gene expression and high reproducibility (false discovery rate < 0.5) across all three biological replications (Tables S1 and S2).
3.3.1. Induction of Transcriptome—Molecular Response of S. Enteritidis to Oxidative Stress
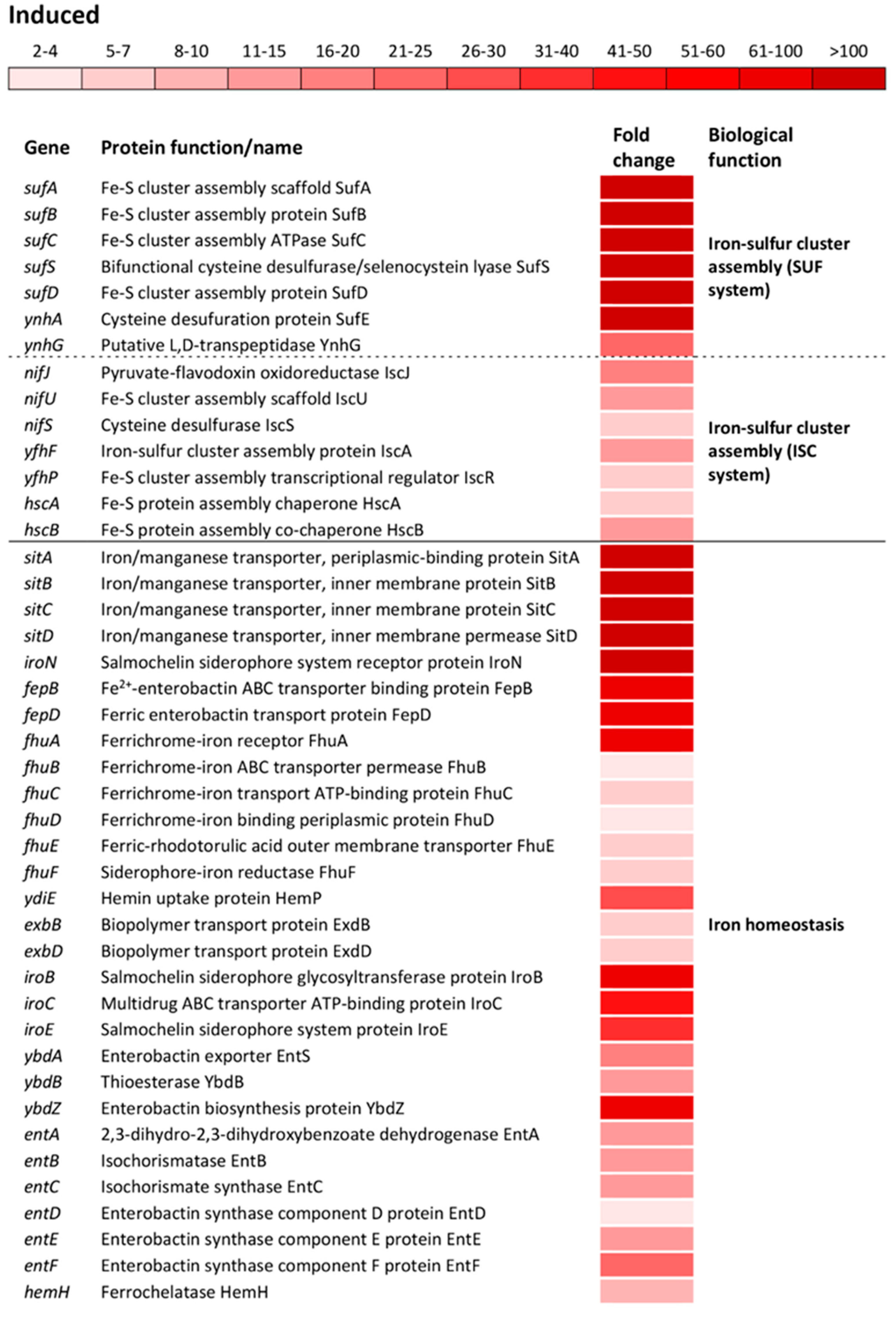
The most altered change in biological processes of oxidatively stressed S. Enteritidis cells was the induction of genes encoding Fe-S cluster biogenesis (Tables S1 and S2). Operons encoding sulfur utilization factor (SUF), sufABCSD and ynhAG, were upregulated from 173- to 903-fold and from 23- to 114-fold, respectively (Figure 3). The SUF operons were profoundly more induced compared to ones encoding iron-sulfur cluster (ISC), nifJUS, yfhFP and hscBA (Figure 3). Another group of highly induced genes were associated with the iron ion homeostasis. The operon sitABCD, iron/manganese ABC transporter, showed the greatest induction among operons/genes associated with this biological function (Figure 3). Besides this Fe/Mn transporter, a group of operons/genes encoding a wide range of iron transporters showed the greatest inductions, including iroN, ferric salmochelin siderophore receptor; fepBD, ferric enterobactin ABC transporter; fhuABCD, ferrichrome outer membrane transporter; ydiE, hemin transporter; exbBD, iron siderophore bacteriocin transporter; fhuE, ferric coprogen and ferric-rhodotorulic acid receptor as well as fhuF, receptor for ferrioxamine B (Figure 3). Also, several operons and a gene responsible for the biosynthesis of salmochelin (iroBCE), enterobactin (ybdABZ, entABCDEF) and hem (hemH) were greatly induced during oxidative stress (Figure 3).
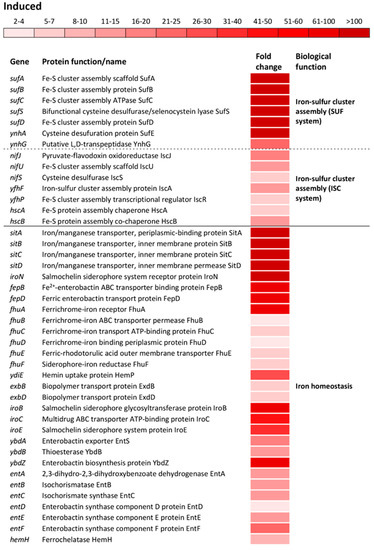
Figure 3.
Differential expression of genes involved in iron-cluster assembly and iron ion homeostasis. Transcriptional profiling of the wild type S. Enteritidis ATCC 13076. Isolation of RNA was carried out during the exponential growth phase. The transcriptomic profiles were determined using a HiSeq 4000 PE100 Illumina platform. The mean fold-increase of three biological replications in each gene expression after exposure to 3 mM of H2O2 compared with controls (no H2O2 treatment and the same growth phase) is indicated by the color scale bar. The biological functions of the induced gene were determined using the Database for Annotation, Visualization and Integrated Discovery (DAVID) as well as the National Center for Biotechnology Information (NCBI). Both sulfur utilization factor (SUF) and iron-sulfur cluster (ISC) belong to the same biological function “Iron-sulfur cluster assembly”.
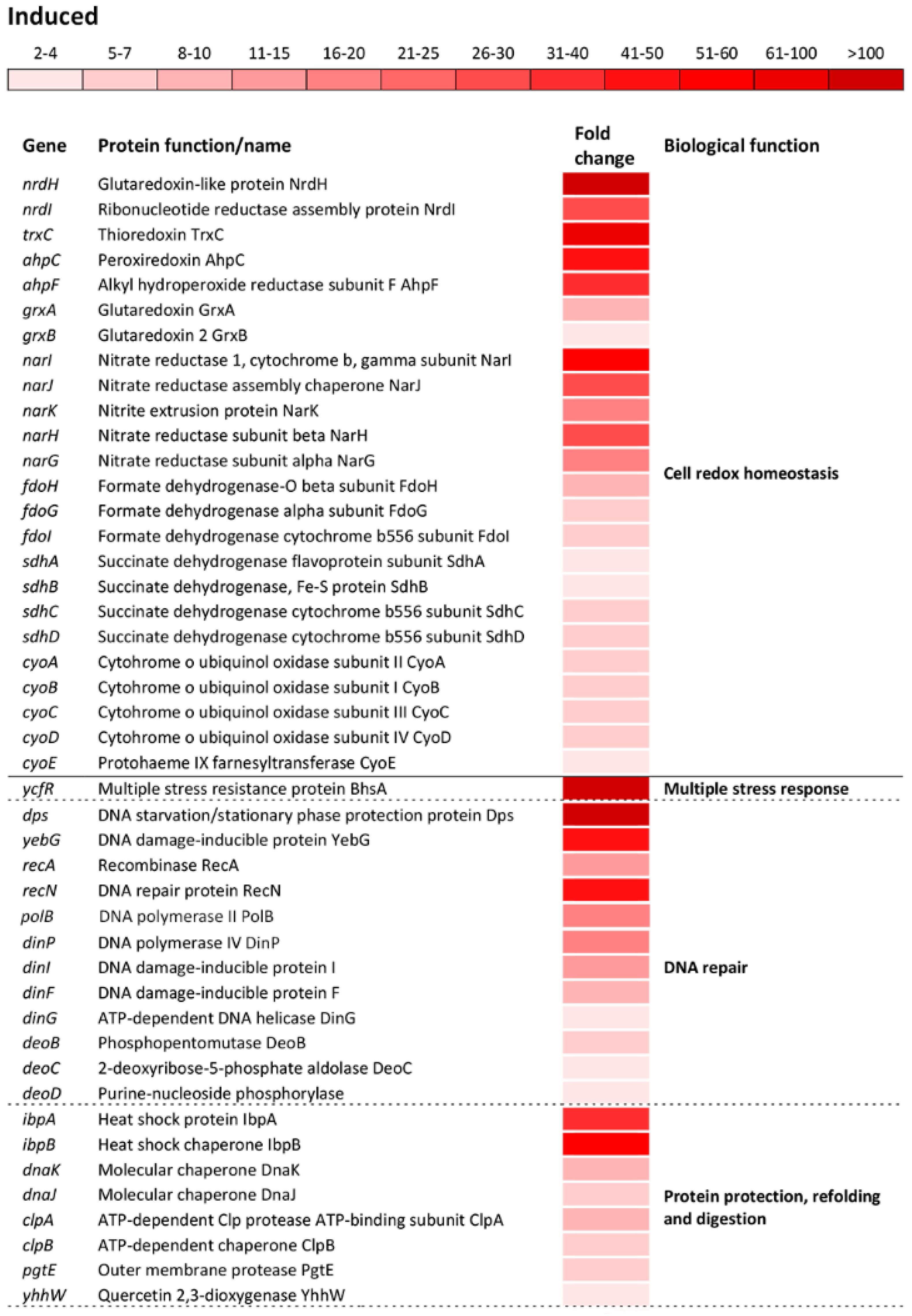
The significant induction showed a group of operons/genes encoding proteins that govern the cell-redox homeostasis. Among the cell-redox encoding genes, the nrdH gene which encodes a classical antioxidant, glutaredoxin-like protein NrdH, showed the greatest induction, resulting in 144.3-fold upregulation compared to the reference strain (Figure 4). The trxC gene that encodes an effective cytoplasmic disulfide-reducing protein, thioredoxin 2, showed 76-fold induction (Figure 4). The grxAB operon, encoding the antioxidants glutaredoxin 1 and 2, showed a moderate induction compared to that of the nrdH gene. A major part of the cell-redox regulon was associated with the genes encoding proteins associated with the electron transport chain (Figure 4). The narIJHKG operon, involved in nitrate assimilation and anaerobic electron transport, exhibited the greatest induction among this group of genes, followed by fdoHGI, dehydrogenases that transfer electrons from the periplasmic to the cytoplasmic heme; sdhABCD, cytochrome b556; cyoABCDE, cytochrome bo3 oxidase complex and sthA, which converts NADPH to NADH (Figure 4).
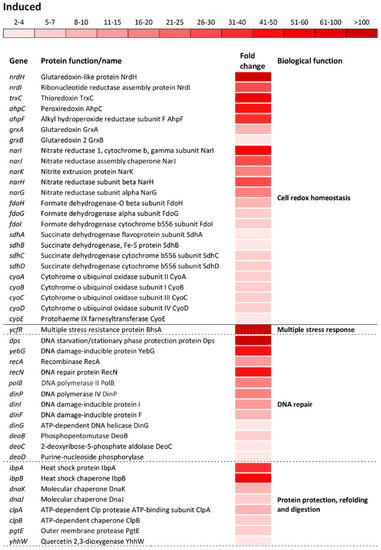
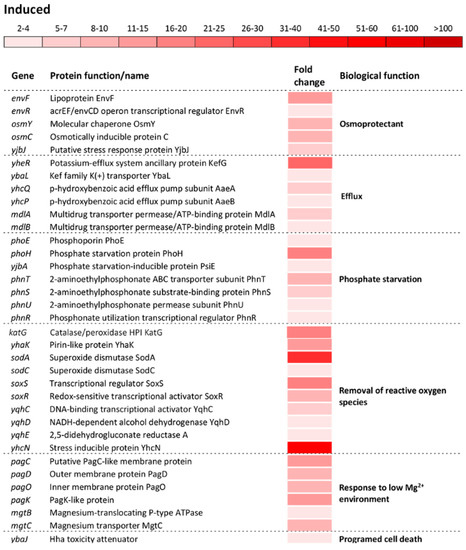
Figure 4.
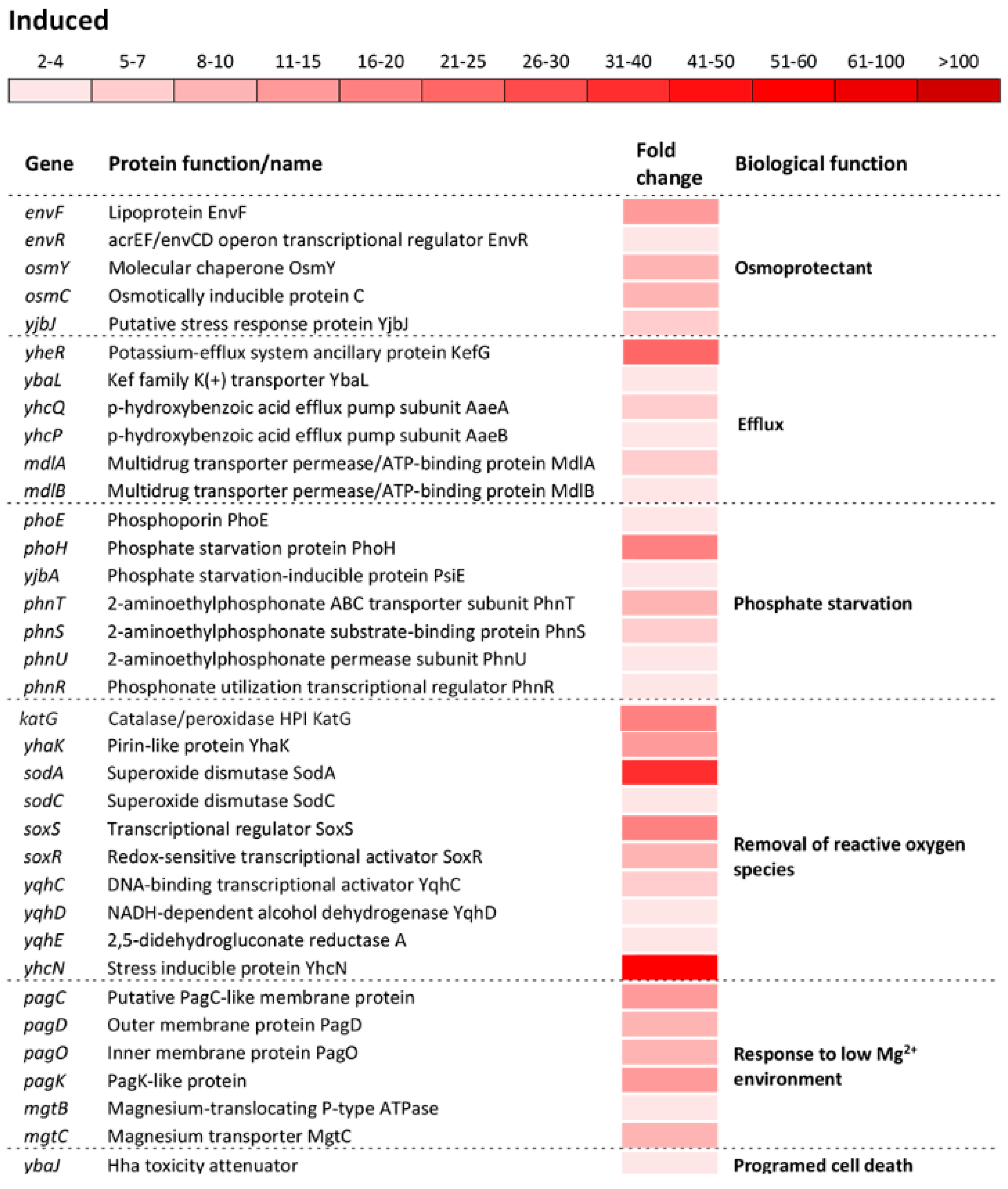
Differential expression of genes involved in cell redox homeostasis and various stress responses. The mean fold-increase in each gene expression after exposure to 3 mM of H2O2 compared with controls is indicated by the color scale bar. Note that a variety of biological functions including multiple stress response, DNA repair, protein protection, refolding and digestion, osmoprotectants, efflux, phosphate starvation, removal of reactive oxygen species, response to low Mg2+ environment and programed cell death belong to a more general biological function called “Stress response”.
The most numerous group of altered operons/genes in the transcriptome of oxidatively stressed S. Enteritidis cells was associated with various stress response processes as a distinct biological function indicated by the GO analysis. The greatest alteration in the entire transcriptome of S. Enteritidis under H2O2 stress was related to the ycfR gene. This gene, encoding the multiple stress resistance outer membrane protein YcfR, was induced 913-fold compared to that of the reference strain (Figure 4). The great induction exhibited the dps gene, which encodes the DNA-binding protein Dps that protects DNA. Besides dps, another group of genes encoding DNA repair proteins was significantly induced, including the DNA damage inducible yebG gene and the recAN, polB, dinPIG, and deoBCD genes (Figure 4). Similarly to genes encoding DNA repair proteins, a group of genes encoding molecular chaperones and proteases was significantly induced. Among this group of genes, the ibpBA gene, encoding small heat shock proteins, showed the greatest induction, followed by molecular chaperones dnaKJ, yhhW, and clpAB, and protease pgtE (Figure 4).
The oxidative stress response regulon included upregulation of: Osmotically inducible genes envFR, osmYC, and yjbJ; efflux encoding genes yheR, yabL, yhcQP, dinF, and mdlAB; phosphate starvation inducible genes phoHE, yjbA, and phnTSUR; sensing and removal of ROS katG, ahpCF, yhaK, sodAC, soxSR, yqhACDE, and yhcN; adaptation to low Mg2+ environment pagCDOK and mgtBC; and the programmed cell death inducible gene ybaJ (Figure 4).
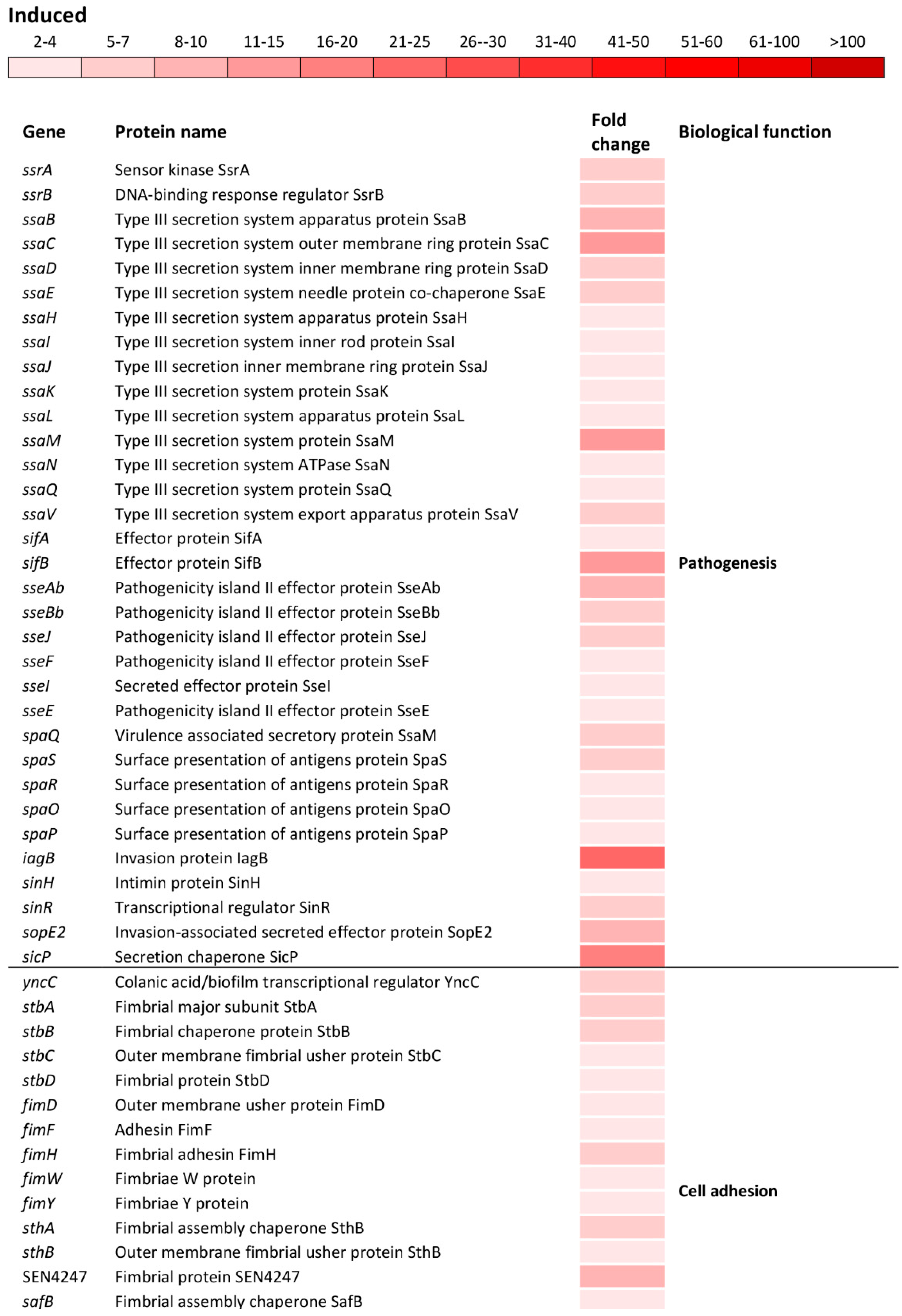
The oxidative stress caused the induction of numerous operons/genes located on Salmonella pathogenicity islands (SPIs). The significant induction was observed for the ssrAB operon that positively controls the expression of the SPI-2 genes (Figure 5)
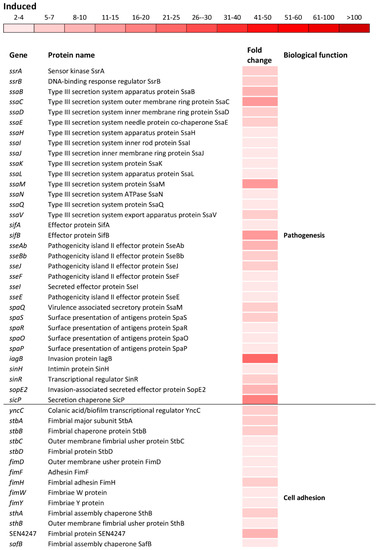
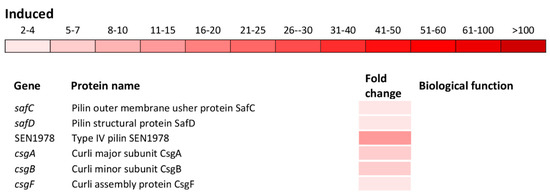
Figure 5.
Differential expression of genes involved in pathogenesis and cell adhesion. The mean fold-increase in each gene expression after exposure to 3 mM of H2O2 compared with controls is indicated by the color scale bar.
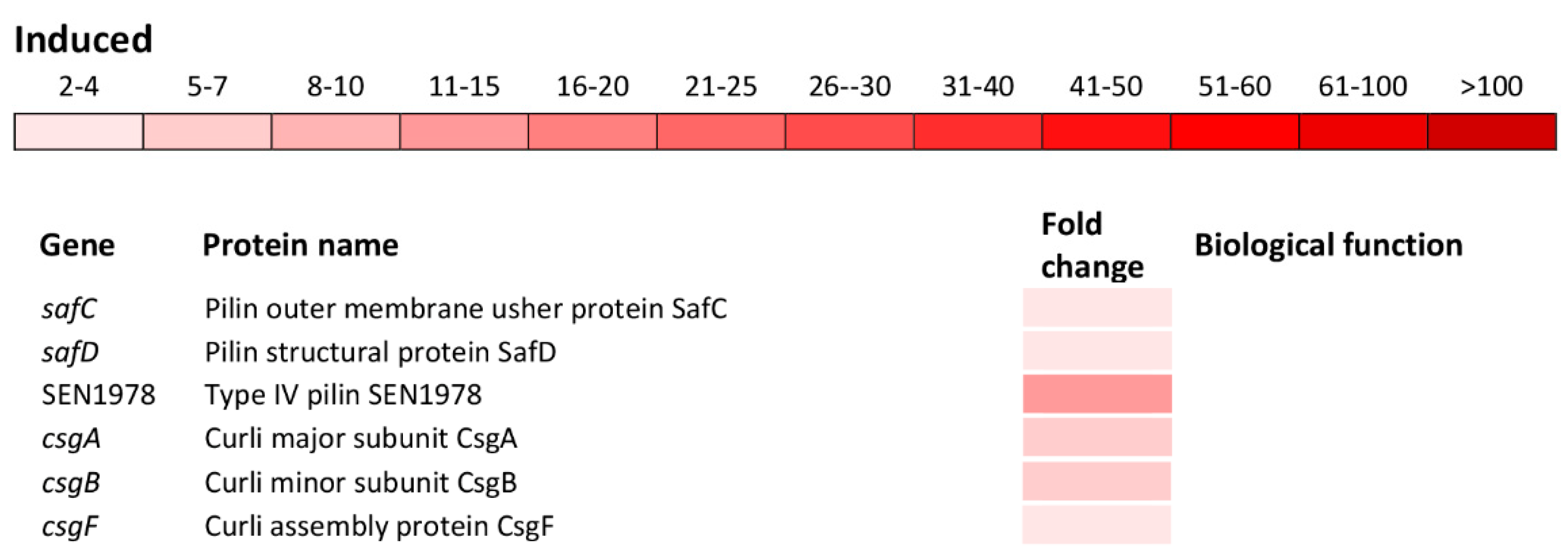
Indeed, a significant induction of the ssaMCBDEVJKHILQN operon encoding the type III secretion system (T3SS) apparatus of the SPI-2 as well as operons sifAB and sseAbJBbFIE encoding the SPI-2 effector proteins (Figure 5) was observed. Also, spaQSROP (translocon), iagB, sinHR, and sopE2 (effectors) as well as sicP (effector chaperone) located on the SPI-1 were significantly induced (Figure 5). Not only genes encoding the T3SS apparatus and effector proteins responsible for the invasion of host cells and intracellular survival, but also genes encoding various cell adhesions were induced. This group of genes included yncC, biofilm transcriptional regulator; stbABCD, fimHYWDF, sthAB and SEN4247, fimbrial operons/genes; safBCD and SEN1978, pilin operon/gene as well as csgABF, curli operon (Figure 5).
Adaptation to oxidative stress involved induction of operons/genes that encode proteins associated with carbohydrate metabolism yjfR, ydiN, nagABCE (glucosamine catabolic processes), pduJKLMDQTNOPHUWSECVXG (carboxysomes formation) and yiaMOGN (uptake of gluconate); amino acid metabolism asnABC, yehEWX, dadAX, yejF, lysA, yneHIG, ybaO, and livCKGFMH (uptake of branched-chain amino acids); nucleoside metabolism xapAB (purine salvage pathway); outer membrane modification pagP, nlpD, ybaN, wcaEFGHID (colonic acid biosynthesis), safA, ompX (porin), and ompA (porin) and genes encoding proteins of unknown functions (Table S1).
3.3.2. Repression of Transcriptome—Molecular Response of S. Enteritidis to Oxidative Stress
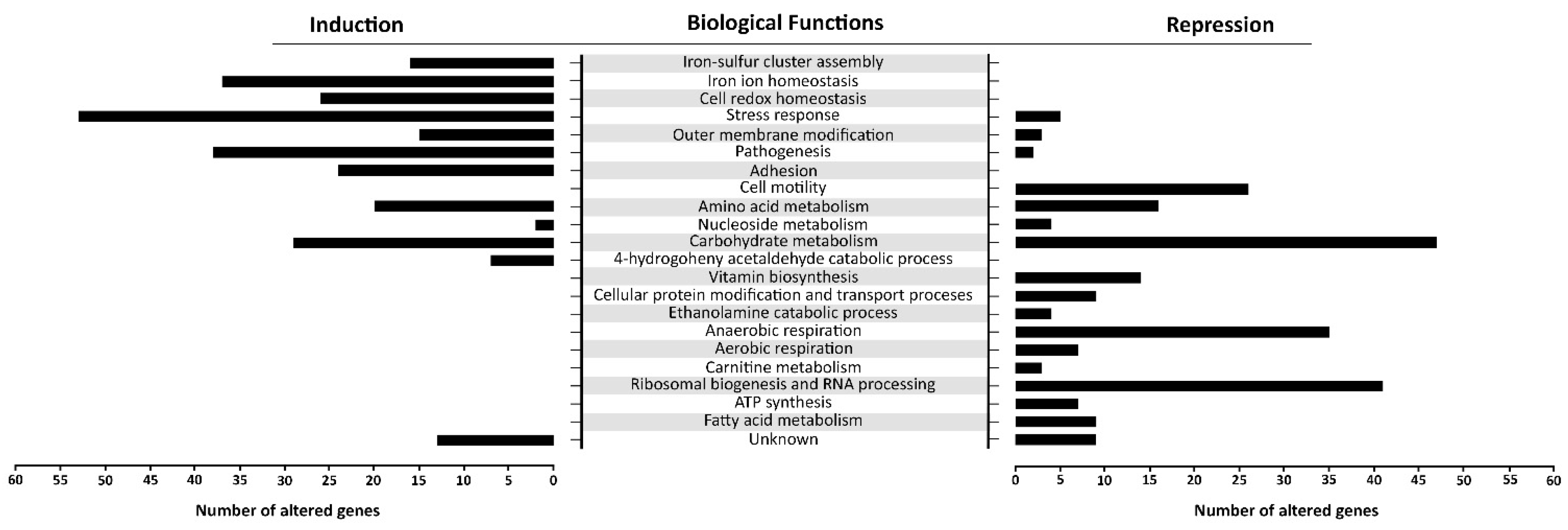
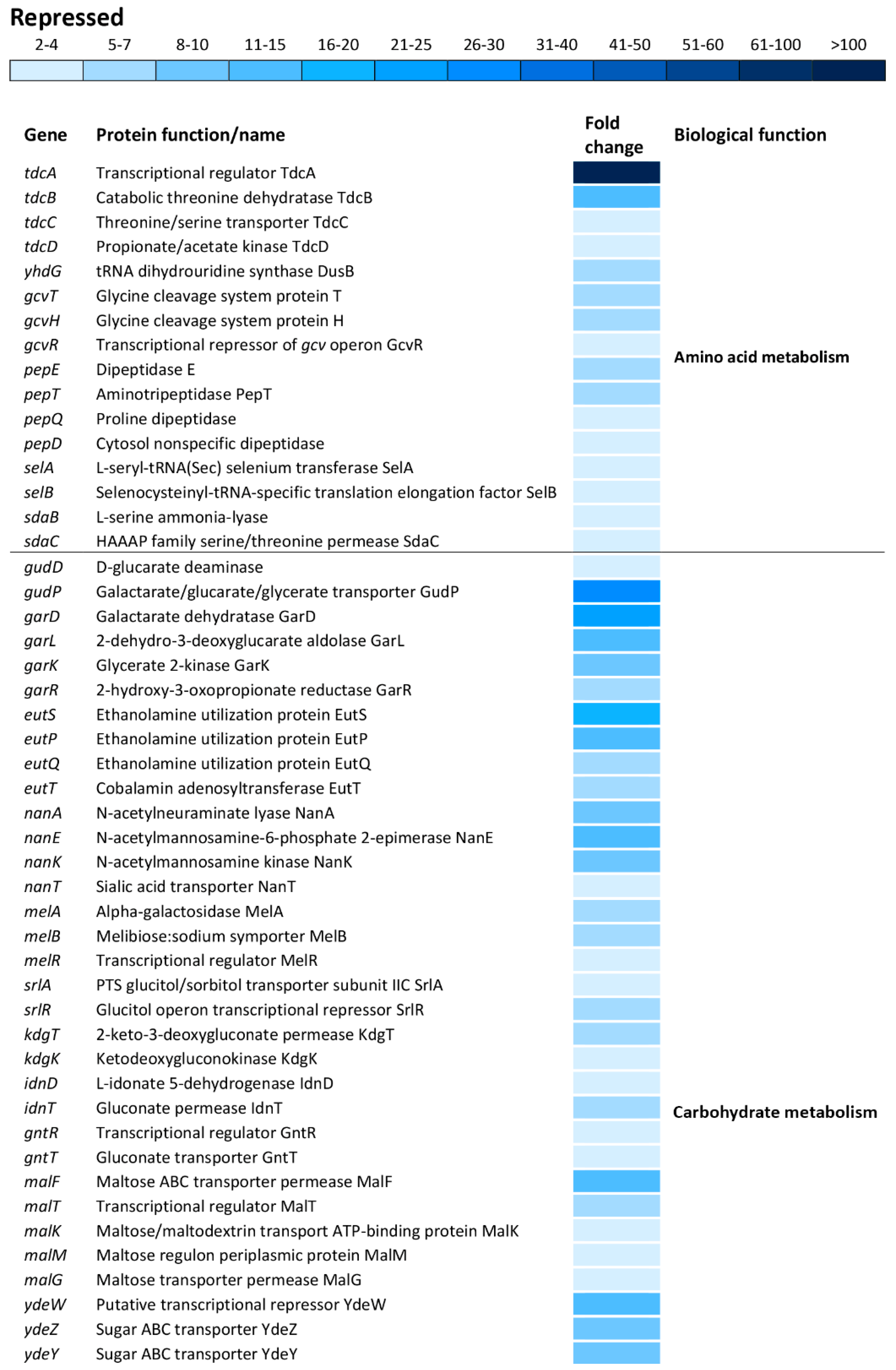
There is a distinct segregation based on the biological functions between the induced and repressed transcriptome of oxidatively stressed S. Enteritidis cells. While the induced transcriptome showed a great association with the iron-sulfur cluster assembly, iron ion homeostasis, cell redox homeostasis, a variety of stress responses, pathogenesis and sessile lifestyle (cell adhesion), the repressed transcriptome was mainly associated with carbohydrate metabolic processes, ribosomal biogenesis, anaerobic respiration and cell motility (Figure 6).
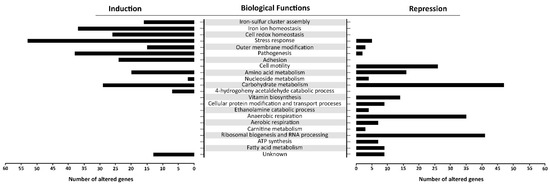
Figure 6.
Gene ontology (GO) enrichment analysis. The figure portrays the most altered biological functions of S. Enteritidis during the H2O2 treatment. In the GO analysis were included operons/genes that showed the greatest alterations compared to their counterparts in the control sample. Each gene showed false discovery rate (FDR) p < 0.05 and high reproducibility across the biological replications.
The tdcA gene, encoding a transcriptional activator for the tdcABCD operon, showed the greatest repression, resulting in 131-fold downregulation compared to that of the reference strain (Figure 7). Indeed, other genes of the tdcABCD operon, which are involved in L-threonine and L-serine catabolic processes, showed significant repression (Figure 7). Also, additional operons/genes associated with amino acid metabolism showed significant repression including yhdG (amino acid permease), gcvTHR (glycine, serine and threonine metabolism), pepETQD (hydrolases of peptide bonds), selAB (selenocysteine synthesis) and sdaBC (serine uptake and degradation) (Figure 7).
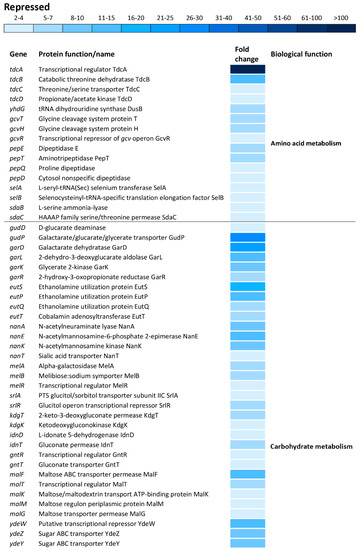
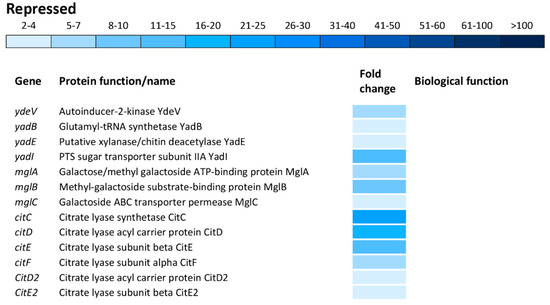
Figure 7.
Differential expression of genes involved in amino acid and carbohydrate metabolisms. The mean fold-decrease in each gene expression after exposure to 3 mM of H2O2 compared with controls is indicated by the color scale bar.
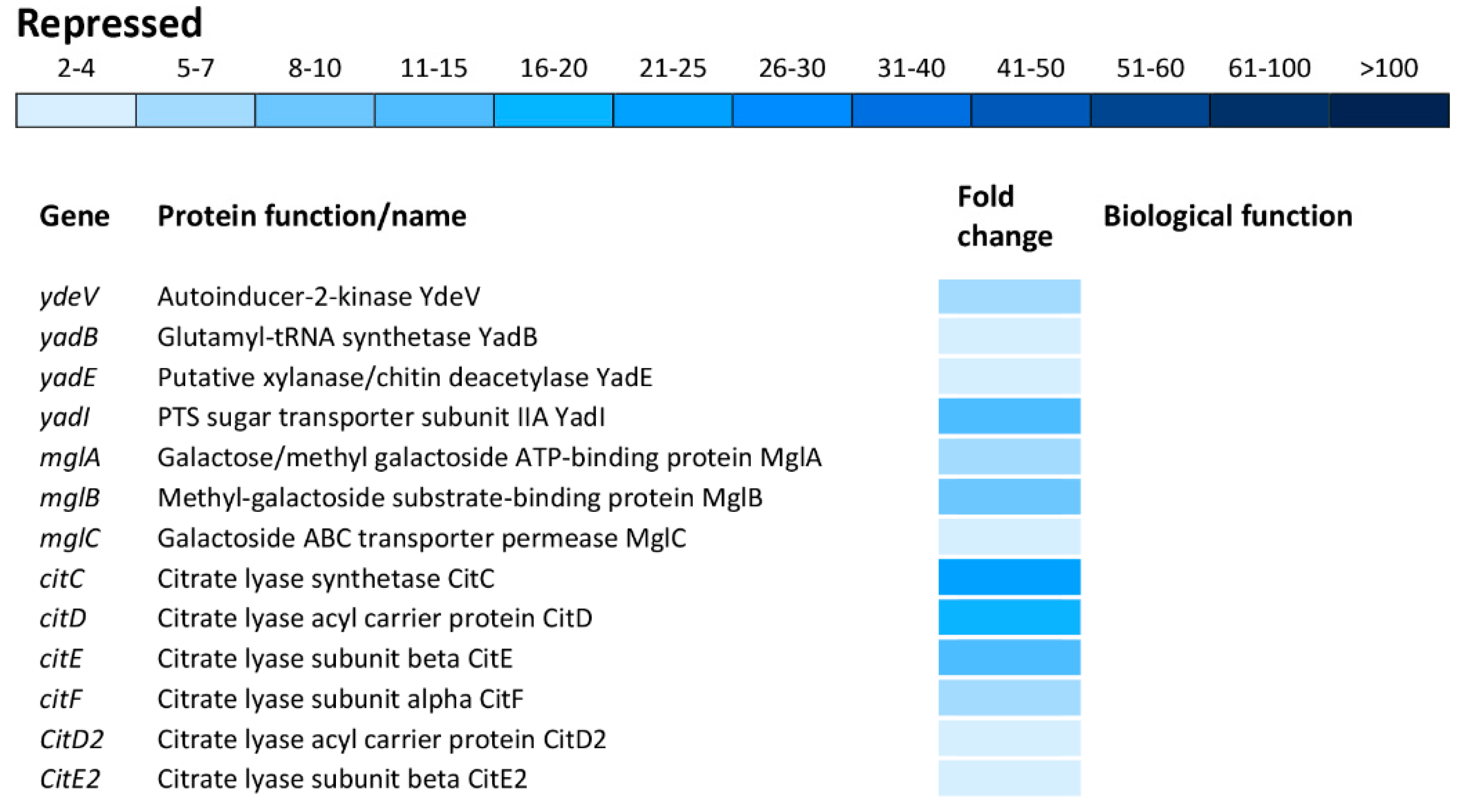
Carbohydrate metabolism was the biological function most commonly affected within the repressed transcriptome. This group of operons/genes, encoding a wide range of proteins, was involved in catabolic pathways, including gudDP (D-glucarate), garDLKR (galactarate), eutSPQT (ethanolamine), nanAEKT (sialic acid), melABR (melibiose), srlAR (sorbitol), kdgTK (D-glucuronate), idnDT, and gntRT (D-gluconate) (Figure 7). Besides this group of operons/genes associated with catabolism, there was repression of operons/genes encoding sugar uptake and anabolism such as malFTKMG (maltose), ydeWZYV (carbohydrate permease), yadBEI (a major carbohydrate active transport system), mglABC (galactose/methyl galactoside) and citCDEFD2E2 (citric operon involved in anabolic processes of the tricarboxylic acid cycle) (Figure 7).
Other central metabolic processes repressed during oxidative stress of S. enterica included anaerobic respiration (napABCDFGH, dmsA2A3A1AB1B, glpABCFTXK, frdABC, nrfABCD, hybABCFG, deuABC), ribosomal biogenesis and rRNA processing (ygjRO, rpsUTPLGSOCFHNJQBR, rplUKSJNXVBCDAEPLWYM, rimJMK, rpmAGBHC), fatty acid metabolic processes (accBD, fadHRL, fabIGBAH), vitamin biosynthesis (pocR, ynfKA, cbiACDETF, menDAB, yfbBSU), ATP synthesis (atpBIEHFGA), cellular protein modification and transport processes (hypAODBCE and oppCBD), aerobic respiration (yhbUTCSPQV), the ethanolamine catabolic process (eutSPQT), the carnitine metabolic process (caiFEA) and the pyrimidine nucleobase biosynthetic process (pyrHDLE) (Figure 6).
3.4. Validation of RNA-seq Data by Real-Time PCR
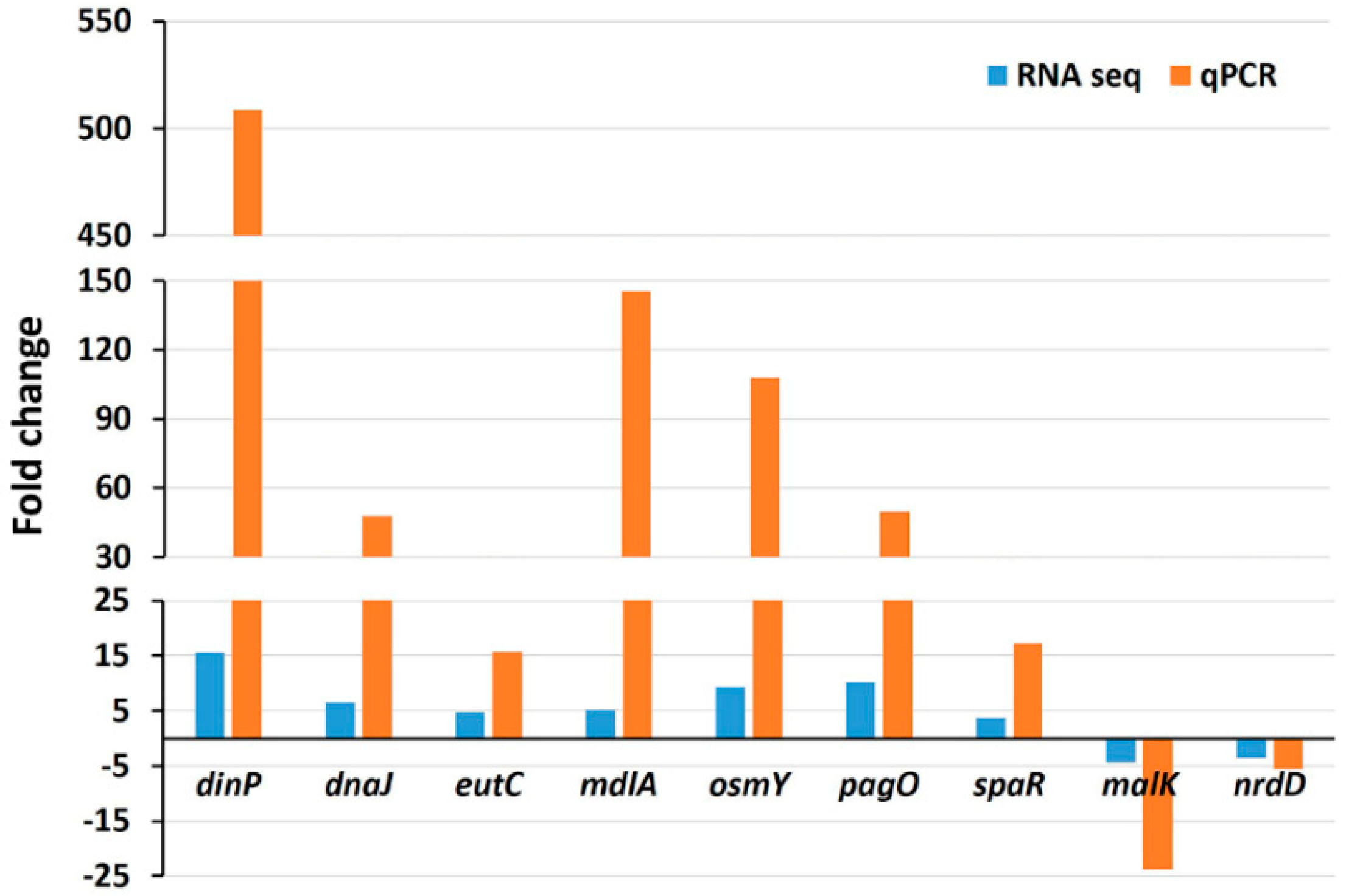
To validate the RNA-seq data, the expressions of nine randomly chosen genes were tested by quantitative real-time PCR (qRT-PCR) under the same experimental conditions. The qRT-PCR showed the same pattern of expressions, including seven upregulated genes (dinP, dnaJ, eutC, mdlA, osmY, pagO, and spaR) and two downregulated genes (malK and nrdD) (Figure 8). It can be observed that generally, the expression values measured by qRT-PCR were greater compared to those measured by RNA-seq analysis. The difference in expression values between these two techniques is consistent with the fact that qRT-PCR generally gives higher expression values compared to RNA-seq [17,22].
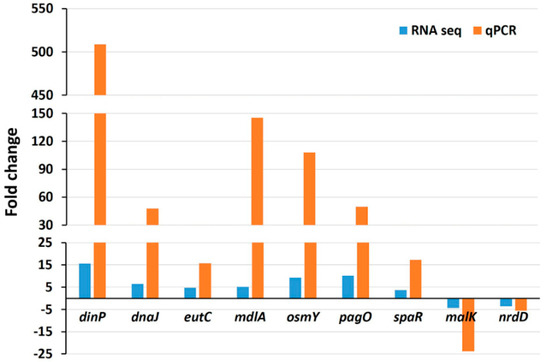
Figure 8.
Validation of RNA-seq data by qRT-PCR analysis. Data represent fold changes in the expression of nine randomly selected genes between the wild type strain without treatment and the same strain treated with 3 mM H2O2. Genes differently expressed between the wild type strain with no treatment and the wild type with H2O2 treatment represent the mean value of three biological replications.
4. Discussion
Salmonella Enteritidis, one of the most important non-typhoidal Salmonella serovars from the public health and veterinary medicine perspectives [23,24,25,26], has developed a robust anti-oxidative response not only to survive endogenous oxidative stress [27] but also to survive exogenous stress imposed by a host [28] or environment [29]. In other words, for successful host invasion, intracellular proliferation and/or environmental survival, possession of an efficient oxidative stress response is one of the key physiological characteristics of this pathogen. In this study, it was shown that incrementally increased concentrations of oxidant agent caused various patterns of gene expressions. While inductions of genes encoding antioxidant (thioredoxin 2) and DNA replication regulator (NrdM) were constant regardless of H2O2 concentrations, induction of a gene encoding iron/manganese transporter, SitA, was proportional with H2O2 concentrations. In contrast to the pattern of sitA induction, the ycfR gene, encoding multiple stress-response protein, exhibited disproportionate induction regarding H2O2 concentration. The greatest concentration of H2O2 resulted in the lowest ycfR induction. Besides these three patterns of gene expressions, the most common gene expression alteration during this assay was associated with an unpredictable pattern, where the middle concentration of H2O2 caused the greatest gene expression alterations, either induction (iroN) or repression (pocR, ompF and dps). Recently, Mitosch et al. [30], using a dual-reported model, demonstrated the importance of timing of the gene expression during exposure of the microbial cell to an oxidative agent. Besides the importance of temporal gene expression response, this study points to a multifaceted effect of oxidative agent concentration on gene expression during oxidative stress, further indicating another dimension of cellular adaptation complexity to oxidative stress.
The comparative transcriptomic analyses revealed that the expression of 45% of the S. Enteritidis genome was altered at least two-fold upon exposure to H2O2. This finding showed that oxidative stress has a much greater impact on the physiology of this pathogen than previously anticipated [18,31,32]. Out of numerous biological functions altered by oxidative stress, the SUF mechanism of Fe-S cluster formation exhibited the greatest change. The Fe-S clusters are cofactors of proteins and they are involved in various critical metabolic processes including DNA replication, regulation, and repair, substrate uptake, respiration, and RNA modification [33]. In general, there are two Fe-S cluster formation systems, the ISC system and the SUF system [33]. In this study, the SUF system showed a significantly greater induction than the ISC system. This finding correlates with previous studies that pointed out that the ISC is a housekeeping system [34], while the SUF was shown to be an oxidative stress or Fe-limited inducible system [35]. Indeed, the transcriptomic analyses showed the significant induction of numerous Fe acquisition mechanisms, including an iron/manganese transporter, ferrichrome, hemin, ferrioxamine, ferric coprogen and ferric-rhodotorulic acid uptake systems, then biosynthesis and uptake of salmochelin, enterobactin, as well as hydroxamate siderophores, clearly indicating a state of Fe-limitation in oxidatively stressed cells. These data showed the activation of the various Fe-acquisition systems in S. Enteritidis cells during oxidative stress, further suggesting their critical importance in cellular adaptation to oxidative stress.
Hydroxyl free radical, a product of the Fenton reaction, represents the most damaging compound during H2O2 treatment [36]. This highly reactive oxygen species interacts with numerous biomolecules further causing multiple effects on the treated prokaryotic cells [37]. The comparative transcriptomics analyses showed multifaceted responses of oxidatively treated cells including induction of not only the OxyR (katG, ahpCF) and the SoxRS (soxR, soxS, sodA, sodC), two oxidative stress regulons, but also stress responses involved in DNA and protein repairs, osmoprotection, multidrug efflux, phosphate and magnesium starvation. During the oxidative stress response, this human pathogen induced a robust DNA repair system, which included base repair (deoBCD), single base mismatch repair (dinIG), early double-strand break repair (yebG), multiple double-strand breaks repair (recAN) and DNA replication (polB, dinP). Also, the dps gene which encodes a multifunctional Dps protein was greatly induced. Dps non-specifically binds DNA, forming a highly stable Dps-DNA nucleoprotein complex which further protects DNA from the deleterious effects of ROS [38]. Similarly to DNA, protein repair systems were induced. The ibpAB genes, encoding small heat-shock proteins IbpA and IbpB, showed the greatest induction among various protein repair systems. Induction of genes encoding molecular chaperones (dnaKJ, clpB) and proteases (clpA, pgtE) indicates a tendency of proteins to aggregate or to undergo a process of denaturation during oxidative stress. Multifaceted responses of oxidatively treated cells was also reflected by induction of key genes associated with osmotic stress (osmYC, envFR), manganese starvation (sitABCD, pagCDOK, mgtBC), phosphate starvation (phoEH, yjbA, phnTSUR), multidrug efflux (mdlAB, yhcQP) and outer membrane modification (wcaEFH, yncCJ, yjbAEHJ). Adaptation of S. Enteritidis to oxidative stress was not only confined to the induced transcriptome but it also included the repressed transcriptome. The significant repression of the major small (rpsUTPLGSOCFHNJQBR) and large (rplUKSJNXVBCDAEPLWYM) units of ribosomal encoding genes indicates that oxidatively stressed cells activated a stringent response. The main purpose of a stringent response is saving cellular energy and precursors required for ribosomal synthesis. If cells continue to synthesize ribosomes, the majority of these organelles would end up being inactive due to the disruption of central metabolic pathways (i.e., lack of amino acids) and this would be an unnecessary and costly expenditure of resources [37].
Besides the alterations of a large pool of genes encoding various central metabolic and stress response pathways, the comparative transcriptome analyses revealed a clear division, during the H2O2 treatment, in the gene expressions associated with the genes encoding for motility (planktonic) and the adhesion genes (sessile) forms of life. The exposure of S. Enteritidis to oxidative stress had an extensive and significant positive effect on the expression of adhesion encoding genes, including fimbrial stbABCD, fimHYWDF, sthB, SEN4247; curli csgABF, and pilin safBCD, SEN1978 (type IV pilin), yibEHG and yncC operons and genes. At the same time, it negatively affected expressions of the major flagellar operons flgHECFIGJDBMN and fliJMRLNKFQPOIHEBG clearly indicating a switch from a planktonic to a sessile (i.e., biofilm) life forms of oxidatively treated cells. Recently, it was shown that a transition from planktonic to sessile life form in S. Enteritidis is followed by repression of the major flagellar operons and induction of numerous fimbrial, curli, and pili operons [22]. This transcriptional change is in agreement with the “swim-or-stick” theory, which postulates that motility and biofilm development are mutually-exclusive processes [39]. Our transcriptomic data indicate that oxidatively stressed cells transition from a planktonic to a biofilm form of life. Another important feature of oxidatively stressed cells was an extensive induction of salmonellae-essential virulence genes located on SPI 1, SPI 2, and SPI 5. Induction of core virulence genes involved genes encoding not only T3SS apparatus of the SPI 2 and translocon of SPI 1 but also numerous effectors (sifAB, sseAbjbBFIE, iagB, sinHR, sopE2, sicP) essential for intracellular survival of this pathogen [40]. Fu and colleagues [18] studying the oxidative stress response of S. Typhimurium found that this organism repressed the synthesis of core virulence proteins encoded on SPI 1. Discrepancy between their and our results can be most likely explained by different experimental setups. While Fu and colleagues [18] exposed the cultures of S. Typhimurium to H2O2 at their mid-exponential growth phases (0.5 at OD600) and harvested them at their early stationary growth phase (0.9 at OD600). We started H2O2 treatment at the mid-exponential growth phase (0.4 at OD600) and harvested the cultures at their still exponential growth phase (~0.5 at OD600). Taken together, our experimental approach ensured that all transcriptional changes will be consequences of oxidative stress, while the approach employed by Fu et al., [18] reflects not only oxidative stress response but additional stress responses associated with the stationary growth phase of this organism [41,42].
5. Conclusions
This study employing a combination of incremental oxidative stress assays and the next generation sequencing approach revealed additional levels of the oxidative stress response complexity in non-typhoidal Salmonella. It was shown that the oxidative stress response is intricately linked with not only Fe-S cluster formation, iron homeostasis, DNA repair, and cell redox homeostasis but also with heat, osmotic, stringent, manganese and phosphate starvation stress responses as well as multidrug efflux and decreased passive influx of small hydrophilic molecules. In addition to these multifaceted cellular adaptation processes, it was shown that S. Enteritidis during oxidative stress simultaneously repressed the key motility encoding genes and massively induced the essential adhesion and salmonellae core-virulence encoding genes, crucial for the biofilm formation and host cell invasion. Based on these data, we hypothesize that the oxidative stress response of non-typhoidal Salmonella is closely associated with intracellular survival and that oxidative stress may act as a stimulus for eukaryotic cell invasion and formation of Salmonella-containing vacuoles. It has been already found that intracellular biofilm formation plays an important step in virulence of uropathogenic Escherichia coli, another facultative intracellular pathogen [43], suggesting that a similar approach can be explored by non-typhoidal Salmonella.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3921/9/9/849/s1, Figure S1. The effect of different H2O2 concentrations on the survivability of S. Enteritidis over 90 min. Error bars correspond to the standard deviations. Table S1. List of induced genes during the 3 mM H2O2 treatment of the S. Enteritidis ATCC 13076 strain compared to the control. Table S2. List of repressed genes during the 3 mM H2O2 treatment of the S. Enteritidis ATCC 13076 strain compared to the same H2O2 untreated strain.
Author Contributions
Conceptualization S.V.; Methodology S.V. and X.L.; Investigation X.L., M.O., and S.V.; Formal analysis S.V., X.L. and J.E.A.; Supply of reagents K.V.N.; Writing—original draft S.V.; Funding acquisition S.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the United States Department of Agriculture, National Institute of Food and Agriculture, Hatch Capacity Grant # 592 and Grant-in-Aid of Research, Artistry and Scholarship, University of Minnesota, USA.
Acknowledgments
The authors gratefully acknowledge the technical support from Daniela Vidovic. The present study was also supported, in part, by resources provided by the Minnesota Supercomputing Institute.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. International Collaboration on Enteric Disease ‘Burden of Illness’ Studies. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.R.; Griffin, P.M.; Cole, D.; Walsh, K.A.; Chai, S.J. Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Elias, S.O.; Decol, L.T.; Tondo, E.C. Foodborne outbreaks in Brazil associated with fruits and vegetables: 2008 through 2014. Food Qual. Saf. 2018, 2, 173–181. [Google Scholar] [CrossRef]
- Bennet, S.D.; Littrell, K.W.; Hill, T.A.; Mahovic, M.; Behravesh, C.B. Multistate foodborne disease outbreaks associated with raw tomatoes, United States, 1990–2010: A recurring public health problem. Epidemiol. Infect. 2015, 143, 1352–1359. [Google Scholar] [CrossRef]
- Greene, S.K.; Daly, E.R.; Talbot, E.A.; Demma, L.J.; Holzbauer, S.; Patel, N.J.; Hill, T.A.; Walderhaug, M.O.; Hoekstra, R.M.; Lynch, M.F.; et al. Recurrent multistate outbreaks of Salmonella Newport associated with tomatoes from contaminated fields, 2005. Epidemiol. Infect. 2008, 136, 157–165. [Google Scholar] [CrossRef]
- Gajraj, R.; Pooransingh, S.; Hawker, J.I.; Olowokure, B. Multiple outbreaks of Salmonella Braenderup associated with consumption of iceberg lettuce. Int. J. Environ. Health Res. 2012, 22, 150–155. [Google Scholar] [CrossRef]
- Reddy, S.P.; Wang, H.; Adams, J.K.; Peter, C.; Feng, H. Prevalence and characteristics of Salmonella serotypes isolated from fresh produce marketed in the United States. J. Food Protec. 2016, 79, 6–16. [Google Scholar] [CrossRef]
- Sivapalasingam, S.; Barrett, E.; Kimura, A.; Van Duyne, S.; De Witt, W.; Ying, M.; Frisch, A.; Phan, O.; Gould, E.; Shillam, P.; et al. A multistate outbreak of Salmonella enterica serotype Newport infection linked to mango consumption: Impact of water-dip disinfestation technology. Clin. Infect. Dis. 2003, 37, 1585–1590. [Google Scholar] [CrossRef]
- Gibbs, R.; Pingault, N.; Mazzucchelli, T.; O’Reilly, L.; MaCkenzie, B.; Green, J.; Mogyorosy, R.; Stafford, R.; Bell, R.; Hiley, L.; et al. An outbreak of Salmonella enterica serotype Litchfield infection in Australia linked to consumption of contaminated papaya. J. Food Prot. 2009, 72, 1094–1098. [Google Scholar] [CrossRef]
- Wei, K.; Zhou, H.; Zhou, T.; Gong, J. Comparison of aqueous ozone and chlorine as sanitizers in the food processing industry: Impact on fresh agricultural produce quality. Ozone Sci. Eng. 2007, 29, 113–120. [Google Scholar] [CrossRef]
- Vidovic, S.; Korber, D.R. Escherichia coli O157: Insights into the adaptive stress physiology and the influence of stressors on epidemiology and ecology of this human pathogen. Crit. Rev. Microbiol. 2016, 42, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Cabezas, C.E.; Briones, A.C.; Aguirre, C.; Pardo-Este, C.; Castro-Severyn, J.; Salinas, C.R.; Baquedano, M.S.; Hidalgo, A.A.; Fuentes, J.A.; Morales, E.H.; et al. The transcription factor SlyA from Salmonella Typhimurium regulates genes in response to hydrogen peroxide and sodium hypochlorite. Res. Microbiol. 2018, 169, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Kurasz, J.E.; Hartman, C.E.; Samuels, D.J.; Mohanty, B.K.; Deleveaux, A.; Mrázek, J.; Karls, A.C. Genotoxic, metabolic, and oxidative stresses regulate the RNA repair operon of Salmonella enterica serovar Typhimurium. J. Bacteriol. 2018, 200, e00476-18. [Google Scholar] [CrossRef]
- Li, J.; Overall, C.C.; Johnson, R.C.; Jones, M.B.; McDermott, J.E.; Heffron, F.; Adkins, J.N.; Cambronne, E.D. ChIP-Seq analysis of the σE regulon of Salmonella enterica serovar Typhimurium reveals new genes implicated in heat shock and oxidative stress response. PLoS ONE 2015, 10, e0138466. [Google Scholar] [CrossRef]
- Vidovic, S.; Elder, J.; Medihala, P.; Lawrence, J.R.; Predicala, B.; Zhang, H.; Korber, D.R. ZnO nanoparticles impose a panmetabolic toxic effect along with strong necrosis, inducing activation of the envelope stress response in Salmonella enterica serovar Enteritidis. Antimicrob. Agents Chemother. 2015, 59, 3317–3328. [Google Scholar] [CrossRef]
- Vidovic, S.; Liu, X.; An, R.; Mendoza, K.M.; Abrahante, J.E.; Johny, A.K.; Reed, K.M. Transcriptional profiling and molecular characterization of the yccT mutant link: A novel STY1099 protein with the peroxide stress response and cell division of Salmonella enterica serovar Enteritidis. Biology 2019, 8, 68. [Google Scholar] [CrossRef]
- Fu, J.; Qi, L.; Hu, M.; Liu, Y.; Yu, K.; Liu, Q.; Liu, X. Salmonella proteomics under oxidative stress reveals coordinated regulation of antioxidant defense with iron metabolism and bacterial virulence. J. Proteom. 2017, 157, 52–58. [Google Scholar] [CrossRef]
- Vidovic, S.; Medihala, P.; Dynes, J.J.; Daida, P.; Vujanovic, V.; Hitchcock, A.P.; Shitty, D.; Zhang, H.; Brown, D.R.; Lawrence, J.R.; et al. Importance of the rpoE regulon in maintaining the lipid bilayer during antimicrobial treatment with the polycationic agent, chlorhexidine. Proteomics 2018, 18, 3–4. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Shitty, D.; Abrahante, J.E.; Chekabab, S.M.; Wu, X.; Korber, D.R.; Vidovic, S. Role of CpxR in biofilm development: Expression of key fimbrial, O-antigen and virulence operons of Salmonella Enteritidis. Int. J. Mol. Sci. 2019, 20, 5146. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, S.D.; Flores, F.S.; dos Santos, L.R.; Brandelli, A. Antimicrobial resistance in Salmonella Enteritidis strains isolated from broiler carcasses, food, human and poultry-related samples. Int. J. Food Microbiol. 2005, 97, 297–305. [Google Scholar] [CrossRef]
- Vaz, C.S.; Streck, A.F.; Michael, G.B.; Marks, F.S.; Rodrigues, D.P.; dos Reis, E.M.F.; Cardoso, M.R.I.; Canal, C.W. Antimicrobial resistance and subtyping of Salmonella enterica subspecies enterica serovar Enteritidis isolated from human outbreaks and poultry is southern Brazil. Poult. Sci. 2010, 89, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Alshalchi, S.; Breimhurst, P.; Munoz-Aguayo, J.; Flores-Figueroa, C.; Vidovic, S. Strong influence of livestock environments on the emergence and dissemination of distinct multidrug-resistant phenotypes among the population of the non-typhoidal Salmonella. PLoS ONE 2017, 12, e0179005. [Google Scholar] [CrossRef] [PubMed]
- Braden, C.R. Salmonella enterica serotype Enteritidis and eggs: A national epidemic in the United States. Clin. Infect. Dis. 2006, 43, 512–517. [Google Scholar] [CrossRef]
- Rhen, M. Salmonella and reactive oxygen species: A love-hate relationship. J. Innate Immun. 2019, 11, 216–226. [Google Scholar] [CrossRef]
- He, H.; Genovese, J.K.; Swaggerty, C.L.; Nisbet, D.J.; Kogut, M.H. A comparative study on invasion, survival, modulation of oxidative burst, and nitric oxide responses of macrophages (HD11), and systemic infection in chickens by prevalent poultry Salmonella serovars. Foodborne Pathog. Dis. 2012, 9, 1104–1110. [Google Scholar] [CrossRef]
- Shah, D.H.; Casavant, C.; Hawley, Q.; Addwebi, T.; Call, D.R.; Guard, J. Salmonella Enteritidis strains from poultry exhibit differential responses to acid stress, oxidative stress, and survival in the egg albumen. Foodborne Pathog. Dis. 2012, 9, 258–264. [Google Scholar] [CrossRef]
- Mitosch, K.; Rieckh, G.; Bollenbach, T. Temporal order and precision of complex stress responses in individual bacteria. Mol. Syst. Biol. 2019, 15, e8470. [Google Scholar] [CrossRef]
- Wang, S.; Phillippy, A.M.; Deng, K.; Rui, X.; Li, Z.; Tortorello, M.L.; Zhang, W. Transcriptomic responses of Salmonella enterica serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl. Environ. Microbiol. 2010, 76, 5013–5024. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yang, E.; Vu, G.P.; Gong, H.; Su, J.; Liu, F.; Lu, S. Mass spectrometry-based quantitative proteomic analysis of Salmonella enterica serovar Enteritidis protein expression upon exposure to hydrogen peroxide. BMC Microbiol. 2010, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Castro, C.; Saini, A.; Outten, F.W. Fe-S cluster assembly pathways in bacteria. Microbiol. Mol. Biol. Rev. 2008, 72, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Lill, R. Function and biogenesis of iron-sulfur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Outten, F.W. Recent advances in the Suf Fe-S cluster biogenesis pathway: Beyond the Proteobacteria. Biochim. Biophys. Acta 2015, 1853, 1464–1469. [Google Scholar] [CrossRef]
- Imlay, J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003, 57, 395–418. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress response in Escherichia coli and Salmonella Typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Wang, G.; Maier, R.J. Helicobacter hepaticus Dps protein plays an important role in protecting DNA from oxidative damage. Free Radic. Res. 2006, 40, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ditmarsch, D.; Boyle, K.E.; Sakhtah, H.; Oyler, J.E.; Nadell, C.D.; Deziel, É.; Dietrich, L.E.P.; Xavier, J.B. Convergent evolution of hyperswarming leads to impaired biofilm formation in pathogenic bacteria. Cell Rep. 2013, 4, 697–708. [Google Scholar] [CrossRef]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008, 6, 53–66. [Google Scholar] [CrossRef]
- Weber, H.; Polen, T.; Heuveling, J.; Wendisch, V.F.; Hengge, R. Genome-wide analysis of the general stress response network in Escherichia coli: σS-dependent genes, promoters, and sigma factor selectivity. J. Bacteriol. 2005, 187, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, J.L.; Uknalis, J.; Oscar, T.P.; Schwarz, J.G.; Vimini, B.; Parveen, S. The effect of previous life cycle phase on the growth kinetics, morphology, and antibiotic resistance of Salmonella Typhimurium DT104 in brain heart infusion and ground chicken extract. Front. Microbiol. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Hunstad, D.A.; Justice, S.S. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu. Rev. Microbiol. 2010, 64, 203–221. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).