The Antioxidant Activities of Betula etnensis Rafin. Ethanolic Extract Exert Protective and Anti-diabetic Effects on Streptozotocin-Induced Diabetes in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
2.2. Plant Material and Preparation of the Extract
2.3. Total Phenolic Content and Total Flavonoids Content
2.4. HPLC/DAD Analysis
2.5. Antioxidant Characterization of the Extract
2.5.1. SOD-like Activity
2.5.2. DPPH Radical Scavenging Activity Assay
2.6. Animals and Treatments
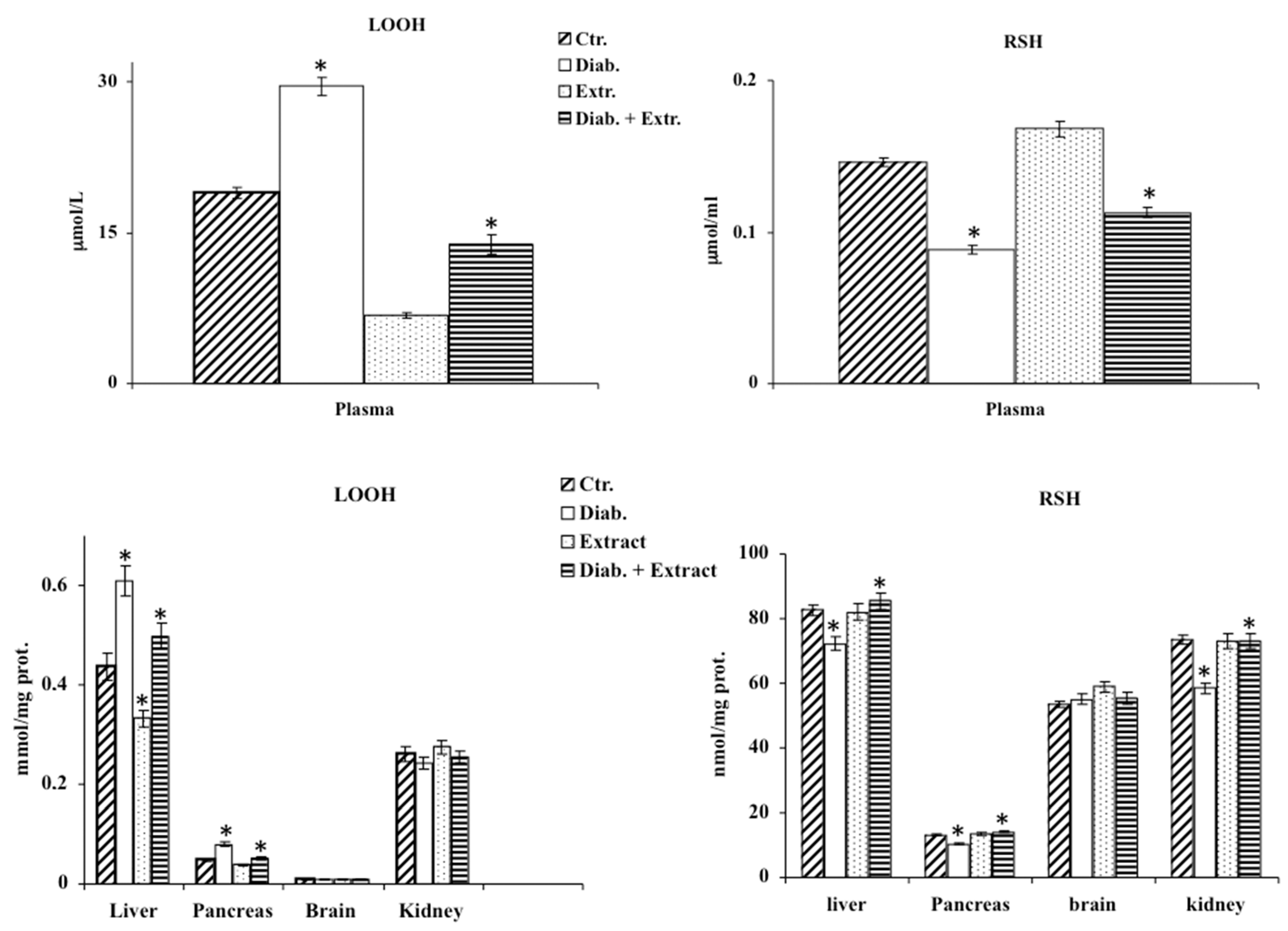
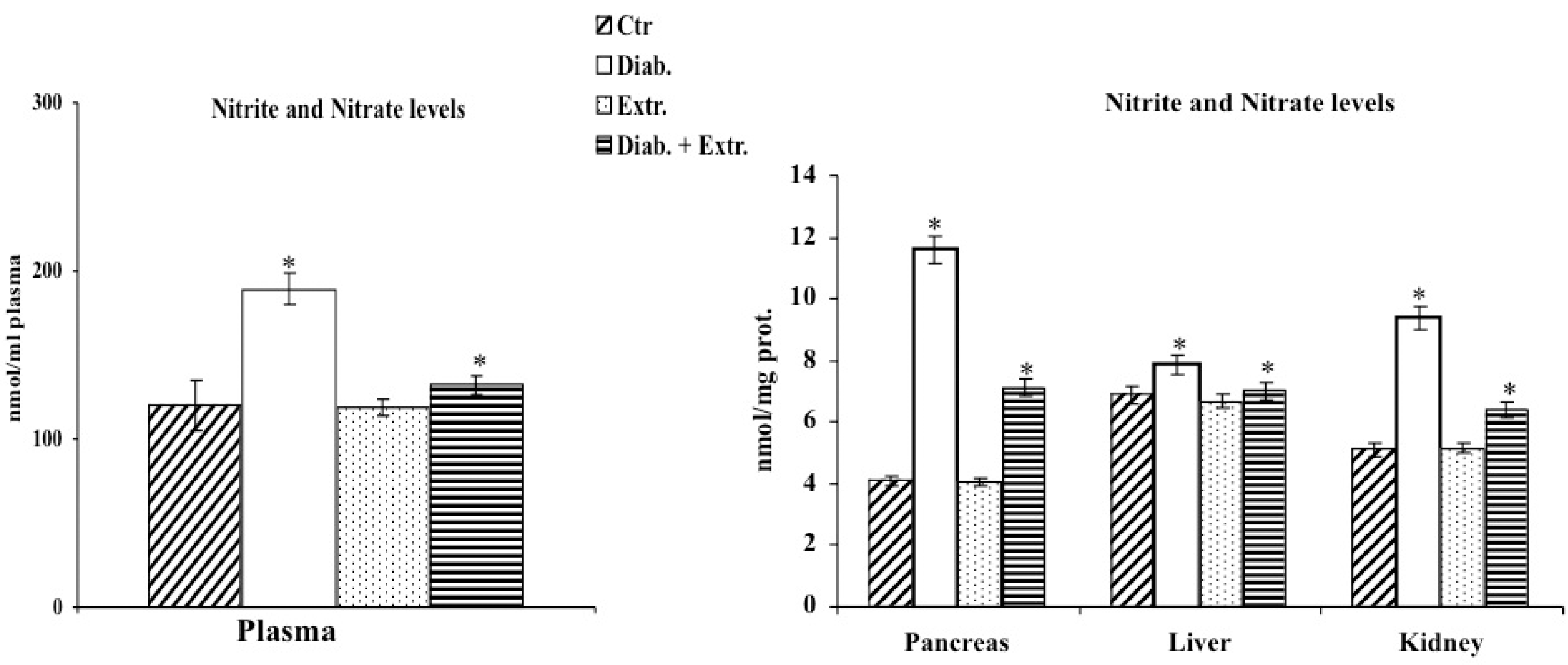
2.7. Determination of LOOH, RSH and of Nitrite/Nitrate Levels in Homogenates
2.8. Western Blotting Analysis
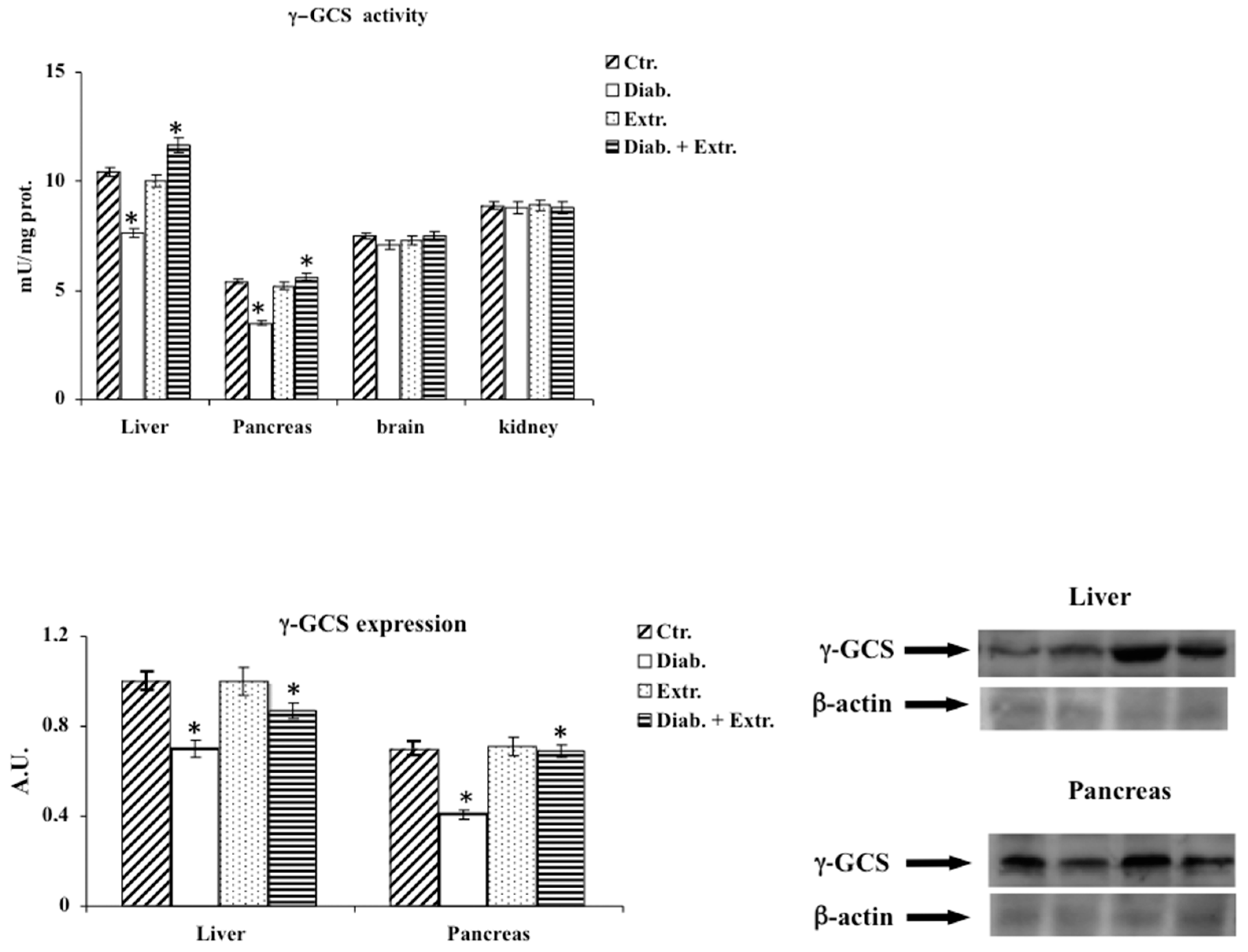
2.9. Enzymatic Activity Assay and Enzyme-Linked Immunosorbent Assay
2.10. Sirt1 Activity
2.11. Statistical Analysis
3. Results
3.1. Phytochemical Contents and Antioxidant Activity
3.2. Effect of Extract Administration on Blood Glucose and Insulin, Body Weight, Volume of Excreted Urine, Amount of Water and Food Intake
3.3. Oxidative Stress in Diabetic Rat
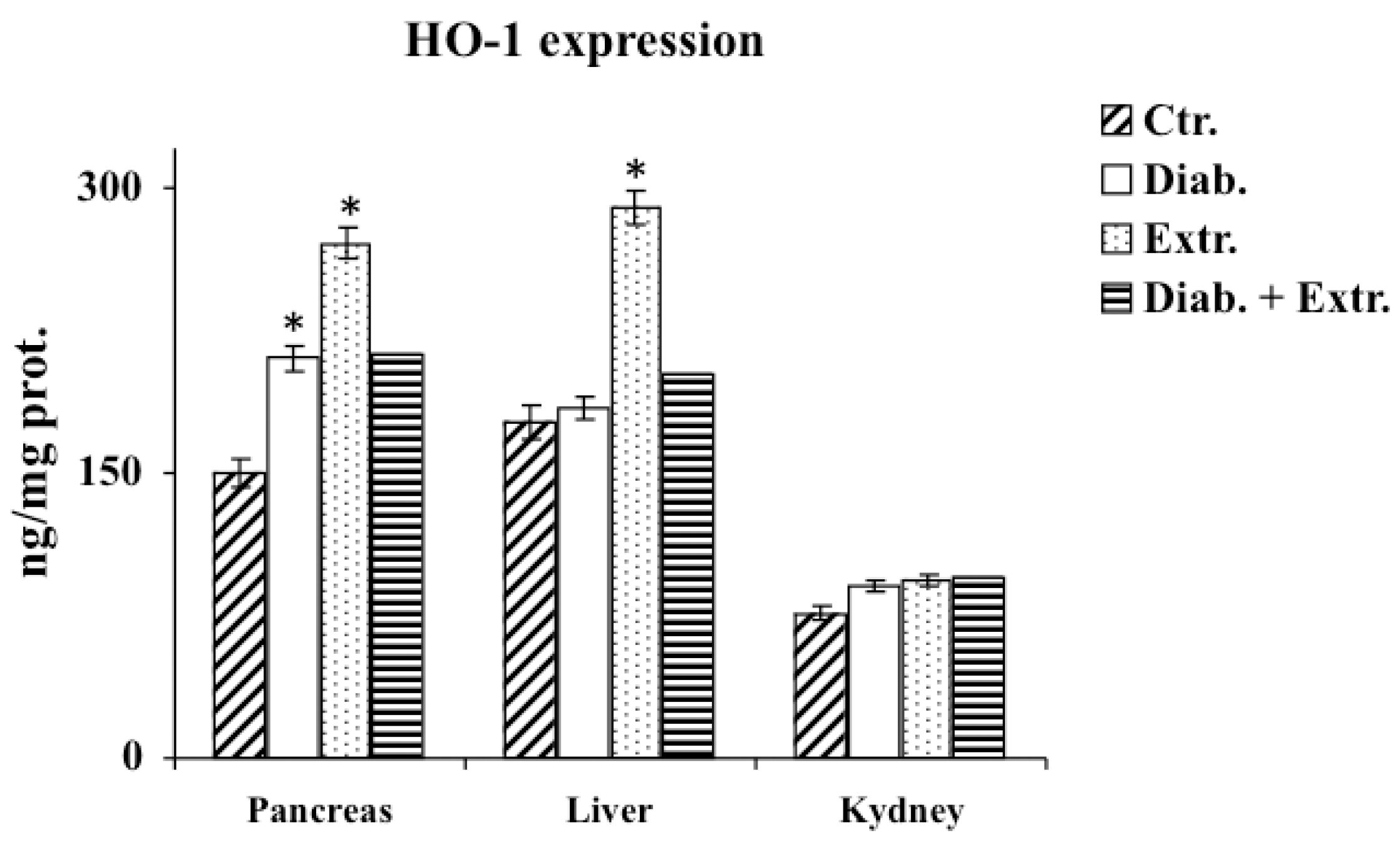
3.4. HO-1, iNOS and eNOS Protein Expression in Pancreas, Liver and Kidney
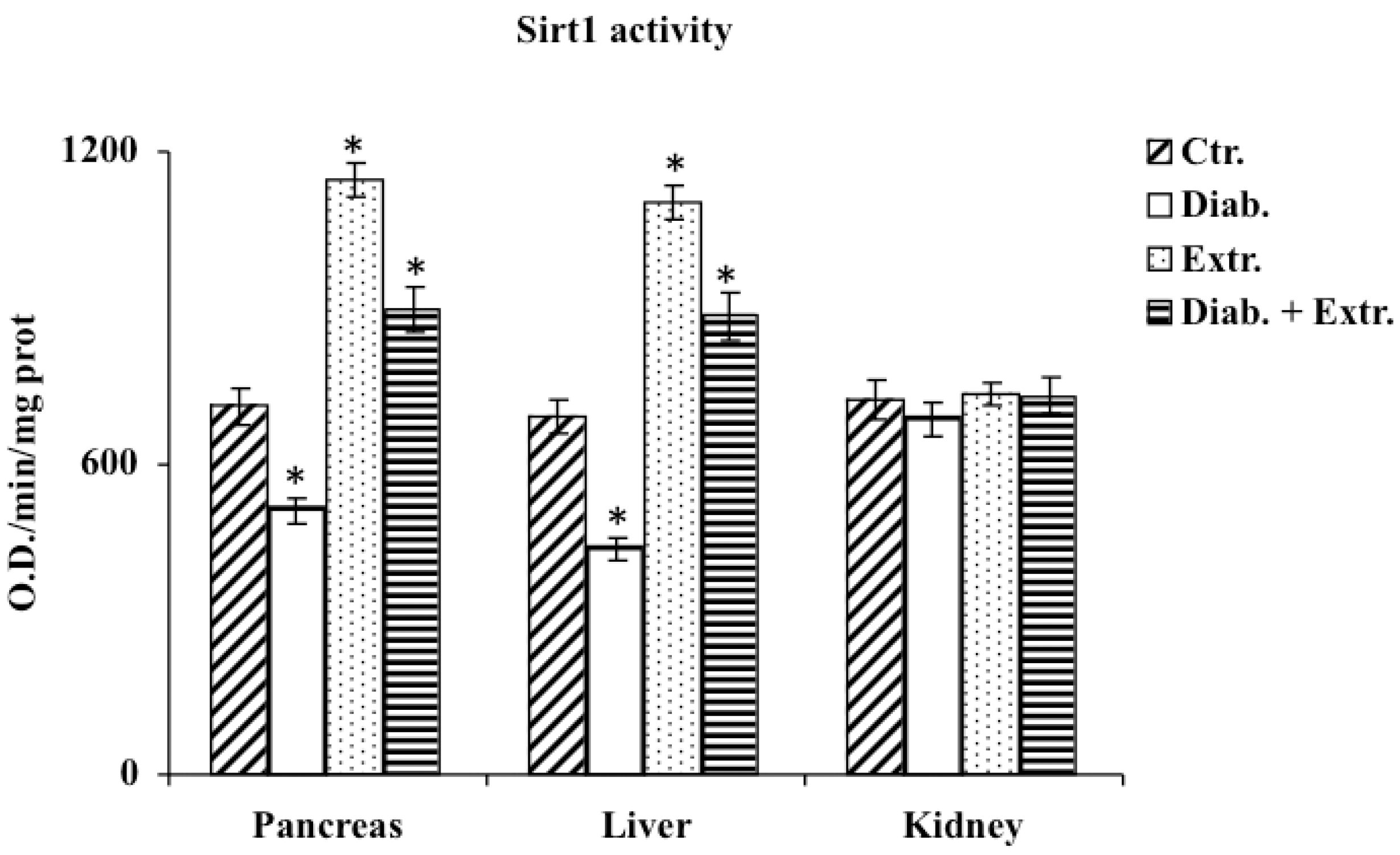
3.5. Sirt1 Activity in Pancreas, Liver and Kidney
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.; Sultana, S. Abrogation of potassium bromate-induced renal oxidative stress and subsequent cell proliferation response by soy isoflavones in Wistar rats. Toxicology 2004, 201, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.; Acharya, J.; Ghaskadbi, S.; Goel, P. Oxidative stress as a covariate of recovery in diabetes therapy. Front. Endocrinol. 2014, 5, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Maritim, A.C.; Sanders, R.A.; Watkins, J.B., 3rd. Diabetes, oxidative stress, and antioxidants: A review. J. Biochem. Mol. Toxicol. 2003, 17, 24–38. [Google Scholar] [CrossRef]
- Mathers, C.D.; Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006, 3, e442. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Hadjzadeh, M.A.; Amin Nya, F.; Davoodi, S. Antihyperglycemic and antihyperlipidemic effects of guar gum on streptozotocin-induced diabetes in male rats. Pharmacogn. Mag. 2012, 8, 65–72. [Google Scholar] [PubMed] [Green Version]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Catechin treatment ameliorates diabetes and its complications in streptozotocin-induced diabetic rats. Dose-Response 2017, 15. [Google Scholar] [CrossRef]
- Samarghandian, S.; Borji, A.; Tabasi, S.H. Effects of Cichorium intybus linn on blood glucose, lipid constituents and selected oxidative stress parameters in streptozotocin-induced diabetic rats. Cardiovasc. Hematol. Disord. Drug Targets 2013, 13, 231–236. [Google Scholar] [CrossRef]
- Farahmand, S.K.; Samini, F.; Samini, M.; Samarghandian, S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 2013, 14, 63–71. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Samini, F. Ameliorative effect of saffron aqueous extract on hyperglycemia, hyperlipidemia, and oxidative stress on diabetic encephalopathy in streptozotocin induced experimental diabetes mellitus. Biomed. Res. Int. 2014, 2014, 920857. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Afshari, R.; Sadati, A. Evaluation of lung and bronchoalveolar lavage fluid oxidative stress indices for assessing the preventing effects of safranal on respiratory distress in diabetic rats. Sci. World J. 2014, 2014, 251378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5’-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Rabey, H.A.; Al-Seeni, M.N.; Bakhashwain, A.S. The Antidiabetic Activity of Nigella sativa and Propolis on Streptozotocin-Induced Diabetes and Diabetic Nephropathy in Male Rats. Evid. Based Complement. Alternat. Med. 2017, 2017, 5439645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miguel, M.G. Antioxidant activity of medicinal and aromatic plants. A review. Flavour Frag. J. 2010, 25, 291–312. [Google Scholar] [CrossRef]
- Ganesan, P.; Arulselvan, P.; Choi, D.K. Phytobioactive compound-based nanodelivery systems for the treatment of type 2 diabetes mellitus—Current status. Int. J. Nanomed. 2017, 12, 1097–1111. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The pentacyclic triterpenoids in herbal medicines and their pharmacological activities in diabetes and diabetic complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar]
- Plini, P.; Tondi, G. La distribuzione appenninica della Betulla bianca. Nat. Mont. 1989, 36, 21–28. [Google Scholar]
- Pignatti, S. Flora d’Italia: Edagricole, New Business Media Italia; Edagricole: Milano, Italy, 2017; Volume II, pp. 676–677. [Google Scholar]
- Acquaviva, R.; Menichini, F.; Ragusa, S.; Genovese, C.; Amodeo, A.; Tundis, R.; Loizzo, M.R.; Iauk, L. Antimicrobial and antioxidant properties of Betula aetnensis Rafin. (Betulaceae) leaves extract. Nat. Prod. Res. 2013, 27, 475–479. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef] [Green Version]
- Acquaviva, R.; Genovese, C.; Amodeo, A.; Tomasello, B.; Malfa, G.; Sorrenti, V.; Tempera, G.; Addamo, A.P.; Ragusa, S.; Tundis, R.; et al. Biological activities of Teucrium flavum L., Teucrium fruticans L., and Teucrium siculum Rafin. crude extracts. Plant Biosyst. 2018, 152, 720–727. [Google Scholar] [CrossRef]
- Tomasello, B.; Malfa, G.A.; La Mantia, A.; Miceli, N.; Sferrazzo, G.; Taviano, M.F.; Di Giacomo, C.; Renis, M.; Acquaviva, R. Anti-adipogenic and anti-oxidant effects of a standardised extract of Moro blood oranges (Citrus sinensis (L.) Osbeck) during adipocyte differentiation of 3T3-L1 preadipocytes. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Chaniad, P.; Techarang, T.; Phuwajaroanpong, A.; Punsawad, C. Antimalarial Activity and Toxicological Assessment of Betula alnoides Extract against Plasmodium berghei Infections in Mice. Evid. Based Complement. Alterna. Med. 2019, 2019, 2324679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhanataev, A.K.; Presnova, G.A.; Chistyakov, A.N.; Durnev, A.D. Effect of Betula bark extract on spontaneous and induced mutagenesis in mice. Bull. Exp. Biol. Med. 2004, 138, 475–478. [Google Scholar] [CrossRef]
- Di Giacomo, C.; Acquaviva, R.; Piva, A.; Sorrenti, V.; Vanella, L.; Piva, G.; Casadei, G.; La Fauci, L.; Ritieni, A.; Bognanno, M.; et al. Protective effect of cyanidin 3-O-beta-D-glucoside on ochratoxin A-mediated damage in the rat. Br. J. Nutr. 2007, 98, 937–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar]
- Tenuta, M.C.; Deguin, B.; Loizzo, M.R.; Dugay, A.; Acquaviva, R.; Malfa, G.A.; Bonesi, M.; Bouzidi, C.; Tundis, R. Contribution of Flavonoids and Iridoids to the Hypoglycaemic, Antioxidant, and Nitric Oxide (NO) Inhibitory Activities of Arbutus unedo L. Antioxidants 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Malfa, G.A.; Tomasello, B.; Sinatra, F.; Villaggio, G.; Amenta, F.; Avola, R.; Renis, M. Reactive response evaluation of primary human astrocytes after methylmercury exposure. J. Neurosci. Res. 2014, 92, 95–103. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamada, K.; Zou, L.B.; Yan, Y.; Mizuno, M.; Nabeshima, T. Interleukin-6 protects PC12 cells from 4-hydroxynonenal-induced cytotoxicity by increasing intracellular glutathione levels. Free Radic. Biol. Med. 2002, 32, 1324–1332. [Google Scholar] [CrossRef]
- Bigagli, E.; Lodovici, M. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid. Med. Cell. Longev. 2019, 2019, 5953685. [Google Scholar] [CrossRef]
- Raghavan, B.; Kumari, S.K. Effect of Terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J. Physiol. Pharmacol. 2006, 50, 133–142. [Google Scholar]
- Puppel, K.; Kapusta, A.; Kuczyńska, B. The etiology of oxidative stress in the various species of animals, a review. J. Sci. Food Agric. 2015, 95, 2179–2184. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.V.; Veskoukis, A.S.; Paschalis, V.; Vrabas, I.S.; Dipla, K.; Zafeiridis, A.; Kyparos, A.; Nikolaidis, M.G. Blood reflects tissue oxidative stress: A systematic review. Biomarkers 2015, 20, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Sono, Y.; Ito, T. Measurement and Clinical Significance of Lipid Peroxidation as a Biomarker of Oxidative Stress: Oxidative Stress in Diabetes, Atherosclerosis, and Chronic Inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newsholme, P.; Rebelato, E.; Abdulkader, F.; Krause, M.; Carpinelli, A.; Curi, R. Reactive oxygen and nitrogen species generation, antioxidant defenses, and β-cell function: A critical role for amino acids. J. Endocrinol. 2012, 214, 11–20. [Google Scholar] [CrossRef]
- Nisoli, E.; Carruba, M.O. Nitric oxide and mitochondrial biogenesis. J. Cell. Sci. 2006, 119, 2855–2862. [Google Scholar] [CrossRef] [Green Version]
- Di Giacomo, G.; Rizza, S.; Montagna, C.; Filomeni, G. Established principles and emerging concepts on the interplay between mitochondrial physiology and S-(De)nitrosylation: Implications in cancer and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 361872. [Google Scholar] [CrossRef] [Green Version]
- Raij, L.; Baylis, C. Glomerular actions of nitric oxide. Kidney Int. 1995, 48, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Soskić, S.S.; Dobutović, B.D.; Sudar, E.M.; Obradović, M.M.; Nikolić, D.M.; Djordjevic, J.D.; Radak, D.J.; Mikhailidis, D.P.; Isenović, E.R. Regulation of Inducible Nitric Oxide Synthase (iNOS) and its Potential Role in Insulin Resistance, Diabetes and Heart Failure. Open Cardiovasc. Med. J. 2011, 5, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Rochette, L.; Zeller, M.; Cottin, Y.; Vergely, C. Redox Functions of Heme Oxygenase-1 and Biliverdin Reductase in Diabetes. Trends Endocrinol. Metab. 2018, 29, 74–85. [Google Scholar] [CrossRef]
- Grochot-Przeczek, A.; Dulak, J.; Jozkowicz, A. Heme oxygenase-1 in neovascularisation: A diabetic perspective. Thromb. Haemost. 2010, 104, 424–431. [Google Scholar] [CrossRef]
- North, B.J.; Verdin, E. Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol. 2004, 5, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordone, L.; Guarente, L. Sirtuins and beta-cell function. Diabetes Obes. Metab. 2007, 9, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, K.A.; Grimm, A.A.; Plueger, M.M.; Bernal-Mizrachi, E.; Ford, E.; Cras-Meneur, C.; Permutt, M.A.; Imai, S.I. Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2005, 2, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef]
- Dihingia, A.; Ozah, D.; Ghosh, S.; Sarkar, A.; Baruah, P.K.; Kalita, J.; Sil, P.C.; Manna, P. Vitamin K1 inversely correlates with glycemia and insulin resistance in patients with type 2 diabetes (T2D) and positively regulates SIRT1/AMPK pathway of glucose metabolism in liver of T2D mice and hepatocytes cultured in high glucose. J. Nutr. Biochem. 2018, 52, 103–114. [Google Scholar] [CrossRef]
- Santos, L.; Escande, C.; Denicola, A. Potential Modulation of Sirtuins by Oxidative Stress. Oxid Med Cell Longev. 2016, 2016, 9831825. [Google Scholar] [CrossRef] [Green Version]
- Corbi, G.; Conti, V.; Komici, K.; Manzo, V.; Filippelli, A.; Palazzo, M.; Vizzari, F.; Davinelli, S.; Di Costanzo, A.; Scapagnini, G.; et al. Phenolic Plant Extracts Induce Sirt1 Activity and Increase Antioxidant Levels in the Rabbit’s Heart and Liver. Oxid Med Cell Longev. 2018. [Google Scholar] [CrossRef]
- Luvizotto, R.A.; Nascimento, A.F.; Miranda, N.C.; Wang, X.D.; Ferreira, A.L. Lycopene-rich tomato oleoresin modulates plasma adiponectin concentration and mRNA levels of adiponectin, SIRT1, and FoxO1 in adipose tissue of obese rats. Hum. Exp. Toxicol. 2015, 34, 612–619. [Google Scholar] [CrossRef]
- Tomasello, B.; Di Mauro, M.D.; Malfa, G.A.; Acquaviva, R.; Sinatra, F.; Spampinato, G.; Laudani, S.; Villaggio, G.; Bielak-Zmijewska, A.; Grabowska, W.; et al. Rapha Myr®, a Blend of Sulforaphane and Myrosinase, Exerts Antitumor and Anoikis-Sensitizing Effects on Human Astrocytoma Cells Modulating Sirtuins and DNA Methylation. Int. J. Mol. Sci. 2020, 21, 5328. [Google Scholar] [CrossRef]
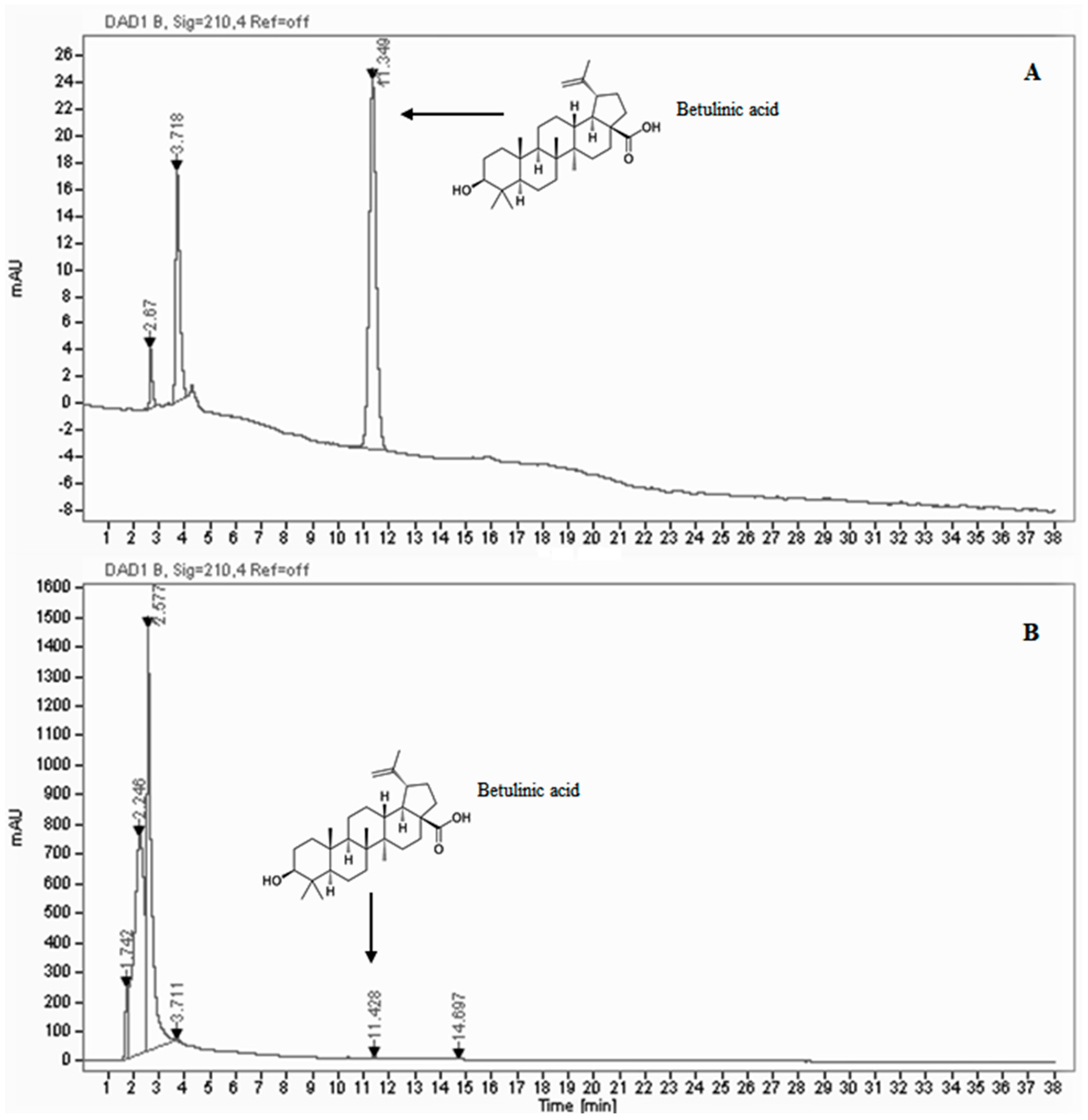

| Sample | Betulinic Acid (mg/mL) | Total Phenolic Gallic Acid (mg/g) | Total Flavonoids Catechin (mg/g) | SOD-like Activity IC50 (μg/mL) | DPPH Test IC50 (μg/mL) |
|---|---|---|---|---|---|
| B. etnensis extract | 0.069 ± 0.007 | 57.30 ± 0.83 | 6.11 ± 0.17 | 0.51 ± 0.80 | 48.35 ± 1.7 |
| Positive control SOD | 50 mU ± 0.85 | ||||
| Trolox | 15 mM ± 0.62 |
| Group | 0 | 8 Days | 15 Days | 21 Days | 28 Days |
|---|---|---|---|---|---|
| Insulin (ng/mL) | |||||
| Ctr. | 0.97 ± 0.04 | 1.0 ± 0.02 | 0.99 ± 0.03 | 1.1 ± 0.04 | 0.99 ± 0.02 |
| Diab. | 0.87 ± 0.06 | 0.34 ± 0.05 * | 0.38 ± 0.04 * | 0.40 ± 0.06 * | 0.39 ± 0.05 * |
| Extr. | 1.0 ± 0.03 | 1.01 ± 0.03 | 1.0 ± 0.05 | 0.99 ± 0.03 | 1.02 ± 0.04 |
| Diab. + Extr. | 0.81 ± 0.02 | 0.60 ± 0.03 * | 0.71 ± 0.03 * | 0.78 ± 0.02 * | 0.68 ± 0.02 * |
| Glycemia (mg/dl) | |||||
| Ctr. | 80 ± 9.02 | 82 ± 7.8 | 90 ± 7.2 | 85 ± 6.98 | 90 ± 9.01 |
| Diab. | 78 ± 10.1 | 600 ± 25 * | 575 ± 31 * | 550 ± 26.1 * | 500 ± 32 * |
| Extr. | 81 ± 8.89 | 81 ± 8.23 | 82 ± 8.01 | 85 ± 8.2 | 87 ± 7.8 |
| Diab. + Extr. | 80 ± 9.03 | 320 ± 13.1 * | 250 ± 14.3 * | 280 ± 12.6 * | 245 ± 10.2 * |
| Food (gr/die) | |||||
| Ctr. | 10 ± 0.5 | 11 ± 1.65 | 10.5 ± 0.5 | 10 ± 0.5 | 12 ± 0.6 |
| Diab. | 10 ± 0.5 | 45 ± 6.75 * | 50 ± 6.25 * | 47 ± 5.87 * | 45 ± 6.07 * |
| Extr. | 12 ± 0.6 | 12 ± 1.8 | 11 ± 0.55 | 11.5 ± 0.57 | 11 ± 0.49 |
| Diab. + Extr. | 11 ± 0.55 | 32 ± 6.3 * * | 28 ± 3.5 * | 27 ± 3.3 * | 24 ± 3.0 * |
| Weight (gr) | |||||
| Ctr. | 330 ± 15 | 330 ± 14 | 350 ± 13 | 370 ± 14 | 382 ± 15 |
| Diab. | 335 ± 12 | 300 ± 12 | 280 ± 12 * | 240 ± 11 * | 210 ± 12 * |
| Extr. | 320 ± 13 | 335 ± 12 | 355 ± 14 | 362 ± 14 | 378 ±15 |
| Diab. + Extr. | 335 ± 12 | 310 ± 10 | 300 ± 10 | 312 ± 9 * * | 321 ± 8 * |
| Water (ml/die) | |||||
| Ctr. | 34 ± 2 | 32 ± 1.5 | 30 ± 2 | 33 ± 1.5 | 34 ± 2 |
| Diab. | 30 ± 2 | 160 ± 4 * | 165 ± 4 * | 158 ± 5 * | 167 ± 4 * |
| Extr. | 35 ± 2 | 33 ± 2 | 35 ± 2 | 31 ± 1.8 | 32 ± 2 |
| Diab. + Extr. | 33 ± 2 | 89 ± 2.5 * | 82 ± 3 * | 75 ± 3 * | 61 ± 3 * |
| Urine (ml/die) | |||||
| Ctr. | 10 ± 1 | 11 ± 1 | 10.5 ± 1.2 | 10 ± 1 | 10 ± 1.3 |
| Diab. | 10 ± 1 | 135 ± 10 ** | 130 ± 9 ** | 138 ± 11 ** | 140 ± 8 ** |
| Extr. | 12 ± 1 | 12 ± 1 | 11 ± 1 | 11.5 ± 0.9 | 9.5 ± 1 |
| Diab. + Extr. | 11 ± 1 | 120 ± 2.5 ** | 78 ± 2 ** | 75 ± 2 ** | 58 ± 2 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Mantia, A.L.; Pappalardo, F.; Ragusa, M.; Renis, M.; Di Giacomo, C. The Antioxidant Activities of Betula etnensis Rafin. Ethanolic Extract Exert Protective and Anti-diabetic Effects on Streptozotocin-Induced Diabetes in Rats. Antioxidants 2020, 9, 847. https://doi.org/10.3390/antiox9090847
Malfa GA, Tomasello B, Acquaviva R, Mantia AL, Pappalardo F, Ragusa M, Renis M, Di Giacomo C. The Antioxidant Activities of Betula etnensis Rafin. Ethanolic Extract Exert Protective and Anti-diabetic Effects on Streptozotocin-Induced Diabetes in Rats. Antioxidants. 2020; 9(9):847. https://doi.org/10.3390/antiox9090847
Chicago/Turabian StyleMalfa, Giuseppe Antonio, Barbara Tomasello, Rosaria Acquaviva, Alfonsina La Mantia, Francesco Pappalardo, Monica Ragusa, Marcella Renis, and Claudia Di Giacomo. 2020. "The Antioxidant Activities of Betula etnensis Rafin. Ethanolic Extract Exert Protective and Anti-diabetic Effects on Streptozotocin-Induced Diabetes in Rats" Antioxidants 9, no. 9: 847. https://doi.org/10.3390/antiox9090847







