Plant Extracts as Possible Agents for Sequela of Cancer Therapies and Cachexia
Abstract
:1. Cancer Therapies and Cachexia
1.1. Limitation of the Current Cancer Therapies
1.2. Plants-Derived Drugs for Side-Effects of Cancer Therapies and Cachexia
2. Sequela of Surgery and Plant Extracts
2.1. Surgery
2.2. Sequela of Surgery
2.3. Plant-Derived Drugs and Sequela of Surgery
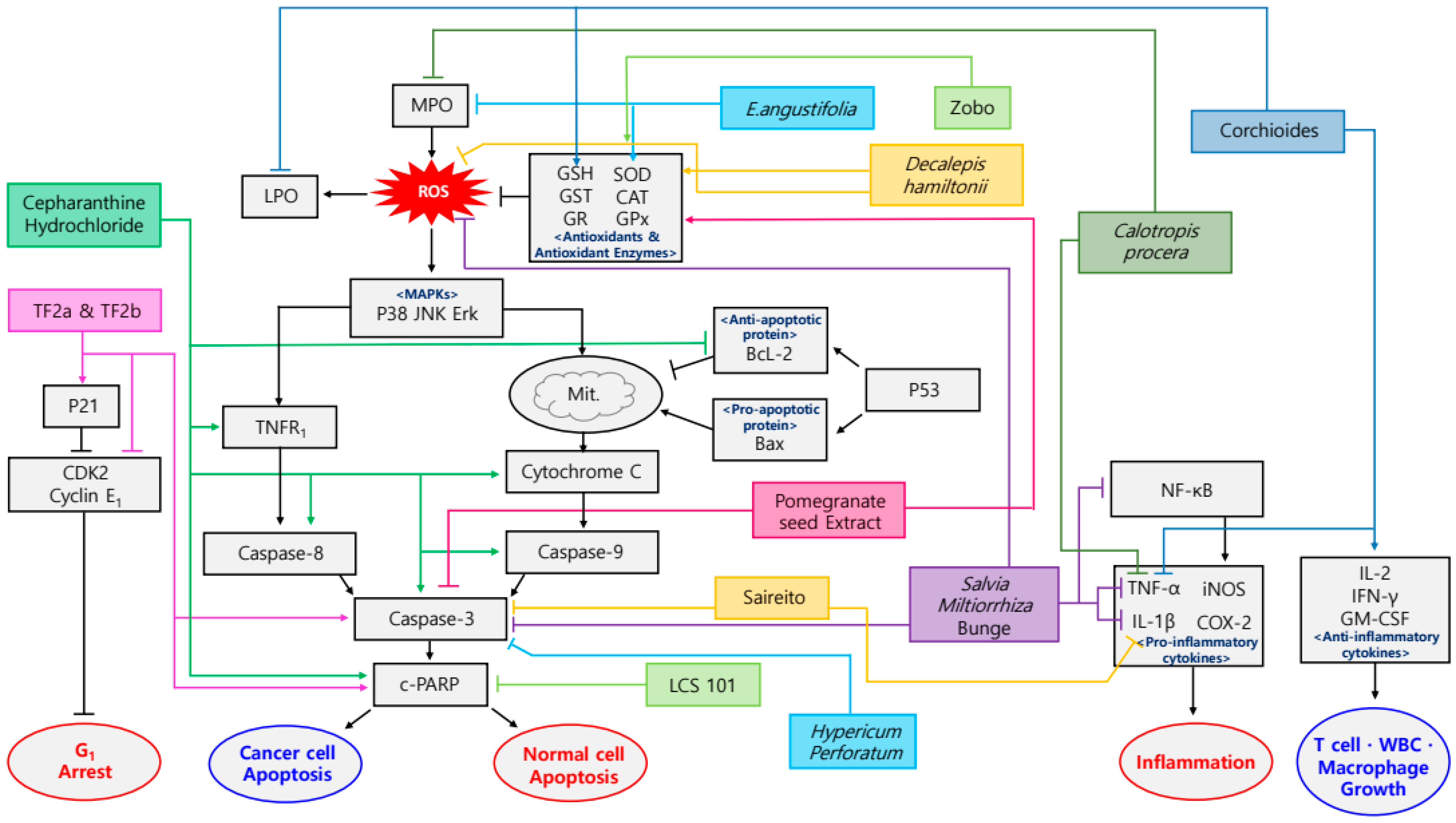
3. Sequela of Chemotherapy and Plant Extracts
3.1. Chemotherapy
3.2. Sequela of Chemotherapy
3.3. Plant-Derived Drugs and Sequela of Chemotherapy
4. Sequela of Radiotherapy and Plant Extracts
4.1. Radiotherapy
4.2. Sequela of Radiotherapy
4.3. Plant-Derived Drugs and Sequela of Radiotherapy
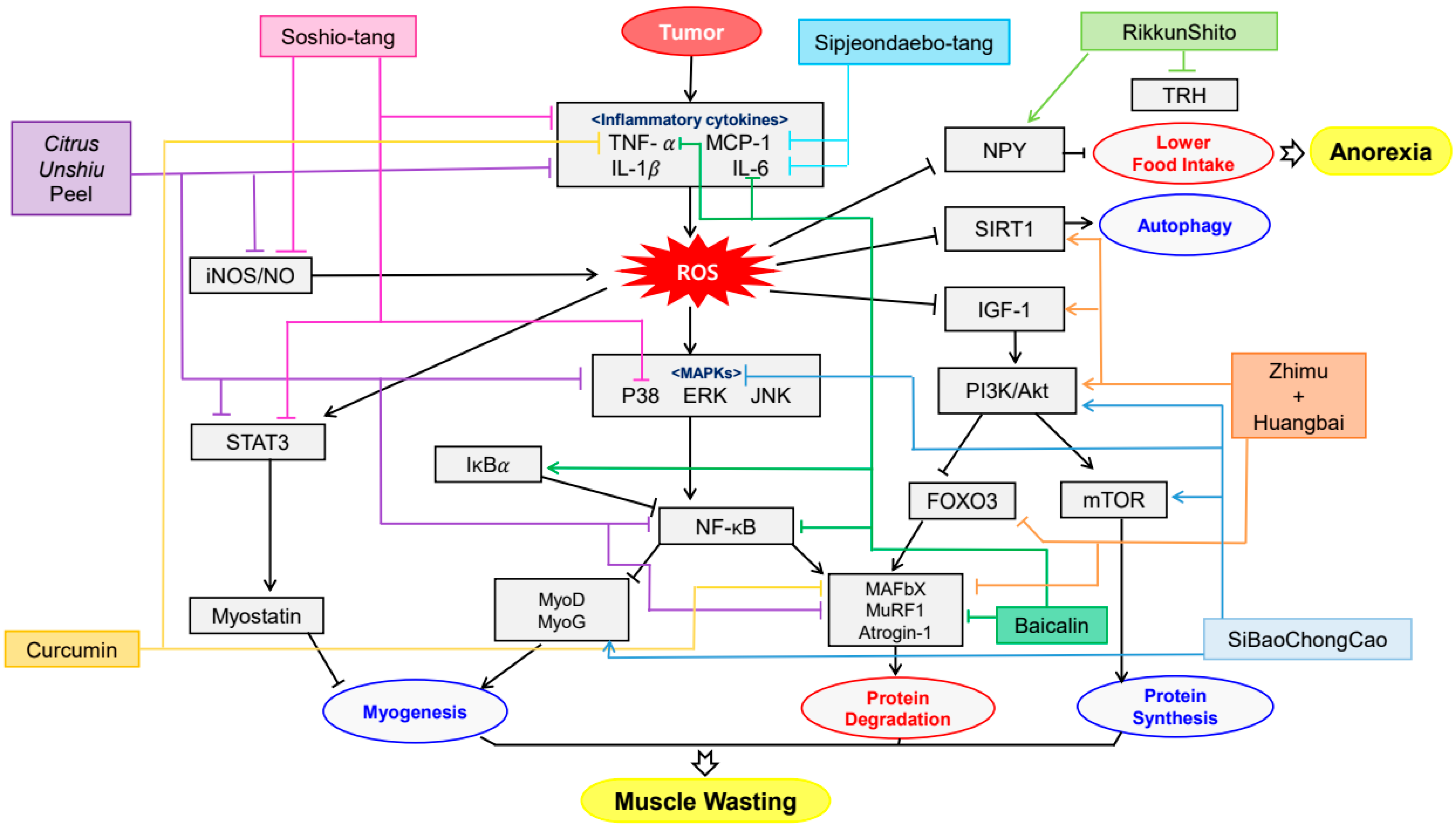
5. Cachexia and Plant Extracts
5.1. Cachexia
5.2. Clinical Difficulties in Treatment of Cachexia
5.3. Plant-Derived Drugs and Cachexia
6. Clinical Trials of Plant-Derived Drugs against Sequela of Cancer Therapies and Cachexia
7. Oxidative Stress and Cancer
8. Limitations of the Studies
9. Hypothesis of a Possible Trial
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyld, L.; Audisio, R.A.; Poston, G.J. The evolution of cancer surgery and future perspectives. Nat. Rev. Clin. Oncol. 2015, 12, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.S.; Han, H.S.; Agarwal, A.; Belli, G.; Itano, O.; Gumbs, A.A.; Yoon, D.S.; Kang, C.M.; Lee, S.E.; Wakai, T.; et al. Survey Results of the Expert Meeting on Laparoscopic Surgery for Gallbladder Cancer and a Review of Relevant Literature. Dig. Surg. 2019, 36, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Cabral, H.; Stylianopoulos, T.; Jain, R.K. Improving cancer immunotherapy using nanomedicines: Progress, opportunities and challenges. Nat. Rev. Clin. Oncol. 2020, 17, 251–266. [Google Scholar] [CrossRef]
- Hwang, W.L.; Pike, L.R.; Royce, T.J.; Mahal, B.A.; Loeffler, J.S. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat. Rev. Clin. Oncol. 2018, 15, 477–494. [Google Scholar] [CrossRef]
- Sharma, R.A.; Plummer, R.; Stock, J.K.; Greenhalgh, T.A.; Ataman, O.; Kelly, S.; Clay, R.; Adams, R.A.; Baird, R.D.; Billingham, L. Clinical development of new drug–radiotherapy combinations. Nat. Rev. Clin. Oncol. 2016, 13, 627–642. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, J.; Couzin-Frankel, J. Cancer immunotherapy sweeps Nobel for medicine. Science 2018, 362, 13. [Google Scholar] [CrossRef]
- Whitmore, W.F., Jr. Hormone therapy in prostatic cancer. Am. J. Med. 1956, 21, 697–713. [Google Scholar] [CrossRef]
- Ito, H.; Nakayama, H.; Yokose, T.; Nagashima, T.; Morohoshi, T.; Tajiri, M.; Maehara, T.; Watanabe, K.; Arai, H.; Yamamoto, T.; et al. A prophylaxis study of acute exacerbation of interstitial pneumonia after lung cancer surgery. Jpn. J. Clin. Oncol. 2020, 50, 198–205. [Google Scholar] [CrossRef]
- Hashimoto, H.; Abe, M.; Yanai, T.; Yamaguchi, T.; Zenda, S.; Uchitomi, Y.; Fukuda, H.; Mori, M.; Iwasa, S.; Yamamoto, N.; et al. Study protocol for j-support 1604 (j-force): A randomized, double blind, placebo-controlled phase iii study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn. J. Clin. Oncol. 2018, 48, 950–952. [Google Scholar]
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T.; et al. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase ii study (jortc kmp-02). J. Gynecol. Oncol. 2017, 28, e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.B.; DeAngelis, L.M. Cancer-treatment-induced neurotoxicity—Focus on newer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef] [Green Version]
- Mi, X.-J.; Hou, J.-G.; Wang, Z.; Han, Y.; Ren, S.; Hu, J.-N.; Chen, C.; Li, W. The protective effects of maltol on cisplatin-induced nephrotoxicity through the ampk-mediated pi3k/akt and p53 signaling pathways. Sci. Rep. 2018, 8, 15922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsao, M.N.; Xu, W.; Wong, R.K.; Lloyd, N.; Laperriere, N.; Sahgal, A.; Rakovitch, E.; Chow, E. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst. Rev. 2018, 1, CD003869. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr. Cancer 2004, 3, 294–300. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular stress responses in radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C.-L.; Blum, J.M.; Kirsch, D.G. Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis. Transl. Cancer Res. 2013, 2, 412–421. [Google Scholar]
- Zhou, J.; Sun, H.C.; Wang, Z.; Cong, W.M.; Wang, J.H.; Zeng, M.S.; Yang, J.M.; Bie, P.; Liu, L.X.; Wen, T.F.; et al. Guidelines for diagnosis and treatment of primary liver cancer in china (2017 edition). Liver Cancer 2018, 7, 235–260. [Google Scholar] [CrossRef]
- Cheng, G.S.; Ilfeld, B.M. A review of postoperative analgesia for breast cancer surgery. Pain Manag. 2016, 6, 603–618. [Google Scholar] [CrossRef] [Green Version]
- Hörske, C.; Weber, K.; Goehl, J.; Hohenberger, W.; Merkel, S. Long-term outcomes and quality of life after rectal carcinoma surgery. Br. J. Surg. 2010, 97, 1295–1303. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, P.; Xu, Y.; Yan, J.; Liu, Z.; Lau, W.B.; Lau, B.; Li, Y.; Zhao, X.; Wei, Y.; et al. Surgical stress and cancer progression: The twisted tango. Mol. Cancer 2019, 18, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffey, J.C.; Wang, J.H.; Smith, M.J.; Bouchier-Hayes, D.; Cotter, T.G.; Redmond, H.P. Excisional surgery for cancer cure: Therapy at a cost. Lancet Oncol. 2003, 4, 760–768. [Google Scholar] [CrossRef]
- Stingl, J.C.; Ettrich, T.; Muche, R.; Wiedom, M.; Brockmoller, J.; Seeringer, A.; Seufferlein, T. Protocol for minimizing the risk of metachronous adenomas of the colorectum with green tea extract (miracle): A randomised controlled trial of green tea extract versus placebo for nutriprevention of metachronous colon adenomas in the elderly population. BMC Cancer 2011, 11, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.Y.; Niu, J.L.; Lu, Y.H. Multidrug resistance reversal effect of dmc derived from buds of cleistocalyx operculatus in human hepatocellular tumor xenograft model. J. Sci. Food Agric. 2012, 92, 135–140. [Google Scholar] [CrossRef]
- Wolf, R.J.; Hilger, R.A.; Hoheisel, J.D.; Werner, J.; Holtrup, F. In vivo activity and pharmacokinetics of nemorosone on pancreatic cancer xenografts. PLoS ONE 2013, 8, e74555. [Google Scholar] [CrossRef]
- Katsuno, H.; Maeda, K.; Ohya, M.; Yoshioka, K.; Tsunoda, A.; Koda, K.; Matsuoka, H.; Ohge, H.; Morita, S.; Saji, S.; et al. Clinical pharmacology of daikenchuto assessed by transit analysis using radiopaque markers in patients with colon cancer undergoing open surgery: A multicenter double-blind randomized placebo-controlled study (jfmc39-0902 additional study). J. Gastroenterol. 2016, 51, 222–229. [Google Scholar] [CrossRef]
- Yamada, T.; Matsumoto, S.; Matsuda, M.K.A.; Shinji, S.; Yokoyama, Y.; Takahashi, G.; Iwai, T.; Takeda, K.; Ohta, K.; Uchida, E. The effect of daikenchuto on postoperative intestinal motility in patients with right-side colon cancer. Surg. Today 2017, 47, 865–871. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharm. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Soliman, A.F.; Anees, L.M.; Ibrahim, D.M. Cardioprotective effect of zingerone against oxidative stress, inflammation and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedeberg Arch. Pharm. 2018, 391, 819–832. [Google Scholar] [CrossRef]
- Olziersky, A.M.; Labidi-Galy, S.I. Clinical development of anti-mitotic drugs in cancer. Adv. Exp. Med. Biol. 2017, 1002, 125–152. [Google Scholar]
- Wang, W.; Tse-Dinh, Y.C. Recent advances in use of topoisomerase inhibitors in combination cancer therapy. Curr. Top. Med. Chem. 2019, 19, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Petrioli, R.; Bonetta, A.; Conca, R.; Rodriquenz, M.G.; Aieta, M. Corticosteroid switch in heavily pre-treated castration-resistant prostate cancer patients progressed on abiraterone acetate plus prednisone. Investig. New Drugs 2018, 36, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. Biomed Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef] [Green Version]
- Kruger, S.; Ilmer, M.; Kobold, S.; Cadilha, B.L.; Endres, S.; Ormanns, S.; Schuebbe, G.; Renz, B.W.; D’Haese, J.G.; Schloesser, H.; et al. Advances in cancer immunotherapy 2019—Latest trends. J. Exp. Clin. Cancer Res. 2019, 38, 268. [Google Scholar] [CrossRef] [Green Version]
- Marin-Acevedo, J.A.; Soyano, A.E.; Dholaria, B.; Knutson, K.L.; Lou, Y. Cancer immunotherapy beyond immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Aung, H.H.; Dey, L.; Mehendale, S.; Xie, J.T.; Wu, J.A.; Yuan, C.S. Scutellaria baicalensis extract decreases cisplatin-induced pica in rats. Cancer Chemother. Pharmacol. 2003, 52, 453–458. [Google Scholar] [CrossRef]
- Sharma, S.S.; Gupta, Y.K. Reversal of cisplatin-induced delay in gastric emptying in rats by ginger (Zingiber officinale). J. Ethnopharmacol. 1998, 62, 49–55. [Google Scholar] [CrossRef]
- Ojo, O.O.; Adegbite, O.S.; Kesinro, M.O.; Womiloju, A.K.; Oluyomi, O.I. Methanol extracts from delonix regia leaves modulate apoptosis in cisplatin-induced nephrotoxicity in male rats. Orient. Pharm. Exp. Med. 2019, 19, 177–186. [Google Scholar] [CrossRef]
- Li, T.; Ito, K.; Sumi, S.; Fuwa, T.; Horie, T. Protective effect of aged garlic extract (age) on the apoptosis of intestinal epithelial cells caused by methotrexate. Cancer Chemother. Pharmacol. 2009, 63, 873–880. [Google Scholar] [CrossRef]
- Horie, T.; Awazu, S.; Itakura, Y.; Fuwa, T. Alleviation by garlic of antitumor drug-induced damage to the intestine. J. Nutr. 2001, 131, 1071S–1074S. [Google Scholar] [CrossRef] [Green Version]
- Senthilnathan, P.; Padmavathi, R.; Banu, S.M.; Sakthisekaran, D. Enhancement of antitumor effect of paclitaxel in combination with immunomodulatory withania somnifera on benzo(a)pyrene induced experimental lung cancer. Chem. Biol. Interact. 2006, 159, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Conte, E.; Bresciani, E.; Rizzi, L.; Cappellari, O.; De Luca, A.; Torsello, A.; Liantonio, A. Cisplatin-induced skeletal muscle dysfunction: Mechanisms and counteracting therapeutic strategies. Int. J. Mol. Sci. 2020, 21, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Allah, A.R.; Al-Majed, A.A.; Al-Yahya, A.A.; Fouda, S.I.; Al-Shabana, O.A. L-carnitine halts apoptosis and myelosuppression induced by carboplatin in rat bone marrow cell cultures (bmc). Arch. Toxicol. 2005, 79, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhou, B.; Xia, X.; Chen, S.; Deng, Y.; Wang, Y.; Wu, L.; Tian, Y.; Zhao, B.; Xu, H.; et al. Prevotella copri is associated with carboplatin-induced gut toxicity. Cell Death Dis. 2019, 10, 714. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Becker, J.C.; Schrama, D. The dark side of cyclophosphamide: Cyclophosphamide-mediated ablation of regulatory t cells. J. Investig. Dermatol. 2013, 133, 1462–1465. [Google Scholar] [CrossRef] [Green Version]
- Jeelani, R.; Khan, S.N.; Shaeib, F.; Kohan-Ghadr, H.R.; Aldhaheri, S.R.; Najafi, T.; Thakur, M.; Morris, R.; Abu-Soud, H.M. Cyclophosphamide and acrolein induced oxidative stress leading to deterioration of metaphase ii mouse oocyte quality. Free Radic. Biol. Med. 2017, 110, 11–18. [Google Scholar] [CrossRef]
- Hamdan, D.; Leboeuf, C.; Le Foll, C.; Bousquet, G.; Janin, A. Re-exploring immune-related side effects of docetaxel in an observational study: Blood hypereosinophilia. Cancer Med. 2019, 8, 2005–2012. [Google Scholar] [CrossRef] [Green Version]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, S.C.M.; Pereira, V.B.M.; Wong, D.V.T.; Santana, A.P.M.; Lucetti, L.T.; Carvalho, L.L.; Barbosa, C.R.N.; Callado, R.B.; Silva, C.A.A.; Lopes, C.D.H.; et al. Amifostine reduces inflammation and protects against 5-fluorouracil-induced oral mucositis and hyposalivation. Braz. J. Med. Biol. Res. 2019, 52, e8251. [Google Scholar] [CrossRef]
- Li, H.L.; Lu, L.; Wang, X.S.; Qin, L.Y.; Wang, P.; Qiu, S.P.; Wu, H.; Huang, F.; Zhang, B.B.; Shi, H.L.; et al. Alteration of gut microbiota and inflammatory cytokine/chemokine profiles in 5-fluorouracil induced intestinal mucositis. Front. Cell Infect. Microbiol. 2017, 7, 455. [Google Scholar] [CrossRef] [PubMed]
- Killu, A.; Madhavan, M.; Prasad, K.; Prasad, A. 5-fluorouracil induced pericarditis. BMJ Case Rep. 2011. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Nagaraja, S.; Ocampo, A.; Tam, L.T.; Wood, L.S.; Pallegar, P.N.; Greene, J.J.; Geraghty, A.C.; Goldstein, A.K.; Ni, L.; et al. Methotrexate chemotherapy induces persistent tri-glial dysregulation that underlies chemotherapy-related cognitive impairment. Cell 2019, 176, 43–55.e13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamorro-Petronacci, C.; Garcia-Garcia, A.; Lorenzo-Pouso, A.I.; Gomez-Garcia, F.J.; Padin-Iruegas, M.E.; Gandara-Vila, P.; Blanco-Carrion, A.; Perez-Sayans, M. Management options for low-dose methotrexate-induced oral ulcers: A systematic review. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e181–e189. [Google Scholar] [CrossRef] [PubMed]
- Branca, J.J.V.; Maresca, M.; Morucci, G.; Becatti, M.; Paternostro, F.; Gulisano, M.; Ghelardini, C.; Salvemini, D.; Di Cesare Mannelli, L.; Pacini, A. Oxaliplatin-induced blood brain barrier loosening: A new point of view on chemotherapy-induced neurotoxicity. Oncotarget 2018, 9, 23426–23438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdem, G.U.; Dogan, M.; Demirci, N.S.; Zengin, N. Oxaliplatin-induced acute thrombocytopenia. J. Cancer Res. 2016, 12, 509–514. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Stavely, R.; Timpani, C.; Petersen, A.C.; Abalo, R.; Bornstein, J.C.; Rybalka, E.; Nurgali, K. Oxaliplatin-induced enteric neuronal loss and intestinal dysfunction is prevented by co-treatment with bgp-15. Br. J. Pharmacol. 2018, 175, 656–677. [Google Scholar] [CrossRef] [Green Version]
- Marks, D.H.; Qureshi, A.; Friedman, A. Evaluation of prevention interventions for taxane-induced dermatologic adverse events: A systematic review. JAMA Derm. 2018, 154, 1465–1472. [Google Scholar] [CrossRef]
- Ogawa, K.; Omatsu, T.; Matsumoto, C.; Tsuchiya, N.; Yamamoto, M.; Naito, Y.; Yoshikawa, T. Protective effect of the japanese traditional medicine juzentaihoto on myelosuppression induced by the anticancer drug ts-1 and identification of a potential biomarker of this effect. BMC Complement. Altern. Med. 2012, 12, 118. [Google Scholar] [CrossRef] [Green Version]
- Tay, C.G.; Lee, V.W.M.; Ong, L.C.; Goh, K.J.; Ariffin, H.; Fong, C.Y. Vincristine-induced peripheral neuropathy in survivors of childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef]
- Yoshida, M.; Ogawa, K.; Sakamoto, H.; Motomura, S.; Ishigatsubo, Y. Syndrome of inappropriate secretion of antidiuretic hormone in a patient with myeloid antigen positive acute lymphoblastic leukemia after systemic chemotherapy including vincristine. Gan Kagaku Ryoho 2000, 27, 99–102. [Google Scholar]
- Chen, X.; Wang, J.; Fu, Z.; Zhu, B.; Wang, J.; Guan, S.; Hua, Z. Curcumin activates DNA repair pathway in bone marrow to improve carboplatin-induced myelosuppression. Sci. Rep. 2017, 7, 17724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ullah, I.; Subhan, F.; Rudd, J.A.; Rauf, K.; Alam, J.; Shahid, M.; Sewell, R.D. Attenuation of cisplatin-induced emetogenesis by standardized Bacopa monnieri extracts in the pigeon: Behavioral and neurochemical correlations. Planta Med. 2014, 80, 1569–1579. [Google Scholar] [PubMed]
- Zhou, P.; Li, Z.; Xu, D.; Wang, Y.; Bai, Q.; Feng, Y.; Su, G.; Chen, P.; Wang, Y.; Liu, H.; et al. Cepharanthine hydrochloride improves cisplatin chemotherapy and enhances immunity by regulating intestinal microbes in mice. Front. Cell Infect. Microbiol. 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Cayır, K.; Karadeniz, A.; Simşek, N.; Yıldırım, S.; Karakuş, E.; Kara, A.; Akkoyun, H.T.; Sengül, E. Pomegranate seed extract attenuates chemotherapy-induced acute nephrotoxicity and hepatotoxicity in rats. J. Med. Food 2011, 14, 1254–1262. [Google Scholar] [CrossRef]
- Pan, H.; Wang, F.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Inhibitory effect of black tea pigments, theaflavin3/3′-gallate against cisplatin-resistant ovarian cancer cells by inducing apoptosis and g1 cell cycle arrest. Int. J. Oncol. 2017, 51, 1508–1520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, R.; Kulkarni, P.; Ganesh, N. Avocado fruit (persea americana mill) exhibits chemo-protective potentiality against cyclophosphamide induced genotoxicity in human lymphocyte culture. J. Exp. Oncol. 2011, 9, 221–230. [Google Scholar]
- Murali, V.P.; Kuttan, G. Enhancement of cancer chemotherapeutic efficacy of cyclophosphamide by Curculigo orchioides gaertn and its ameliorative effects on cyclophosphamide-induced oxidative stress. Integr. Cancer 2015, 14, 172–183. [Google Scholar] [CrossRef]
- Shathish, K.; Reena, D.; Guruvayoorappan, C. Chemoprotective effect of Decalepis hamiltonii against cyclophosphamide induced toxicity. J. Exp. Oncol. 2012, 9, 291–301. [Google Scholar]
- Zarei, M.; Shivanandappa, T. Neuroprotective effect of Decalepis hamiltonii on cyclophosphamide-induced oxidative stress in the mouse brain. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 341–348. [Google Scholar] [CrossRef]
- Wang, P.; Henning, S.M.; Heber, D.; Vadgama, J.V. Sensitization to docetaxel in prostate cancer cells by green tea and quercetin. J. Nutr. Biochem. 2015, 26, 408–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendanha, D.M.; Ferreira, H.D.; Felicio, L.P.; Silva, E.M.; Pereira, D.G.; Nunes, W.B.; Carvalho, S. Modulatory effect of Byrsonima verbascifolia (malpighiaceae) against damage induced by doxorubicin in somatic cells of drosophila melanogaster. Genet. Mol. Res. 2010, 9, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.G.; Jeon, B.M.; Yun, Y.J.; Cui, C.H.; Kim, S.C. Ginsenoside rh2 ameliorates doxorubicin-induced senescence bystander effect in breast carcinoma cell mda-mb-231 and normal epithelial cell mcf-10a. Int. J. Mol. Sci. 2019, 20, 1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, Z.; Maimon, Y.; Yoeli-Lerner, M.; Yang, P.; Samuels, N.; Berger, R. Selective anticancer effects and protection from chemotherapy by the botanical compound lcs101: Implications for cancer treatment. Int. J. Oncol. 2015, 46, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freitas, A.P.; Bitencourt, F.S.; Brito, G.A.; de Alencar, N.M.; Ribeiro, R.A.; Lima-Junior, R.C.; Ramos, M.V.; Vale, M.L. Protein fraction of calotropis procera latex protects against 5-fluorouracil-induced oral mucositis associated with downregulation of pivotal pro-inflammatory mediators. Naunyn Schmiedeberg Arch. Pharm. 2012, 385, 981–990. [Google Scholar] [CrossRef]
- Xi, S.; Hong, R.; Huang, J.; Lu, D.; Qian, L.; Li, P.; Wen, L.; Wang, Y. Effects of ciji hua’ai baosheng granule formula (chbgf) on life time, pathology, peripheral blood cells of tumor chemotherapy model mouse with h22 hepatoma carcinoma cells. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2014, 11, 94–100. [Google Scholar] [CrossRef]
- Koohi-Hosseinabadi, O.; Ranjbar, Z.; Sepehrimanesh, M.; AndisheTadbir, A.; Poorbaghi, S.L.; Bahranifard, H.; Tanideh, N.; Koohi-Hosseinabadi, M.; Iraji, A. Biochemical, hematological and pathological related healing effects of Elaeagnus angustifolia hydroalcoholic extract in 5-fluorouracil-induced oral mucositis in male golden hamster. Environ. Sci. Pollut. Res. Int. 2017, 24, 24447–24453. [Google Scholar] [CrossRef]
- Kato, S.; Hayashi, S.; Kitahara, Y.; Nagasawa, K.; Aono, H.; Shibata, J.; Utsumi, D.; Amagase, K.; Kadowaki, M. Saireito (tj-114), a japanese traditional herbal medicine, reduces 5-fluorouracil-induced intestinal mucositis in mice by inhibiting cytokine-mediated apoptosis in intestinal crypt cells. PLoS ONE 2015, 10, e0116213. [Google Scholar] [CrossRef]
- Kim, D.R.; Kim, J.; Oh, J.Y.; Kim, H.Y.; Kim, Y.J.; Chang, M.S. Protective effect of Salvia miltiorrhiza bunge on 5-fluorouracil-induced oral mucositis. Int. J. Mol. Med. 2017, 40, 39–46. [Google Scholar] [CrossRef]
- Famurewa, A.C.; Folawiyo, A.M.; Epete, M.A.; Igwe, E.C.; Okike, P.I.; Maduagwuna, E.K. Abrogation of hepatic damage induced by anticancer drug methotrexate by zobo (Hibiscus sabdariffa extract) supplementation via targeting oxidative hepatotoxicity in rats. J. Diet. Suppl. 2019, 16, 318–330. [Google Scholar] [CrossRef]
- Hao, Y.; Luo, X.; Ba, X.; Wang, J.; Zhou, S.; Yang, S.; Fang, C.; Jiang, C.; Sun, W. Huachansu suppresses trpv1 up-regulation and spinal astrocyte activation to prevent oxaliplatin-induced peripheral neuropathic pain in rats. Gene 2019, 680, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Cinci, L.; Di Cesare Mannelli, L.; Maidecchi, A.; Mattoli, L.; Ghelardini, C. Effects of Hypericum perforatum extract on oxaliplatin-induced neurotoxicity: In vitro evaluations. Z. Nat. C J. Biosci. 2017, 72, 219–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riccio, G.; Sommella, E.; Badolati, N.; Salviati, E.; Bottone, S.; Campiglia, P.; Dentice, M.; Tenore, G.C.; Stornaiuolo, M.; Novellino, E. Annurca apple polyphenols protect murine hair follicles from taxane induced dystrophy and hijacks polyunsaturated fatty acid metabolism toward beta-oxidation. Nutrients 2018, 10, 1808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.J.; Lee, H.G.; Kim, Y.S.; Lee, J.Y.; Jeon, J.P.; Park, C.; Moon, D.E. Ginkgo biloba extract attenuates hyperalgesia in a rat model of vincristine-induced peripheral neuropathy. Anesth. Analg. 2012, 115, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Cheah, K.Y.; Howarth, G.S.; Yazbeck, R.; Wright, T.H.; Whitford, E.J.; Payne, C.; Butler, R.N.; Bastian, S.E. Grape seed extract protects iec-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol. 2009, 8, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.M.; Lin, K.C.; Yuan, K.S.; Chang, C.L.; Chow, J.M.; Wu, S.Y. Treatment of advanced nasopharyngeal cancer using low- or high-dose concurrent chemoradiotherapy with intensity-modulated radiotherapy: A propensity score-matched, nationwide, population-based cohort study. Radiother. Oncol. 2018, 129, 23–29. [Google Scholar] [CrossRef]
- Yin, Z.; Li, C.; Wang, J.; Xue, L. Myeloid-derived suppressor cells: Roles in the tumor microenvironment and tumor radiotherapy. Int. J. Cancer 2019, 144, 933–946. [Google Scholar] [CrossRef]
- Lomax, M.E.; Folkes, L.K.; O’Neill, P. Biological consequences of radiation-induced DNA damage: Relevance to radiotherapy. Clin. Oncol. 2013, 25, 578–585. [Google Scholar] [CrossRef] [Green Version]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Murro, D.; Jakate, S. Radiation esophagitis. Arch. Pathol. Lab. Med. 2015, 139, 827–830. [Google Scholar] [CrossRef]
- Rosenthal, A.; Israilevich, R.; Moy, R. Management of acute radiation dermatitis: A review of the literature and proposal for treatment algorithm. J. Am. Acad. Dermatol. 2019, 81, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Skiba-Tatarska, M.; Kusa-Podkanska, M.; Surtel, A.; Wysokinska-Miszczuk, J. The side-effects of head and neck tumors radiotherapy. Pol. Merkur. Lek. Organ. Pol. Tow. Lek. 2016, 41, 47–49. [Google Scholar]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A review of our current understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Marinko, T. Pericardial disease after breast cancer radiotherapy. Radiol. Oncol. 2018, 53, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Esplugas, R.; Arenas, M.; Serra, N.; Belles, M.; Bonet, M.; Gascon, M.; Vallve, J.C.; Linares, V. Effect of radiotherapy on the expression of cardiovascular disease-related mirna-146a, -155, -221 and -222 in blood of women with breast cancer. PLoS ONE 2019, 14, e0217443. [Google Scholar] [CrossRef]
- Yahya, N.; Ebert, M.A.; Bulsara, M.; House, M.J.; Kennedy, A.; Joseph, D.J.; Denham, J.W. Urinary symptoms following external beam radiotherapy of the prostate: Dose-symptom correlates with multiple-event and event-count models. Radiother. Oncol. 2015, 117, 277–282. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Wang, F.X.; Jia, K.K.; Kong, L.D. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.J.; Lee, H.J.; Kim, J.D.; Jung, H.J.; Bae, S.H.; Ryoo, H.M.; Kim, S.G. Changes of peripheral blood lymphocyte subtypes in patients with end stage cancer administered localized radiotherapy and bojungikki-tang. Evid. Based Complement. Altern. Med. 2014, 2014, 207613. [Google Scholar] [CrossRef]
- Hamilton, K.; Bennett, N.C.; Purdie, G.; Herst, P.M. Standardized cranberry capsules for radiation cystitis in prostate cancer patients in New Zealand: A randomized double blinded, placebo controlled pilot study. Support. Care Cancer 2015, 23, 95–102. [Google Scholar] [CrossRef]
- Bonetta, A.; Di Pierro, F. Enteric-coated, highly standardized cranberry extract reduces risk of utis and urinary symptoms during radiotherapy for prostate carcinoma. Cancer Manag. Res. 2012, 4, 281–286. [Google Scholar]
- Saberi, H.; Keshavarzi, B.; Shirpoor, A.; Gharalari, F.H.; Rasmi, Y. Rescue effects of ginger extract on dose dependent radiation-induced histological and biochemical changes in the kidneys of male wistar rats. Biomed. Pharm. 2017, 94, 569–576. [Google Scholar] [CrossRef]
- Ji, K.; Fang, L.; Zhao, H.; Li, Q.; Shi, Y.; Xu, C.; Wang, Y.; Du, L.; Wang, J.; Liu, Q. Ginger oleoresin alleviated gamma-ray irradiation-induced reactive oxygen species via the nrf2 protective response in human mesenchymal stem cells. Oxidative Med. Cell. Longev. 2017, 2017, 1480294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeena, K.; Liju, V.B.; Ramanath, V.; Kuttan, R. Protection against whole body gamma-irradiation induced oxidative stress and clastogenic damage in mice by ginger essential oil. Asian Pac. J. Cancer Prev. APJCP 2016, 17, 1325–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamide, D.; Yamashita, T.; Araki, K.; Tomifuji, M.; Shiotani, A. Hangeshashinto (tj-14) prevents radiation-induced mucositis by suppressing cyclooxygenase-2 expression and chemotaxis of inflammatory cells. Clin. Transl. Oncol. 2017, 19, 1329–1336. [Google Scholar] [CrossRef]
- Jayachandran, S.; Balaji, N. Evaluating the effectiveness of topical application of natural honey and benzydamine hydrochloride in the management of radiation mucositis. Indian J. Palliat. Care 2012, 18, 190–195. [Google Scholar] [PubMed]
- Kim, H.G.; Jang, S.S.; Lee, J.S.; Kim, H.S.; Son, C.G. Panax ginseng meyer prevents radiation-induced liver injury via modulation of oxidative stress and apoptosis. J. Ginseng Res. 2017, 41, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, S.; Grove, T.A.; Liu, Y.; Cheng, Y.C.; Higgins, S.A.; Booth, C.J. Preclinical studies of the chinese herbal medicine formulation phy906 (kd018) as a potential adjunct to radiation therapy. Int. J. Radiat. Biol. 2013, 89, 16–25. [Google Scholar] [CrossRef]
- Ghassemi, L.; Zabihi, E.; Mahdavi, R.; Seyedmajidi, M.; Akram, S.; Motallebnejad, M. The effect of ethanolic extract of propolis on radiation-induced mucositis in rats. Saudi Med. J. 2010, 31, 622–626. [Google Scholar]
- Zhang, J.; Tong, F.; Cai, Q.; Chen, L.J.; Dong, J.H.; Wu, G.; Dong, X.R. Shenqi fuzheng injection attenuates irradiation-induced brain injury in mice via inhibition of the nf-kappab signaling pathway and microglial activation. Acta Pharmacol. Sin. 2015, 36, 1288–1299. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.L.; Lee, C.L.; Korivi, M.; Liao, J.W.; Rajendran, P.; Wu, J.J.; Hseu, Y.C. Zerumbone protects human skin keratinocytes against uva-irradiated damages through nrf2 induction. Biochem. Pharmacol. 2018, 148, 130–146. [Google Scholar] [CrossRef]
- Wang, L.J.; Lu, J.Z.; Cai, B.N.; Li, M.W.; Qu, B.L. Effect of compound zhuye shigao granule ( ) on acute radiation-induced esophagitis in cancer patients: A randomized controlled trial. Chin. J. Integr. Med. 2017, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Oh, S.W.; Shin, S.W.; Lee, K.W.; Cho, J.Y.; Lee, J. Zingerone protects keratinocyte stem cells from uvb-induced damage. Chem. Biol. Interact. 2018, 279, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Meimeti, E.; Kafanas, A.; Pavlou, P.; Evangelatou, A.; Tsouparelou, P.; Kanellopoulos, S.; Kipouros, P.; Koliarakis, N.; Leonis, G.; Ioannou, E.; et al. Topical treatment of skin injury inflicted in mice by x-ray irradiation. Skin Pharm. Physiol. 2018, 31, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Strasser, F. Diagnostic criteria of cachexia and their assessment: Decreased muscle strength and fatigue. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 417–421. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Aoyagi, T.; Terracina, K.P.; Raza, A.; Matsubara, H.; Takabe, K. Cancer cachexia, mechanism and treatment. World J. Gastrointest. Oncol. 2015, 7, 17–29. [Google Scholar] [CrossRef]
- Marceca, G.P.; Londhe, P.; Calore, F. Management of cancer cachexia: Attempting to develop new pharmacological agents for new effective therapeutic options. Front. Oncol. 2020, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Kumar, A. Er stress and unfolded protein response in cancer cachexia. Cancers 2019, 11, 1929. [Google Scholar] [CrossRef] [Green Version]
- Oakes, S.A.; Papa, F.R. The role of endoplasmic reticulum stress in human pathology. Annu. Rev. Pathol. 2015, 10, 173–194. [Google Scholar] [CrossRef] [Green Version]
- Mallinson, J.E.; Marimuthu, K.; Murton, A.; Selby, A.; Smith, K.; Constantin-Teodosiu, D.; Rennie, M.J.; Greenhaff, P.L. Statin myalgia is not associated with reduced muscle strength, mass or protein turnover in older male volunteers but is allied with a slowing of time to peak power output, insulin resistance and differential muscle mrna expression. J. Physiol. 2015, 593, 1239–1257. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elkina, Y.; von Haehling, S.; Anker, S.D.; Springer, J. The role of myostatin in muscle wasting: An overview. J. Cachexia Sarcopenia Muscle 2011, 2, 143–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.L.; Carroll, J.K.; Ryan, E.P.; Mustian, K.M.; Fiscella, K.; Morrow, G.R. Mechanisms of cancer-related fatigue. Oncologist 2007, 12 (Suppl. 1), 22–34. [Google Scholar] [CrossRef] [Green Version]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Wan, L.; Li, Y.; Yu, Q.; Chen, P.; Gan, R.; Yang, Q.; Han, Y.; Guo, C. Baicalin, a component of scutellaria baicalensis, alleviates anorexia and inhibits skeletal muscle atrophy in experimental cancer cachexia. Tumor Biol. 2014, 35, 12415–12425. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Im, M.; Gu, M.J.; Ma, J.Y. Citrus unshiu peel extract alleviates cancer-induced weight loss in mice bearing ct-26 adenocarcinoma. Sci. Rep. 2016, 6, 24214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirza, K.A.; Luo, M.; Pereira, S.; Voss, A.; Das, T.; Tisdale, M.J. In vitro assessment of the combined effect of eicosapentaenoic acid, green tea extract and curcumin c3 on protein loss in c2c12 myotubes. In Vitro Cell. Dev. Biol.-Anim. 2016, 52, 838–845. [Google Scholar] [CrossRef] [Green Version]
- Terawaki, K.; Kashiwase, Y.; Sawada, Y.; Hashimoto, H.; Yoshimura, M.; Ohbuchi, K.; Sudo, Y.; Suzuki, M.; Miyano, K.; Shiraishi, S.; et al. Development of ghrelin resistance in a cancer cachexia rat model using human gastric cancer-derived 85as2 cells and the palliative effects of the kampo medicine rikkunshito on the model. PLoS ONE 2017, 12, e0173113. [Google Scholar] [CrossRef]
- Shen, Q.; Miao, C.-X.; Zhang, W.-L.; Li, Y.-W.; Chen, Q.-Q.; Li, X.-X.; Liu, X.; Zhang, X.-W. Sibaochongcao exhibited anti-fatigue activities and ameliorated cancer cachexia in mice. RSC Adv. 2019, 9, 17440–17456. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.K.; Jung, K.Y.; Woo, S.M.; Yun, Y.J.; Jun, C.Y.; Park, J.H.; Shin, Y.C.; Cho, S.G.; Ko, S.G. Effect of sipjeondaebo-tang on cancer-induced anorexia and cachexia in ct-26 tumor-bearing mice. Mediat. Inflamm. 2014, 2014, 736563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, A.; Im, M.; Ma, J.Y. Sosihotang ameliorates cachexiarelated symptoms in mice bearing colon 26 adenocarcinoma by reducing systemic inflammation and muscle loss. Oncol. Rep. 2016, 35, 1841–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, P.; Zhang, J.; Wang, Y.; Zhang, M.; Song, L.; Lu, Z.; Zhang, L.; Zhang, F.; Wang, J.; Zhang, Y.; et al. Reversal of muscle atrophy by zhimu and huangbai herb pair via activation of igf-1/akt and autophagy signal in cancer cachexia. Support. Care Cancer 2016, 24, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Katsuno, H.; Maeda, K.; Kaiho, T.; Kunieda, K.; Funahashi, K.; Sakamoto, J.; Kono, T.; Hasegawa, H.; Furukawa, Y.; Imazu, Y. Clinical efficacy of daikenchuto for gastrointestinal dysfunction following colon surgery: A randomized, double-blind, multicenter, placebo-controlled study (jfmc39-0902). Jpn. J. Clin. Oncol. 2015, 45, 650–656. [Google Scholar] [CrossRef]
- Shimada, M.; Morine, Y.; Nagano, H.; Hatano, E.; Kaiho, T.; Miyazaki, M.; Kono, T.; Kamiyama, T.; Morita, S.; Sakamoto, J. Effect of tu-100, a traditional japanese medicine, administered after hepatic resection in patients with liver cancer: A multi-center, phase iii trial (jfmc40-1001). Int. J. Clin. Oncol. 2015, 20, 95–104. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Shimada, M.; Wakabayashi, G.; Ishida, K.; Kaiho, T.; Kitagawa, Y.; Sakamoto, J.; Shiraishi, N.; Koeda, K.; Mochiki, E. Effect of daikenchuto, a traditional japanese herbal medicine, after total gastrectomy for gastric cancer: A multicenter, randomized, double-blind, placebo-controlled, phase ii trial. J. Am. Coll. Surg. 2015, 221, 571–578. [Google Scholar] [CrossRef]
- Henning, S.M.; Wang, P.; Said, J.W.; Huang, M.; Grogan, T.; Elashoff, D.; Carpenter, C.L.; Heber, D.; Aronson, W. Randomized clinical trial of brewed green and black tea in men with prostate cancer prior to prostatectomy. Prostate 2015, 75, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase i/ii study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Marx, W.; McCarthy, A.; Ried, K.; McKavanagh, D.; Vitetta, L.; Sali, A.; Lohning, A.; Isenring, E.J.N. The effect of a standardized ginger extract on chemotherapy-induced nausea-related quality of life in patients undergoing moderately or highly emetogenic chemotherapy: A double blind, randomized, placebo controlled trial. Nutrients 2017, 9, 867. [Google Scholar] [CrossRef]
- Sanaati, F.; Najafi, S.; Kashaninia, Z.; Sadeghi, M. Effect of ginger and chamomile on nausea and vomiting caused by chemotherapy in Iranian women with breast cancer. Asian Pac. J. Cancer Prev. 2016, 17, 4125–4129. [Google Scholar]
- Ryan, J.L.; Heckler, C.E.; Roscoe, J.A.; Dakhil, S.R.; Kirshner, J.; Flynn, P.J.; Hickok, J.T.; Morrow, G. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: A urcc ccop study of 576 patients. Support. Care Cancer 2012, 20, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, C.; Munemoto, Y.; Mishima, H.; Nagata, N.; Oshiro, M.; Kataoka, M.; Sakamoto, J.; Aoyama, T.; Morita, S.; Kono, T.; et al. Double-blind, placebo-controlled, randomized phase ii study of tj-14 (hangeshashinto) for infusional fluorinated-pyrimidine-based colorectal cancer chemotherapy-induced oral mucositis. Cancer Chemother. Pharmacol. 2015, 76, 97–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoyama, T.; Nishikawa, K.; Takiguchi, N.; Tanabe, K.; Imano, M.; Fukushima, R.; Sakamoto, J.; Oba, M.S.; Morita, S.; Kono, T.; et al. Double-blind, placebo-controlled, randomized phase ii study of tj-14 (hangeshashinto) for gastric cancer chemotherapy-induced oral mucositis. Cancer Chemother. Pharmacol. 2014, 73, 1047–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arany, I.; Safirstein, R.L. Cisplatin nephrotoxicity. Semin Nephrol. 2003, 23, 460–464. [Google Scholar] [CrossRef]
- Mahmoodnia, L.; Mohammadi, K.; Masumi, R. Ameliorative effect of lycopene effect on cisplatin-induced nephropathy in patient. J. Nephropathol. 2017, 6, 144. [Google Scholar] [CrossRef] [Green Version]
- Kooshyar, M.M.; Mozafari, P.M.; Amirchaghmaghi, M.; Pakfetrat, A.; Karoos, P.; Mohasel, M.R.; Orafai, H.; Azarian, A.A. A randomized placebo-controlled double blind clinical trial of quercetin in the prevention and treatment of chemotherapy-induced oral mucositis. J. Clin. Diagn. Res. 2017, 11, ZC46. [Google Scholar] [CrossRef]
- Sahebjamee, M.; Mansourian, A.; Mohammad, M.; Zadeh, M.T.; Bekhradi, R.; Kazemian, A.; Manifar, S.; Ashnagar, S.; Doroudgar, K. Comparative efficacy of aloe vera and benzydamine mouthwashes on radiation-induced oral mucositis: A triple-blind, randomised, controlled clinical trial. Oral Health Prev. Dent. 2015, 13, 309–315. [Google Scholar]
- Sahebnasagh, A.; Ghasemi, A.; Akbari, J.; Alipour, A.; Lashkardoost, H.; Ala, S.; Salehifar, E.; Medicine, C. Successful treatment of acute radiation proctitis with aloe vera: A preliminary randomized controlled clinical trial. J. Altern. Complement. Med. 2017, 23, 858–865. [Google Scholar] [CrossRef]
- Ryan, J.L.; Heckler, C.E.; Ling, M.; Katz, A.; Williams, J.P.; Pentland, A.P.; Morrow, G. Curcumin for radiation dermatitis: A randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat. Res. 2013, 180, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Heydarirad, G.; Rezaeizadeh, H.; Choopani, R.; Mosavat, S.H.; Ameri, A. Efficacy of a traditional persian medicine preparation for radiation-induced xerostomia: A randomized, open-label, active-controlled trial. J. Integr. Med. 2017, 15, 201–208. [Google Scholar] [CrossRef]
- Charalambous, M.; Raftopoulos, V.; Paikousis, L.; Katodritis, N.; Lambrinou, E.; Vomvas, D.; Georgiou, M.; Charalambous, A. The effect of the use of thyme honey in minimizing radiation-induced oral mucositis in head and neck cancer patients: A randomized controlled trial. Eur. J. Oncol. Nurs. 2018, 34, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Peppone, L.J.; Inglis, J.E.; Mustian, K.M.; Heckler, C.E.; Padula, G.D.; Mohile, S.G.; Kamen, C.S.; Culakova, E.; Lin, P.-J.; Kerns, S. Multicenter randomized controlled trial of omega-3 fatty acids versus omega-6 fatty acids for the control of cancer-related fatigue among breast cancer survivors. JNCI Cancer Spectr. 2019, 3, pkz005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Ábrigo, J.; Elorza, A.A.; Riedel, C.A.; Vilos, C.; Simon, F.; Cabrera, D.; Estrada, L.; Cabello-Verrugio, C. Role of oxidative stress as key regulator of muscle wasting during cachexia. Oxidative Med. Cell. Longev. 2018, 2018, 2063179. [Google Scholar] [CrossRef] [PubMed]
- Assi, M.; Rébillard, A. The janus-faced role of antioxidants in cancer cachexia: New insights on the established concepts. Oxidative Med. Cell. Longev. 2016, 2016, 9579868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marucci, L.; Farneti, A.; Di Ridolfi, P.; Pinnaro, P.; Pellini, R.; Giannarelli, D.; Vici, P.; Conte, M.; Landoni, V.; Sanguineti, G. Double-blind randomized phase iii study comparing a mixture of natural agents versus placebo in the prevention of acute mucositis during chemoradiotherapy for head and neck cancer. Head Neck 2017, 39, 1761–1769. [Google Scholar] [CrossRef]
- Spradlin, J.N.; Hu, X.; Ward, C.C.; Brittain, S.M.; Jones, M.D.; Ou, L.; To, M.; Proudfoot, A.; Ornelas, E.; Woldegiorgis, M.; et al. Harnessing the anti-cancer natural product nimbolide for targeted protein degradation. Nat. Chem. Biol. 2019, 15, 747–755. [Google Scholar] [CrossRef]

| Surgery | Compound/Extract | Source | Cancer type | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Colorectal polyp removal surgery | Epigallocatechin gallate green tea extract | Amellia sinensis O. Kuntze | Colon adenoma | Human | 150 mg; 3 years | Prevention of metachronous adenomas in colorectum | ↓P450 isoenzymes | [23] |
| Hepatocellular tumor xenograft surgery | 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone | Cleistocalyx operculatus (Roxb.) | Hepatocellular tumor cancer | BEL-7402/5-FU, nude mice | 40 mg/kg; 16 days | Decrease of 5-FU resistance | ↑caspase-3 | [24] |
| Pancreatic cancer xenografts surgery | Nemorosone | Pancreatic cancer | NMRI nu/nu mice | 50 mg/kg; 1 day | Decrease of side-effects and cancer growth | ↑CYP3A4 | [25] | |
| Sigmoid or rectosigmoid cancer surgery | Daickenchuto | Sigmoid or rectosigmoid cancer | Human | 15.0 g; 6 days | Decrease of delayed gastric emptying and paralytic ileus | [26] | ||
| Sigmoid colon cancer surgery | Daickenchuto | Sigmoid colon cancer | Human | 7.5 g; 2 h | Increase of postoperative intestinal motility | ↑CRP | [27] |
| Chemotherapy | Compound/Extract | Source | Cancer Type | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Carboplatin | Curcumin | Curcuma longa Linn | T241-bearing mice | 5 mM; 15 days | Alleviation of myelosuppression | ↑BRCA1, BRCA2, ERCC1 | [62] | |
| Cisplatin | Bacopa monnieri extract | Bacopa monnieri | Pigeon | 10, 20, 40 mg/kg; 24 h | Alleviation of vomiting | ↓dopamine, 5-HT, 5-HIAA | [63] | |
| Cisplatin | Cepharanthine hydrochloride | Esophageal squamous cell carcinoma | Eca109 cells inoculated BALB/c nude mice | 10 mg/kg; 13 days | Increase of anticancer properties and decrease of side effects | ↑c-PARP, c-caspase-3, 8, 9, TNFR1, p-JNK, cytochrome C ↓Bcl-2 | [64] | |
| Cisplatin | Pomegranate seed extract | Punica granatum | SD rats | 300 mg/kg; 15 days | Alleviation of acute nephrotoxicity and hepatotoxicity | ↑GSH, GST, GPx, SOD ↓Lipid peroxidation, MDA, caspase-3 | [65] | |
| Cisplatin | Theaflavin-3-gallate, theaflavin-3′-gallate | Ovarian cancer | A2780/CP70, IOSE-364 | 5, 10, 20 µM; 24 h | Prevention of ovarian cancer | ↑caspase-3, -7, c-PARP, p21, p38, p53 ↓CDK2, CDK4, cyclin E1 | [66] | |
| Cyclophosphamide | Avocado methanol extract | Persea Americana Mill | Human lymphocyte | 100, 200, 300 mg/kg; 70 h | Decrease of chromosomal aberrations | [67] | ||
| Cyclophosphamide | Curculigo orchioides methanolic extract | Curculigo orchioides Gaertn. | Balb/c, Swiss albino mice | 25, 50, 100, 200 mg/kg; 14 days | Increase of anticancer properties and decrease of side effects | ↑α-esterase, IL-2, GM-CSF, IFN-γ, GSH ↓TNF-α, LPO, ALP, GPT | [68] | |
| Cyclophosphamide | Decalepis hamiltonii methanolic extract | Decalepis hamiltonii | BALB/c mice | 0.5 mg; 10 days | Alleviation of side effects | ↑GSH ↓SGOT, SGPT | [69] | |
| Cyclophosphamide | Decalepis hamiltonii aqueous extract | Decalepis hamiltonii Wight & Arn | Swiss albino mice | 50, 100 mg/kg; 10 days | Alleviation of side effects | ↑GSH, SOD, CAT, GPx, GR, GST ↓ROS | [70] | |
| Docetaxel | (1) Green tea (2) Quercetin | Camellia sinensis | Prostate cancer | LAPC-4-AI, PC-3 | (1) 40, 5 μM; 48 h | Increase of therapeutic effect and decrease of chemoresistance | ↑Bax/Bcl-2 ↓PI3K/Akt, STAT3, MRP1, CD44+/CD24− | [71] |
| Doxorubicin | Byrsonima verbascifolia water extract | Byrsonima verbascifolia | Somatic cells of Drosophila melanogaster | 25, 50, 100 mg/mL; 2 days | Alleviation of doxorubicin-induced damage | [72] | ||
| Doxorubicin | Ginsenoside Rh2 | Breast cancer | MDA-MB-231 | 20 μg/mL; 48 h | Alleviation of cellular senescence | ↓Vimentin, beta-catenin, Snail, caspase 3/7, MCP-1, CXCL1, IL-6, IL-8, p-MEK1, p-p38, p-STAT3, p-NF-κB p65 | [73] | |
| MCF-10A | ↓caspase 3/7, MCP-1, CXCL1, IL-6, IL-8, p-p38, p-STAT3 | |||||||
| Doxorubicin, 5-fluorouracil | LCS101 | Astragalus membranaceus, Atractylodes macrocephala, Citrus reticulate, Glehnia littoralis, Ligustrum lucidum, Lycium chinense, Milletia reticulata, Oldenlandia diffusa, Ophiopogon japonicus, Paeonia lactiflora, Paeonia obovata, Poriae cocos, Prunella vulgaris, Scutellaria barbata | Breast, Colorectal, Prostate cancer | MCF7, MDA-MB-231, HCT116, PC-3, DU-145, MCF10A, EP#2 | 1, 2, 3 mg/mL; 24, 48, 72 h | Regulation of tumorigenic and non-tumorigenic cells | ↓c-PARP-1 | [74] |
| 5-Fluorouracil | Calotropis procera latex | Calotropis procera | Golden hamsters | 0.25, 1, 5, 25 mg/kg; 24 h before, 24 h after mechanical trauma | Alleviation of oral mucositis | ↓COX-2, iNOS, TNF- α, IL-1β, MPO | [75] | |
| 5-Fluorouracil | Ciji Hua’ai Baosheng Granule Formula | Radix Codonopsis, Radix astragali Mongolici, Bulbus fritillariae Thunbergii, Rhizoma Arisaematis Erubescentis, Pericarpium Citri Reticulatae, Poria, Cortex Magnoliae Officinalis, Fructus Aurantii Submaturus, Rhizoma Atractylodis Macrocephalae, Fructus Amomi, Fructus Alpiniae Oxyphyllae, Semen Lablab Album, Fructus Hordei Germinatus, Rhizoma sparganii, Spina Gleditsiae, Cortex Albiziae, Concha Ostreae, Ganoderma Lucidum, Fructus Psoraleae | Hepatic cancer | Kunming mice | 16, 32, 64 g/kg; 21 days | Alleviation of tumor growth and appearance | [76] | |
| 5-Fluorouracil | E. angustifolia hydroalcoholic extract | Echinacea angustifolia de Candolle | Golden hamsters | 3000 mg/kg; 5 days | Healing stimulatory and anti-inflammatory properties in oral mucositis | ↑SOD ↓MPO | [77] | |
| 5-Fluorouracil | Saireito | C57BL/6 mice | 100, 300, 1000 mg/kg; 6 days | Prevention of intestinal mucositis | ↓Caspase-3, TNF-α, IL-1β | [78] | ||
| 5-Fluorouracil | Salvia miltiorrhiza Bunge | Salvia miltiorrhiza Bunge | Golden Syrian hamsters | 100, 500, 1000 mg/kg; 10 days | Prevention of oral mucositis | ↑DPPH ↓NF-κB, caspase-3, ROS, TNF-α, IL-1β | [79] | |
| Methotrexate | Hibiscus sabdariffa extract (Zobo) | Hibiscus sabdariffa Linn | Albino Wistar rats | 10 mL/kg; 14 days | Alleviation of oxidative hepatotoxicity | ↑SOD, CAT, GPx ↓MDA | [80] | |
| Oxaliplatin | Toad skin aqueous extract (Huachansu) | Bufo bufo gargarizans Cantor | SD rats | 1.25, 2.5 g/kg; 21 days | Prevention of allodynia and hyperalgesia | ↑TRPV4 ↓TRPV1 | [81] | |
| Oxaliplatin | Hypericum perforatum hydrophilic extract | Hypericum perforatum Linn | Rat astrocytes, HT-29 | 5, 50, 250 μg/mL; 4, 8 h | Alleviation of chemotherapy-induced neuropathy | ↓Caspase-3 | [82] | |
| Taxane | Annurca Apple polyphenolic Extract | Malus Pumila Miller cv. Annurca | Wild-type C57BL/6 mice | 400 mg/L; 7 days | Protection of murine hair follicles from dystrophy | ↑PGF2α ↓PPP | [83] | |
| TS-1 | Juzentaihoto | Astragali Radix, Cinamomi Cortex, Rehmanniae Radix, Paeoniae Radix, Cnidii Rhizoma, Angelicae Radix, Ginseng Radix, Hoelen, Glycyrrhizae Radix, Atractylodis Lanceae Rhizoma | Balb/c mice | 1 g/kg; 3, 5, 7 days | Activation of hematopoiesis | ↑CD34+ BMC ratio ↓WBC | [59] | |
| Vincristine | Ginkgo biloba extract | Ginkgo biloba | SD rats | 50, 100, 150 mg/kg; 15, 30, 60, 90, 120, 150, 180 m | Alleviation of mechanical and cold hyperalgesia | [84] |
| Compound/Extract | Source | Cancer Type | Target Tissue | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|---|---|
| Bojungikki-tang water extract | Panax ginseng C. A. Meyer, Atractylodes macrocephala Koidz., Astragalus membranaceus Bunge, Angelicae gigantis Radix, Citrus aurantium Linne, Ziziphus jujuba var. inermis, Bupleurum falcatum Linne, Glycyrrhiza uralensis Fisch., Zingiber officinale Roscoe, Cimicifuga heracleifolia KOM. | Human | 9 g; 4 weeks | Restoration of B cells decrease | [98] | |||
| Cranberry capsules | Vaccinium macrocarpon Ait. | Prostate cancer | Urothelium | Human | 72 mg/day; 2 weeks | Alleviation of cystitis | [99] | |
| Cranberry | Vaccinium macrocarpon Ait. | Prostate cancer | Prostate | Human | 200 mg; 6, 7 weeks | Alleviation of lower urinary tract infections and urinary symptoms | [100] | |
| Ginger hydro-alcoholic extract | Zingiber officinale | Kidney | Wistar rats | 50 mg/kg; 10 days | Prevention of γ-ray induced kidney damage | ↑TAC ↓8-OhdG, CRP | [101] | |
| Ginger oleoresin | Zingiberofficinale | Human mesenchymal stem cells | Human | 1, 10, 100, 1000 μg/mL; 24, 48, 72 h | Prevention of cell injury | ↑HO-1, NQO-1 ↓ROS | [102] | |
| Ginger essential oil | tabke | Balb/C mice | 500 mg/kg; 19 days | Prevention of γ-irradiation induced damage | ↑SOD, catalase, GPx, GSH, MNPCE, MNNCE | [103] | ||
| Hangeshashinto (TJ-14) | Pinellia ternata, Scutellaria baicalensis, Zingiber officinale Roscoe, Glycyrrhiza uralensis Fisch., Zizyphus jujuba Mill., Panax ginseng C. A. Meyer, Coptis chinensis Franch. | Head, neck cancer | Buccal mucosa | Syrian golden hamsters | 2%; 28 days | Prevention of radiation mucositis | ↓COX-2 | [104] |
| Pure natural honey (Dabur honey) | Apis mellifera Linnaeus | Head, neck cancer | Head, neck | Human | 60 m/L; 7 weeks | Alleviation of radiation mucositis | [105] | |
| Panax ginseng water extract | Panax ginseng C. A. Meyer | Liver | C57BL/6N mice | 25, 50, 100 mg/kg; 4 days | Prevention of liver injury | ↑TAC, GSH, GSH-Rd, SOD, catalase, Bcl-2, Bcl-xL ↓4-HNE, ROS, MDA, TNF-α, IL-6, p53, Bax | [106] | |
| PHY906 | Scutellaria baicalensis, Glycyrrhiza uralensis Fisch., Paeonia lactiflora, Ziziphus jujuba var. inermis | N/A | Abdomen | EMT6, BALB/c Rw mice | 500 mg/kg; 4 days | Alleviation of abdominal irradiation induced toxicity | [107] | |
| Propolis ethanolic extract | Apis mellifera Linnaeus | Head, neck | Wistar rats | 100, 200 mg/kg; 10 days | Alleviation and delay of mucositis | [108] | ||
| Shenqi Fuzheng | Codonopsis pilosula, Astragalus membranaceus Bunge | Brain | C57BL/6J mice | 20 mL/kg/d; 28 days | Alleviation of brain injury | ↓HRP, TNF-α, IL-1β, NF-κB, PIDD-C, PIDD-CC, p65 | [109] | |
| Zerumbone | Zingiber zerumbet Smith | Skin | HaCaT | 2–10 μM; 24 h | Dermato-protective efficacies | ↑Bcl-2, Nrf2, HO-1, γ-GCLC, GSH, p38 MAPK, PI3K ↓LDH, ROS, Bax | [110] | |
| BALB/c-nu mice | 55, 110 μg/day; 14 days | |||||||
| Zhuye Shigao Granule | Lophatherum gracile Brongn., Gypsum, Panax ginseng C. A. Meyer, Liriope platyphylla, Pinellia ternate (Thunb.) Breit., Glycyrrhiza uralensis Fisch., Rabdosia serra (Maxim.) Hara, Hedyotis diffusa Willd., Scutellaria barbata D. Don, Coix lacryma-jobi, Curcuma longa Linne | Lung, esophagus, mediastinal cancer | Chest, mediastinum | Human | 12 mg; 4 weeks | Alleviation of acute esophagitis | [111] | |
| Zingerone | Zingiberofficinale | Albino rats | 25 mg/kg; 21 days | Prevention of cardiotoxicity | ↑GSH, CAT, ETC complex I, II, IV ↓cTnT, LDH, CK-MB, MDA, TNF-α, MPO, caspase-3 | [29] | ||
| Zingerone | Zingiberofficinale | Skin | Keratinocyte stem cells | 10, 20, 100 μM; 24 h | Prevention of UVB-induced keratinocyte damages | ↑PCNA, VEGF, TERT, HDAC1, DNMT1 ↓TNF-α, IL-1β, IL-6, p21, p42/44 MAPK, p38 MAPK | [112] | |
| Gel containing Pinus halepensis bark aqueous extract | Pinus halepensis Mill. | Breast cancer | Skin | SKH-HR2 hairless mice | 5%; 60 days | Alleviation of chronic and granulomatous inflammation | [113] | |
| Ointment containing marine isopod Ceratothoa oestroides olive oil extract | Ceratothoa oestroides Risso. | Breast cancer | Skin | SKH-HR2 hairless mice | 10%; 60 days | Alleviation of chronic and granulomatous inflammation | [113] |
| Compound/Extract | Source | Cell Line/Animal Model | Dose; Duration | Efficacy | Mechanism | Reference |
|---|---|---|---|---|---|---|
| Baicalin | Scutellaria baicalensis | CT26 adenocarcinoma inoculated BALB/c mice | 50, 150 mg/kg; 15 days | Amelioration of anorexia, weight loss and muscle atrophy | ↑IκBα ↓NF-κB, TNF-α, IL-6, MURF1, Atrogin-1, p65 | [126] |
| Citrus unshiu peel water extract | Citrus unshiu Markovich | CT26 adenocarcinoma-induced cancer cachexia BALB/c mice | 250, 500 mg/kg; 17 days | Amelioration of weight loss, muscle wasting and Hb loss | ↑MyH, p-Akt ↓MAFbx, MuRF-1, IL-6, NO, iNOS, IL-1β, TNF-α, p-p38, ERK, JNK, IκBα, STAT3, p-p65, | [127] |
| Curcumin green tea extract | Curcuma longa, Camellia sinensis | C2C12 myotubes | 10 μg/mL; 24 h | Amelioration of weight loss and muscle wasting | ↓20S proteasome subunits, p42, MuRF1, MAFbx, PIF, TNF-α | [128] |
| Rikkunshito | Atractylodes lancea, Panax ginseng, Pinellia ternate, Poria cocos, Zizyphus jujuba, Citrus unshiu, Glycyrrhiza uralensis, Zingiber officinale | 85As2 cells inoculated F344/NJcl-rnu/rnu rats | 1 g/kg/day; 7 days | Amelioration of anorexia and weight loss | ↑NPY ↓TRH | [129] |
| SiBaoChongCao | Cordyceps sinensis | C26 tumor-bearing BALB/c mice | 1, 2 g/kg; 20 days | Amelioration of weight loss, muscle wasting and adipocyte cell reduction | ↑MHC, MyoD, MyoG, p-AKT, p-mTOR, AMPKα, ERK, TORC1, PGC-1α ↓IL-6, TG, AMPK, p38 MAPK, p-HSL, UCP1 | [130] |
| Sipjeondaebo-tang | Angelica gigas, Astragalus membranaceus, Atractylodes japonica, Cinnamomum cassia, Cnidium officinale, Glycyrrhiza uralensis, Paeonia lactiflora, Panax ginseng, Poria cocos, Rehmannia glutinosa | CT-26 inoculated- BALB/c mice | 6.784, 67.84, 678.4 mg/kg; 21 days | Amelioration of anorexia, weight loss, muscle wasting and anemia | ↓IL-6, MCP-1, PYY, GLP-1 | [131] |
| Soshio-tang | Bupleurum falcatum, Glycyrrhiza uralensis, Panax ginseng, Pinellia ternata, Scutellaria baicalensis, Zingiber officinale, Ziziphus jujuba | J774A.1 macrophage cell line inoculated CT-26-bearing mice | 50, 100 mg/kg; 18 days | Alleviation of weight loss, muscle wasting and appetite loss | ↓NO, iNOS, IL-6, IL-1α, IL-1β, TNF-α, p38, NF-κB, IκBα, IKKαβ, STAT3 | [132] |
| Zhimu and Huangbai herb pair | Anemarrhena asphodeloides, Phellodendron amurense | colon-26 adenocarcinoma inoculated C57BL/6 mice | 104 mg/kg; 18 days | Amelioration of weight loss and muscle wasting | ↑IGF-1, Akt, LC3B, SIRT1 ↓TNF-α, IL-6, atrogin-1, MuRF1, FOXO3 | [133] |
| Treatment | Compound/Extract | Source | Phase | Patients | Status | Number | Efficacy | Reference |
|---|---|---|---|---|---|---|---|---|
| Surgery | Daikenchuto | Zingiber officinale Roscoe, Zanthoxylum piperitum De Candolle, Panax ginseng Carl Anton Meyer, maltose | III | 336 | Completed | UMIN000001592 | Improvement of gastrointestinal dysfunction | [134] |
| Surgery | Daikenchuto | Zingiber officinale Roscoe, Zanthoxylum piperitum De Candolle, Panax ginseng Carl Anton Meyer, maltose | III | 209 | Completed | UMIN000003103 | Improvement of gastrointestinal dysmotility | [135] |
| Surgery | Daikenchuto | Zingiber officinale Roscoe, Zanthoxylum piperitum De Candolle, Panax ginseng Carl Anton Meyer, maltose | III | 195 | Completed | UMIN000004693 | Improvement of postoperative bowel function | [136] |
| Surgery | Daikenchuto | Zingiber officinale Roscoe, Zanthoxylum piperitum De Candolle, Panax ginseng Carl Anton Meyer, maltose | III | 71 | Completed | UMIN000001793 | Alleviation of postoperative paralytic ileus | [26] |
| Surgery | Green tea | Camellia sinensis | II | 93 | Active, not recruiting | NCT00685516 | Increase of systemic antioxidant activity | [137] |
| Chemotherapy | Curcumin | Curcuma longa Linn | I, II | 21 | Completed | UMIN000001386 | Sensitization of pancreatic cancer cells to gemcitabine | [138] |
| Chemotherapy | Ginger | Zingiber officinale Roscoe | II | 34 | Completed | ACTRN12613000120774 | Improvement of chemotherapy-induced nausea | [139] |
| Chemotherapy | Ginger, Matricaria Chamomilla extract | Zingiber officinale Roscoe, Matricaria chamomilla Linné | II | 45 | Completed | IRCT2013020912404N1 | Alleviation of nausea and vomiting | [140] |
| Chemotherapy | Ginger purified liquid extract | Zingiber officinale Roscoe | II, III | 576 | Completed | NCT00040742 | Alleviation of acute nausea | [141] |
| Chemotherapy | Hangeshashinto | Pinellia ternata Breitenbach, Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fischer, Zizyphus jujuba Miller var. inermis Rehder, Panax ginseng Carl Anton Meyer, Zingiber officinale Roscoe, Coptis rhizome | II | 90 | Completed | UMIN000004287 | Improvement of mucositis | [142] |
| Chemotherapy | Hangeshashinto | Pinellia ternata Breitenbach, Scutellaria baicalensis Georgi, Glycyrrhiza uralensis Fischer, Zizyphus jujuba Miller var. inermis Rehder, Panax ginseng Carl Anton Meyer, Zingiber officinale Roscoe, Coptis rhizome | II | 91 | No longer recruiting | UMIN000004214 | Alleviation of oral mucositis | [143] |
| Chemotherapy | Lycopene | Momordica cochinchinensis Spreng, Elaeagnus umbellata, Lycopersicon esculentum etc. | II- III | 120 | Completed | IRCT2016050427745N1 | Alleviation of nephrotoxicity related complications | [145] |
| Chemotherapy | Oral quercetin capsules | Plant flavonol from the flavonoid group of polyphenols | I, II | 20 | Completed | NCT01732393 | Prevention of oral mucositis | [146] |
| Chemotherapy | Rikkunshito | Atractylodes lancea De Candlle, Panax ginseng Carl Anton Meyer, Pinellia ternata Breitenbach, Poria cocos Wolf, Zizyphus jujuba Miller var. inermis Rehder, Citrus reticulata Blanco, Glycyrrhiza uralensis Fischer, Zingiber officinale Roscoe | II | 36 | Completed | UMIN000011227 | Improvement of nausea, vomiting and anorexia | [11] |
| Radiotherapy | Aloe vera | Aloe vera | II | 26 | Completed | IRCT2012072410377N1 | Alleviation of mucositis | [147] |
| Radiotherapy | Aloe vera ointment | Aloe vera | II | 20 | Completed | IRCT201606042027N6 | Improvement of acute proctitis | [148] |
| Radiotherapy | Curcumin C3 Complex | Curcuma longa Linn | II | 30 | Completed | NCT01042938 | Alleviation of dermatitis | [149] |
| Radiotherapy | Dry flowers of Alcea digitata Alef, Malva sylvestris | Alcea digitata Alef, Malva sylvestris Carl Linnaeus | II | 60 | Completed | NCT02854358 | Improvement of xerostomia | [150] |
| Radiotherapy | Thyme honey | Thymus Capitatus, Thymus Vularis, Thymus Serpyllum etc. | II | 64 | Completed | NCT01465308 | Improvement of oral mucositis | [151] |
| Cachexia | Omega-6 polyunsaturated fatty acids | Soybean oil | II | 81 | Completed | NCT02352779 | Alleviation of cancer-related fatigue | [152] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Jeong, M.I.; Kim, H.-R.; Park, H.; Moon, W.-K.; Kim, B. Plant Extracts as Possible Agents for Sequela of Cancer Therapies and Cachexia. Antioxidants 2020, 9, 836. https://doi.org/10.3390/antiox9090836
Lee J, Jeong MI, Kim H-R, Park H, Moon W-K, Kim B. Plant Extracts as Possible Agents for Sequela of Cancer Therapies and Cachexia. Antioxidants. 2020; 9(9):836. https://doi.org/10.3390/antiox9090836
Chicago/Turabian StyleLee, Jinjoo, Myung In Jeong, Hyo-Rim Kim, Hyejin Park, Won-Kyoung Moon, and Bonglee Kim. 2020. "Plant Extracts as Possible Agents for Sequela of Cancer Therapies and Cachexia" Antioxidants 9, no. 9: 836. https://doi.org/10.3390/antiox9090836