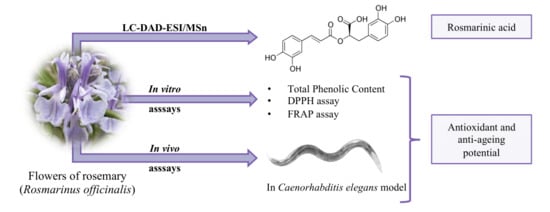
Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
2.2. Samples and Preparation of Extract
2.3. Analysis of Phenolic Compounds
2.4. C. elegans Assays
2.4.1. C. elegans Strains and Maintenance
2.4.2. Acute Toxicity Assay
2.4.3. Oxidative Stress Resistance Assay
2.4.4. Lifespan Assay
2.5. In Vitro Reducing/Antiradical Activity
2.6. Statistical Analyses
3. Results and Discussion
3.1. Phenolic Compounds of Rosemary Flowers
3.2. Evaluation of Rosemary Flowers Acute Toxicity
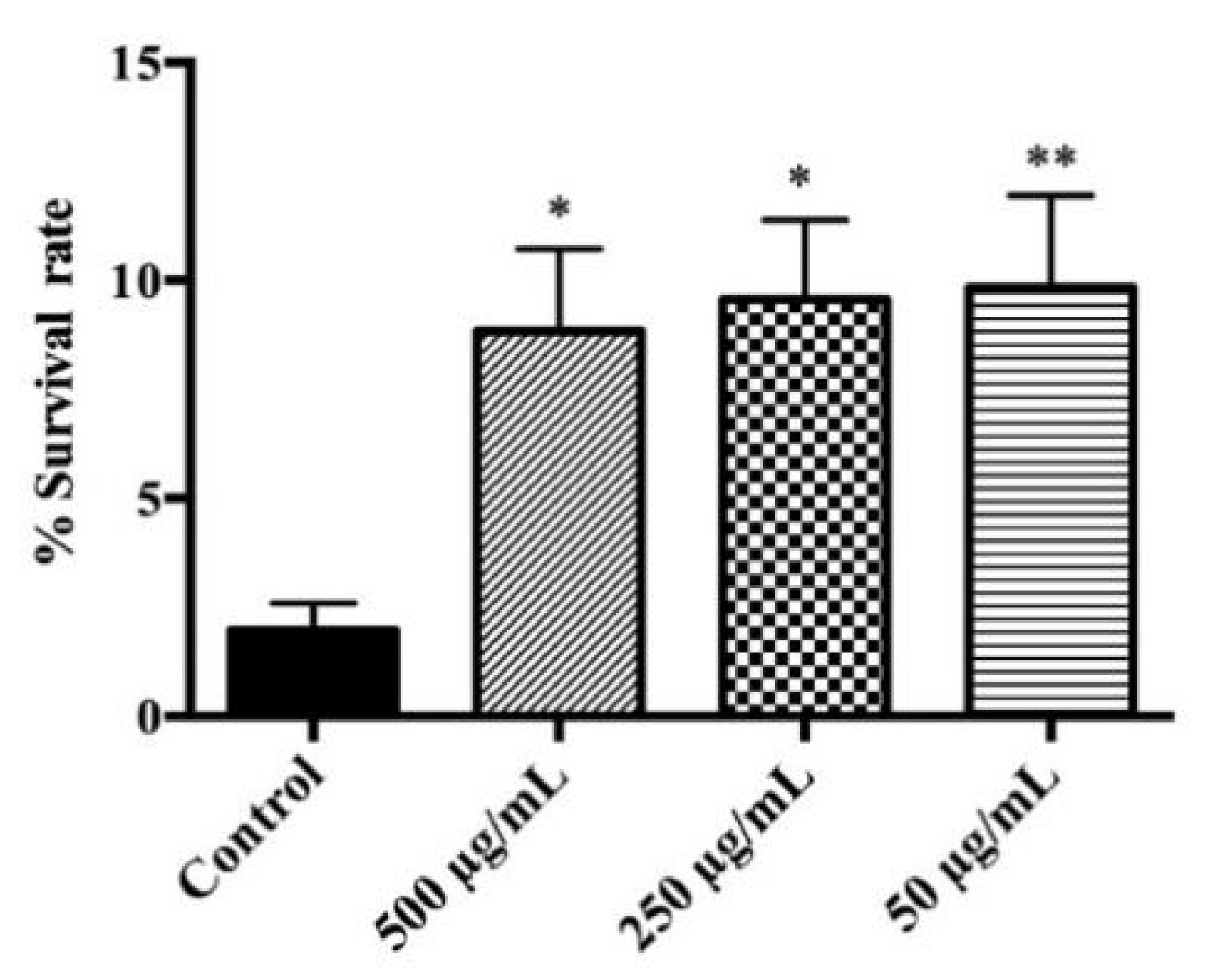
3.3. Evaluation of Protective Effects on C. elegans under Lethal Oxidative Stress
3.4. Evaluation of C. elegans Lifespan
3.5. In Vitro Reducing/Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Takahashi, J.A.; Rezende, F.A.G.G.; Moura, M.A.F.; Dominguete, L.C.B.; Sande, D. Edible flowers: Bioactive profile and its potential to be used in food development. Food Res. Int. 2020, 129, 108868. [Google Scholar] [CrossRef] [PubMed]
- Del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Del Río, J.A.; Ortuño, A.; Quirin, K.-W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef]
- Del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Marín, M.P.; Del Río, J.A.; Ortuño, A.; Ibarra, I. Flavonoid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus officinalis. Postulation of a Biosynthetic Pathway. J. Agric. Food Chem. 2003, 52, 4987–4992. [Google Scholar] [CrossRef]
- Lu, B.; Li, M.; Yin, R. Phytochemical Content, Health Benefits, and Toxicology of Common Edible Flowers: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, S130–S148. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Tenuta, C.; Menichini, F.; Xiao, J.; Tundis, R. Edible Flowers: A Rich Source of Phytochemicals with Antioxidant and Hypoglycaemic Activity. J. Agric. Food Chem. 2015, 64, 2467–2474. [Google Scholar] [CrossRef]
- Wang, F.; Miao, M.; Xia, H.; Yang, L.-G.; Wang, S.-K.; Sun, G.J. Antioxidant activities of aqueous extracts from 12 Chinese edible flowers in vitro and in vivo. Food Nutr. Res. 2017, 61, 1265324. [Google Scholar] [CrossRef] [Green Version]
- Committee on Herbal Medicinal Products (HMPC). Assessment Report on Rosmarinus officinalis L., Aetheroleum and Rosmarinus officinalis L., Folium; Final Report; European Medicines Agency: London, UK, 2010; Volume EMA/HMPC/1, Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Herbal_-_HMPC_assessment_report/2011/02/WC500101693.pdf (accessed on 13 October 2019).
- Calvo, D.R.; Martorell, P.; Genovés, S.; Gosálbez, L. Development of novel functional ingredients: Need for testing systems and solutions with Caenorhabditis elegans. Trends Food Sci. Technol. 2016, 54, 197–203. [Google Scholar] [CrossRef]
- Martorell, P.; Llopis, S.; Gonza, N.; Monto, F.; Ortiz, P.; Genove, S. Caenorhabditis elegans as a Model to Study the E FF ectiveness and Metabolic Targets of Dietary Supplements Used for Obesity Treatment: The Speci FI C Case of a Conjugated Linoleic Acid Mixture (Tonalin). J. Agric. Food Chem. 2012, 60, 11071–11079. [Google Scholar] [CrossRef]
- Bessada, S.; Barreira, J.C.; Barros, L.; Ferreira, I.C.; Oliveira, M.B.P. Phenolic profile and antioxidant activity of Coleostephus myconis (L.) Rchb. F.: An underexploited and highly disseminated species. Ind. Crop. Prod. 2016, 89, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Stiernagle, T. Maintenance of C. elegans. WormBook 1999, 2, 51–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donkin, S.G.; Williams, P.L. Influence of developmental stage, salts and food presence on various end points using Caenorhabditis Elegans for aquatic toxicity testing. Environ. Toxicol. Chem. 1995, 14, 2139–2147. [Google Scholar] [CrossRef]
- Surco-Laos, F.; Cabello, J.; Gómez-Orte, E.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Dueñas, M.; Dueñas, M. Effects of O-methylated metabolites of quercetin on oxidative stress, thermotolerance, lifespan and bioavailability on Caenorhabditis elegans. Food Funct. 2011, 2, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Virk, B.; Correia, G.D.S.; Dixon, D.; Feyst, I.; Jia, J.; Oberleitner, N.; Briggs, Z.; Hodge, E.; Edwards, R.; Ward, J.M.; et al. Excessive folate synthesis limits lifespan in the C. elegans: E. coli aging model. BMC Biol. 2012, 10, 67. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- López, V.; Akerreta, S.; Casanova, E.; García-Mina, J.; Cavero, R.; Calvo, M. Screening of Spanish Medicinal Plants for Antioxidant and Antifungal Activities Screening of Spanish Medicinal Plants for Antioxidant and Antifungal Activities. Pharm. Biol. 2008, 46, 602–609. [Google Scholar] [CrossRef]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant Activity of Dietary Polyphenols As Determined by a Modified Ferric Reducing / Antioxidant Power Assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, A.; Caleja, C.; Barros, L.; Santos-Buelga, C.; Barreiro, M.F.; Ferreira, I.C. Rosemary extracts in functional foods: Extraction, chemical characterization and incorporation of free and microencapsulated forms in cottage cheese. Food Funct. 2016, 7, 2185–2196. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, G.D.A.; De Sá-Nakanishi, A.B.; Comar, J.F.; Bracht, L.; Dias, M.I.; Barros, L.; Peralta, R.M.; Ferreira, I.C.; Bracht, A. Water soluble compounds of: Rosmarinus officinalis L. improve the oxidative and inflammatory states of rats with adjuvant-induced arthritis. Food Funct. 2018, 9, 2328–2340. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Corrêa, R.C.; Barros, L.; Dias, M.I.; Calhelha, R.C.; Correa, V.G.; Bracht, A.; Peralta, R.M.; Ferreira, I.C. Effects of in vitro gastrointestinal digestion and colonic fermentation on a rosemary (Rosmarinus officinalis L.) extract rich in rosmarinic acid. Food Chem. 2019, 271, 393–400. [Google Scholar] [CrossRef] [Green Version]
- Ożarowski, M.; Mikołajczak, P.Ł.; Bogacz, A.; Gryszczyńska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Llorent-Martínez, E.J.; Spínola, V.; Gouveia, S.; Castilho, P. HPLC-ESI-MSn characterization of phenolic compounds, terpenoid saponins, and other minor compounds in Bituminaria bituminosa. Ind. Crop. Prod. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Mena, P.; Cirlini, M.; Tassotti, M.; Herrlinger, K.A.; Dall’Asta, C.; Del Rio, D. Phytochemical profiling of flavonoids, phenolic acids, terpenoids, and volatile fraction of a rosemary (Rosmarinus officinalis L.) extract. Molecules 2016, 21, 1576. [Google Scholar] [CrossRef]
- Linares, I.B.; Arráez-Román, D.; Herrero, M.; Ibanez, E.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time. J. Chromatogr. A 2011, 1218, 7682–7690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulinacci, N.; Innocenti, M.; Bellumori, M.; Giaccherini, C.; Martini, V.; Michelozzi, M. Storage method, drying processes and extraction procedures strongly affect the phenolic fraction of rosemary leaves: An HPLC/DAD/MS study. Talanta 2011, 85, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Linares, I.; Stojanovic, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis leaves as a natural source of bioactive compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Gallego, P.P.; Ferrer-Gallego, R.; Roselló, R.; Peris, J.B.; Guillén, A.; Gómez, J.; Laguna, E. A new subspecies of Rosmarinus officinalis (Lamiaceae) from the eastern sector of the Iberian Peninsula. Phytotaxa 2014, 172, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free. Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Carvalho-Costa, D.; Cavaleiro, C.; Costa, H.S.; Albuquerque, T.G.; Castilho, M.C.; Ramos, F.; Melo, N.R.; Sanches-Silva, A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.). Trends Food Sci. Technol. 2015, 45, 355–368. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Xiao, Y.; Fu, N.-L. Antioxidant capacities and total phenolic contents of 30 flowers. Ind. Crop. Prod. 2018, 111, 430–445. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (EFSA ANS Panel). Refined exposure assessment of extracts of rosemary (E 392) from its use as food additive. EFSA J. 2018, 16, e05373. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Pluci, B.; Kuczy, P. The oxidative stress in allelopathy: Participation of prenyllipid antioxidants in the response to juglone in Chlamydomonas reinhardtii. Phytochemistry 2017, 144, 171–179. [Google Scholar]
- Zamberlan, D.; Amaral, G.; Arantes, L.P.; Machado, M.L.; Mizdal, C.; De Campos, M.M.A.; Soares, F.A.A. Rosmarinus officinalis L. increases Caenorhabditis elegans stress resistance and longevity in a DAF-16, HSF-1 and SKN-1-dependent manner. Braz. J. Med. Biol. Res. 2016, 49, e5235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankel, E.N.; Huang, S.-W.; Aeschbach, R.; Prior, E. Antioxidant Activity of a Rosemary Extract and Its Constituents, Carnosic Acid, Carnosol, and Rosmarinic Acid, in Bulk Oil and Oil-in-Water Emulsion. J. Agric. Food Chem. 1996, 44, 131–135. [Google Scholar] [CrossRef]
- Lin, C.; Xiao, J.; Xi, Y.; Zhang, X.; Zhong, Q.; Zheng, H.; Cao, Y.; Chen, Y.-J. Rosmarinic acid improved antioxidant properties and healthspan via the IIS and MAPK pathways in Caenorhabditis elegans. BioFactors 2019, 45, 774–787. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Q.D.; Wang, L.; Zhang, Q.; Hua, Z.T. The Molecular Mechanism of Rosmarinic Acid Extending the Lifespan of Caenorhabditis elegans. Appl. Mech. Mater. 2011, 140, 469–472. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Sturzenbaum, S.; Menzel, R.; Steinberg, C.E.W. Hormetins, antioxidants and prooxidants: Defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Jęsko, H.; Stępień, A.; Lukiw, W.J.; Strosznajder, R.P. The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol. Neurobiol. 2019, 56, 3501–3521. [Google Scholar] [CrossRef] [Green Version]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. A thorough study of reactivity of various compound classes towards the Folin-Ciocalteu. J. Agric. Food Chem. 2014, 58, 8139–8144. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-N.; Li, S.; Li, H.-B.; Xu, N.-P.; Xu, X.-R.; Chen, F. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Foods. 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Del Bo’, C.; Bernardi, S.; Marino, M.; Porrini, M.; Tucci, M.; Guglielmetti, S.; Cherubini, A.; Carrieri, B.; Kirkup, B.M.; Kroon, P.; et al. Systematic Review on Polyphenol Intake and Health Outcomes: Is there Sufficient Evidence to Define a Health-Promoting Polyphenol-Rich Dietary Pattern? Nutrients 2019, 11, 1355. [Google Scholar]
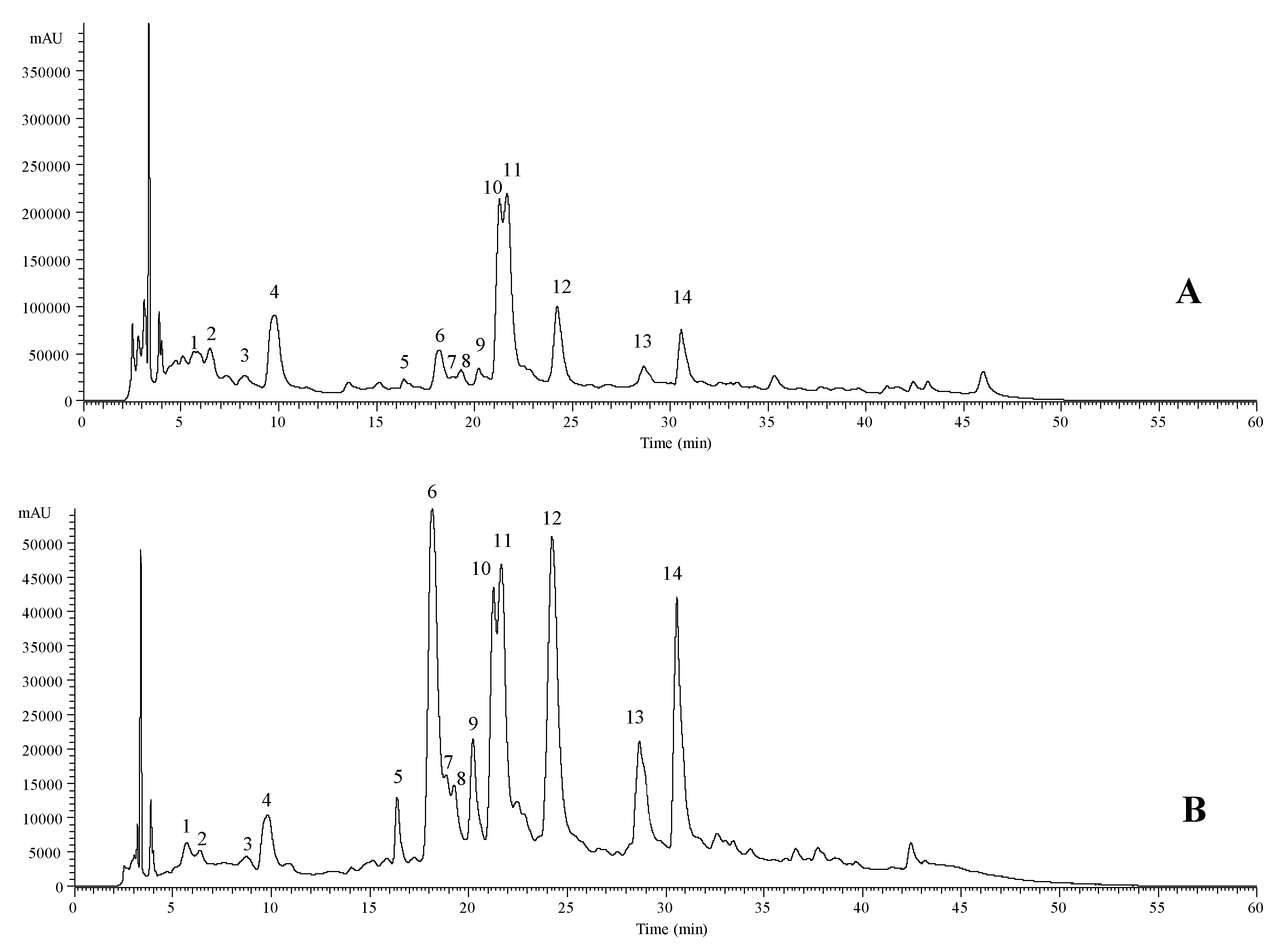

| Peak | Rt (min) | λmax (nm) | Molecular Ion [M − H]− (m/z) | MS2 (m/z) | Tentative Identification | Quantification (mg/g of Extract) |
|---|---|---|---|---|---|---|
| 1 | 5.65 | 322 | 353 | 191(20),179(57),173(100),155(5),135(10) | cis 4-O-Caffeoylquinic acid A | 0.656 ± 0.003 |
| 2 | 6.47 | 324 | 353 | 191(17),179(52),173(100),155(3),135(8) | trans 4-O-Caffeoylquinic acid A | 0.91 ± 0.03 |
| 3 | 8.27 | 312 | 387 | 207(100),179(5),163(42) | Medioresinol B | 0.28 ± 0.02 |
| 4 | 9.8 | 327 | 179 | 135(100) | Caffeic acid C | 0.76 ± 0.02 |
| 5 | 16.39 | 340 | 609 | 285(100) | Luteolin-O-di-hexoside D | 0.52 ± 0.01 |
| 6 | 18.17 | 245/266/345 | 461 | 285(100) | Luteolin-7-O-glucuronide D | 0.99 ± 0.01 |
| 7 | 18.96 | 350 | 463 | 301(100) | Quercetin-3-O-glucoside E | 0.54 ± 0.01 |
| 8 | 19.28 | 350 | 623 | 315(100),301(42) | Isorhamnetin-3-O-rutinoside E | 0.55 ± 0.01 |
| 9 | 20.26 | 350 | 477 | 315(100) | Isorhamnetin-3-O-glucoside E | 0.62 ± 0.02 |
| 10 | 21.25 | 327 | 359 | 197(36),179(42),161(100),135(5) | cis Rosmarinic acid F | 2.64 ± 0.02 |
| 11 | 21.68 | 328 | 359 | 197(33),179(44),161(100),135(5) | trans Rosmarinic acid F | 3.4 ± 0.1 |
| 12 | 24.25 | 345 | 461 | 285(100) | Luteolin-O-glucuronide D | 1.03 ± 0.01 |
| 13 | 28.68 | 332 | 503 | 285(100) | Luteolin-3′-acetyl-O-glucuronide D | 0.60 ± 0.03 |
| 14 | 30.57 | 330 | 503 | 285(100) | Luteolin-3′-acetyl-O-glucuronide D | 0.9 ± 0.1 |
| Total phenolic acids | 8.69 ± 0.05 | |||||
| Total flavonoids | 5.69 ± 0.04 | |||||
| Total phenolic compounds | 14.3 ± 0.1 | |||||
| Assay | Mean ± SEM |
|---|---|
| TPC mg PE/g extract | 48 ± 2 |
| DPPH IC50 [µg/mL] | 67 ± 5 |
| FRAP µmol Fe2+/g extract | 34 ± 2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moliner, C.; López, V.; Barros, L.; Dias, M.I.; Ferreira, I.C.F.R.; Langa, E.; Gómez-Rincón, C. Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans. Antioxidants 2020, 9, 811. https://doi.org/10.3390/antiox9090811
Moliner C, López V, Barros L, Dias MI, Ferreira ICFR, Langa E, Gómez-Rincón C. Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans. Antioxidants. 2020; 9(9):811. https://doi.org/10.3390/antiox9090811
Chicago/Turabian StyleMoliner, Cristina, Víctor López, Lillian Barros, Maria Inês Dias, Isabel C. F. R. Ferreira, Elisa Langa, and Carlota Gómez-Rincón. 2020. "Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans" Antioxidants 9, no. 9: 811. https://doi.org/10.3390/antiox9090811
APA StyleMoliner, C., López, V., Barros, L., Dias, M. I., Ferreira, I. C. F. R., Langa, E., & Gómez-Rincón, C. (2020). Rosemary Flowers as Edible Plant Foods: Phenolic Composition and Antioxidant Properties in Caenorhabditis elegans. Antioxidants, 9(9), 811. https://doi.org/10.3390/antiox9090811