Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease
Abstract
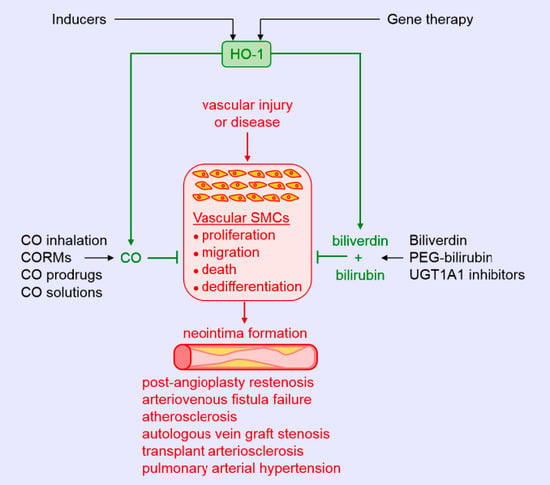
:1. Introduction
2. Regulation of HO-1 Activity and Expression
3. Effect of HO-1 on Vascular SMC Function
4. Role of HO-1 in the Arterial Response to Mechanical Injury
5. Role of HO-1 in Atherosclerosis and Transplant Arteriosclerosis
6. Role of HO-1 in Pulmonary Arterial Hypertension
7. Therapeutic Potential of HO-1 and Its Products in Vascular Remodeling
8. Conclusions
Funding
Conflicts of Interest
References
- Mazurek, R.; Dave, J.M.; Chandran, R.R.; Misra, A.; Sheikh, A.Q.; Grief, D.M. Vascular cells in blood vessel wall development and disease. Adv. Pharmacol. 2017, 78, 323–350. [Google Scholar] [PubMed] [Green Version]
- Van Varick, B.J.; Rennenberg, R.J.M.W.; Reutelingsperger, C.P.; Kroon, A.A.; de Leeuw, P.W.; Schurgers, L.J. Mechanisms of arterial remodeling: Lessons from genetic diseases. Front. Genet. 2012, 3, 290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, G.K.; Kumar, M.S.; Wamhoff, B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol. Rev. 2004, 84, 767–801. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Gomez, D. Smooth muscle cell phenotypic diversity. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1715–1723. [Google Scholar] [CrossRef]
- Dzau, V.J.; Brau-Dellaeus, R.C.; Shedding, D.G. Vascular proliferation and atherosclerosis: New perspectives and therapeutic approaches. Nat. Med. 2002, 8, 1249–1256. [Google Scholar] [CrossRef]
- Jeffrey, T.K.; Wanstall, J.C. Pulmonary vascular remodeling: A target for therapeutic intervention in pulmonary hypertension. Pharmacol. Ther. 2001, 92, 1–20. [Google Scholar] [CrossRef]
- Schiffin, E.L. Vascular remodeling in hypertension: Mechanisms and treatment. Hypertension 2012, 59, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Roostalu, U.; Wong, J.K. Arterial smooth muscle dynamics in development and repair. Dev. Biol. 2018, 435, 109–121. [Google Scholar] [CrossRef]
- Abraham, N.G.; Kappas, A. Heme oxygenase and the cardiovascular-renal system. Free Rad. Biol. Med. 2005, 39, 1–25. [Google Scholar] [CrossRef]
- Durante, W. Targeting heme oxygenase-1 in vascular disease. Curr. Drug. Targets 2010, 11, 1504–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durante, W. Protective role of heme oxygenase-1 against inflammation in atherosclerosis. Front. Biosci. 2011, 16, 2372–2388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayer, A.; Zarjou, A.; Agarwal, A.; Stocker, R. Heme oxygenase in cardiovascular health and disease. Physiol. Rev. 2016, 96, 1449–1508. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 2019. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, B.; Nemeth, Z.; Correa-Costa, M.; Bulmer, A.C.; Otterbein, L.E. Heme oxygenase-1: A metabolic nike. Antioxid. Redox Signal. 2014, 20, 1709–1722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitti, M.; Furfaro, A.L.; Mann, G.E. Heme oxygenase dependent bilirubin generation in vascular cells: A role in preventing endothelial dysfunction in local tissue environment? Front. Physiol. 2020, 11, 23. [Google Scholar] [CrossRef]
- Facchinetti, M.M. Heme oxygenase-1. Antioxid. Redox Signal. 2020, 32, 1239–1242. [Google Scholar] [CrossRef]
- Bellner, L.; Lebovics, N.B.; Rubinstein, R.; Buchen, Y.D.; Sinatra, G.; Abraham, N.G.; McClung, J.A.; Thompson, E.A. Heme oxygenase-1 upregulation: A novel approach in the treatment of cardiovascular disease. Antioxid. Redox Signal. 2020, 32, 1045–1060. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. Microsomal heme oxygenase, characterization of the enzyme. J. Biol. Chem. 1969, 44, 6388–6394. [Google Scholar]
- Kappas, A.; Drummond, G. Control of heme metabolism with synthetic metalloporphyrins. J. Clin. Investig. 1986, 77, 335–339. [Google Scholar] [CrossRef]
- Tenhunen, R.; Ross, M.E.; Marver, S.; Schmid, R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: Partial purification and characterization. Biochemistry 1970, 20, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Harrison, P.M.; Arosio, P. The ferritins: Molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta 1996, 1275, 161–203. [Google Scholar] [CrossRef] [Green Version]
- Huber, W.J., 3rd; Backes, W.L. Expression and characterization of full-length human heme oxygenase-1: The presence of intact membrane-binding region leads to increased binding affinity for NADPH cytochrome P450 reductase. Biochemistry 2007, 46, 12212–12219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Converso, D.P.; Taille, C.; Carreras, M.C.; Jaitovich, A.; Poderoso, J.J.; Boczkowski, J. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006, 20, 1236–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.P.; Wang, X.; Galbiati, F.; Ryter, S.W.; Choi, A.M. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004, 18, 1080–1089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, N.-H.; Kim, H.P.; Kim, B.-R.; Cha, S.H.; Kim, G.A.; Ha, H.; Na, Y.E.; Cha, Y.-N. Evidence for heme oxygenase-1 association with caveolin-1 and-2 in mouse mesangial cells. IUBMB Life 2003, 55, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Biswasm, C.; Shah, N.; Muthu, M.; La, P.; Fernando, A.P.; Sengupta, S.; Yang, G.; Dennery, P.A. Nuclear heme oxygenase-1 (HO-1) modulates subcellular distribution and activation of Nrf2, impacting metabolic and antioxidant defenses. J. Biol. Chem. 2014, 289, 26882–26894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taira, J.; Sugishima, M.; Kida, Y.; Oda, E.; Noguchi, M. Caveolin-1 is a competitive inhibitor of heme oxygenase-1 (HO-1) with heme: Identification of a minimum sequence in caveolin-1 for binding to HO-1. Biochemistry 2011, 50, 6824–6831. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Devesa, I. Inducers of heme oxygenase-1. Curr. Pharm. Des. 2008, 14, 473–486. [Google Scholar] [CrossRef]
- Alam, J.; Cook, J.L. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr. Pharm. Des. 2003, 9, 2499–24511. [Google Scholar] [CrossRef]
- Exner, M.; Minar, E.; Wagner, O.; Schillinger, M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Rad. Biol. Med. 2004, 37, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Goto, Y.; Takagi, S.; Baba, S.; Tago, N.; Nonogi, H.; Iwai, N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis 2004, 173, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Chen, S.M.; Wang, D.M.; Huang, X.S. 413A/T polymorphism in heme oxygenase-1 gene promoter is related to susceptibility of coronary artery disease in patients with dyslipidemia. Chin. J. Arterioscler. 2010, 18, 63–66. [Google Scholar]
- Duckers, H.J.; Boehm, M.; True, A.L.; Yet, S.-F.; Park, J.L.; Webb, R.C.; Lee, M.-E.; Nabel, G.J.; Nabel, E.G. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001, 7, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Li Volti, G.; Wang, J.; Traganos, F.; Kappas, A.; Abraham, N.G. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell progression. Biochem. Biophys. Res. Commun. 2002, 296, 1077–1088. [Google Scholar] [CrossRef]
- Cheng, C.; Haasdijk, R.A.; Tempel, D.; den Dekker, W.K.; Chriif, I.; Blonden, L.A.J.; va de Kamp, E.S.H.; de Boer, M.; Burgisser, P.E.; Noorderloos, A.; et al. PDGF_induced migration of vascular smooth muscle cells is inhibited by heme oxygenase-1 via VEGFR2 upregulation and subsequent assembly of inactive VEGFR2/PDGFRβ heterodimers. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1289–1298. [Google Scholar] [CrossRef] [Green Version]
- Peyton, K.J.; Reyna, S.V.; Chapman, G.B.; Ensenat, D.; Liu, X.; Wang, H.; Schafer, A.I.; Durante, W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood 2002, 51, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Otterbein, L.E.; Zuckerbraun, B.S.; Haga, M.; Liu, F.; Song, R.; Usheva, A.; Stachulak, C.; Bodyak, N.; Neil Smith, R.; Czismadia, E.; et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003, 9, 183–190. [Google Scholar] [CrossRef]
- Morita, T.; Perrella, M.A.; Lee, M.A.; Kourembanas, S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular smooth muscle cGMP. Proc. Natl. Acad. Sci. USA 1995, 92, 1475–1479. [Google Scholar] [CrossRef] [Green Version]
- Christodoulides, N.; Durante, W.; Kroll, M.H.; Schafer, A.I. Vascular smooth muscle cell heme oxygenases generate guanylyl cyclase-stimulatory carbon monoxide. Circulation 1995, 91, 2306–2309. [Google Scholar] [CrossRef]
- Duckles, H.; Boycott, H.E.; Al-Owais, M.M.; Elies, J.; Johnson, E.; Dallas, M.L.; Porter, K.E.; Giuntini, F.; Boyle, J.P.; Scragg, J.L.; et al. Heme oxygenase-1 regulates cell proliferation via carbon monoxide-mediated inhibition of T-type Ca2+ channels. Pflugers Arch. 2015, 467, 415–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.P.; Wang, X.; Nakao, A.; Kim, S.I.; Murase, N.; Choi, M.E.; Ryter, S.W.; Choi, A.M. Caveolin-1 expression by means of p38β mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc. Natl. Acad. Sci. USA 2005, 102, 11319–11324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ollinger, R.; Bilban, M.; Erat, A.; Froio, A.; McDaid, J.; Tyagi, S.; Csizmadia, E.; Graca-Souza, A.V.; Liloia, A.; Soares, M.P.; et al. Bilirubin: A natural inhibitor of vascular smooth muscle cell proliferation. Circulation 2005, 112, 1030–1039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, A.; Murase, N.; Ho, C.; Toyokawa, H.; Billiar, T.R.; Kanno, S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation 2005, 112, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Peyton, K.J.; Shebib, A.R.; Azam, M.A.; Liu, X.M.; Tulis, D.A.; Durante, W. Bilirubin inhibits neointima formation and vascular smooth muscle cell proliferation and migration. Front. Pharmacol. 2012, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Stoeckius, M.; Erat, A.; Fujikawa, T.; Hiromura, M.; Koulova, A.; Otterbein, L.E.; Bianchi, C.; Tobiasch, E.; Dagon, Y.; Selke, F.W.; et al. Essential roles of Raf/extracellular signal-regulated kinase/mitogen-activated protein kinase pathway, YY1, and Ca2+ influx in growth arrest of human vascular smooth muscle cells by bilirubin. J. Biol. Chem. 2012, 287, 15418–15426. [Google Scholar] [CrossRef] [Green Version]
- Ollinger, R.; Yamashita, K.; Bilban, M.; Erat, A.; Kogler, P.; Thomas, M.; Csizmadia, E.; Usheva, A.; Margreiter, R.; Bach, F.H. Bilirubin and biliverdin treatment of atherosclerotic disease. Cell Cycle 2007, 6, 39–43. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Chapman, G.B.; Wang, H.; Durante, W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation 2002, 105, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, A.I.; Gangopadhyay, A.; Kelley, E.E.; Pagano, P.J.; Zuckerbraun, B.S.; Bauer, P.M. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 98–104. [Google Scholar] [CrossRef]
- Tsai, M.H.; Lee, C.W.; Hsu, L.F.; Li, S.Y.; Chiang, Y.C.; Lee, M.H.; Chen, C.H.; Liang, H.F.; How, J.M.; Chang, P.J.; et al. CO-releasing molecules CORM2 attenuates angiotensin II-induced human aortic smooth muscle cell migration through inhibition of ROS/IL-6 generation and matrix metalloproteinase-9 expression. Redox Biol. 2017, 12, 377–388. [Google Scholar] [CrossRef]
- Liu, X.M.; Chapman, G.B.; Peyton, K.J.; Schafer, A.I.; Durante, W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2002, 55, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Chapman, G.B.; Peyton, K.J.; Schafer, A.I.; Durante, W. Antiapoptotic action of carbon monoxide in cultured vascular smooth muscle cells. Exp. Biol. Med. (Maywood) 2003, 228, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Peyton, K.J.; Ensenat, D.; Wang, H.; Schafer, A.I.; Alam, J.; Durante, W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. Role in cell survival. J. Biol. Chem. 2005, 280, 872–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Wang, H.; Ma, J.; Yao, L.; Xing, H.; Zhang, L.; Liao, L.; Zhu, D. Biliverdin reductase/bilirubin mediates the anti-apoptotic effect of hypoxia in pulmonary arterial smooth muscle cells through ERK1/2 pathway. Exp. Cell Res. 2013, 319, 1973–1987. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Peyton, K.J.; Ensenat, D.; Wang, H.; Hannink, M.; Alam, J.; Durante, W. Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival. Cardiovasc. Res. 2007, 75, 381–389. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.J.; Park, K.M.; Lee, S.; Lim, H.J.; Go, S.H.; Eom, S.M.; Park, H.Y. Preconditioning with low concentrations NO attenuates subsequent NO-induced apoptosis in vascular smooth muscle cells via HO-1-dependent mitochondrial death pathway. Toxicol. Appl. Pharmacol. 2006, 217, 176–184. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, B.H.; Chen, L.; An, W. Overexpression of heme oxygenase-1 protects smooth muscle cells against oxidative injury and inhibits cell proliferation. Cell Res. 2002, 12, 123–132. [Google Scholar] [CrossRef]
- Brunt, K.R.; Fenrich, K.K.; Kiani, G.; Tse, M.Y.; Pang, S.C.; Ward, C.A.; Melo, L.G. Protection of human vascular smooth muscle cells from H2O2-induced apoptosis through functional codependence between HO-1 and AKT. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2027–2034. [Google Scholar] [CrossRef] [Green Version]
- Gabunia, K.; Ellison, S.P.; Singh, H.; Datta, P.; Kelemen, S.E.; Rizzo, V.; Autieri, M.V. Interleukin-19 (IL-19) induces heme oxygenase-1 (HO-1) expression and decreases reactive oxygen species in human vascular smooth muscle cells. J. Biol. Chem. 2012, 287, 2477–2484. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, M.; Bockmann, S.; Hinz, B. Up-regulation of heme oxygenase-1 expression and inhibition of disease-associated features of cannabinoid in vascular smooth muscle cells. Oncotarget 2018, 9, 34595–34616. [Google Scholar] [CrossRef] [Green Version]
- Stulnig, G.; Frisch, M.T.; Crnkovic, S.; Stiegler, P.; Sereinigg, M.; Stacher, E.; Olschewski, A.; Frank, S. Docahexaenoic acid (DHA)-induced heme oxygenase-1 attenuates cytotoxic effects of DHA in vascular smooth muscle cells. Atherosclerosis 2013, 230, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tao, W.; Jiang, F.; Li, C.; Lin, J.; Liu, C. Celastrol attenuates hypertension-induced inflammation and oxidative stress in vascular smooth muscle cells via the induction of HO-1. Am. J. Hypertens. 2010, 23, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Ishizaka, N.; Taguchi, J.; Kimura, S.; Kurokawa, K.; Ohno, M. Balloon injury does not induce heme oxygenase-1 gene expression, but administration of hemin inhibits neointimal formation in balloon injured rat carotid arteries. Biochem. Biophys. Res. Commun. 1999, 261, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Togane, Y.; Toshisuki, M.; Suematsu, M.; Ishimura, Y.; Yamazaki, J.; Katayama, S. Protective roles of endogenous carbon monoxide in neointimal development elicited by arterial injury. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H623–H632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulis, D.A.; Durante, W.; Peyton, K.J.; Evans, A.J.; Schafer, A.I. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis 2001, 155, 113–122. [Google Scholar] [CrossRef]
- Hyvelin, J.M.; Maurel, B.; Uzbekov, R.; Motterlini, R.; Lermusiaux, P. Hemin prevents in-stent stenosis in rat and rabbit models by inducing heme oxygenase-1. J. Vasc. Surg. 2010, 51, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.M.; Wu, B.J.; Witting, P.K.; Stocker, R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation 2004, 110, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.M.; Azam, M.A.; Peyton, K.J.; Ensenat, D.; Keswani, A.; Wang, H.; Durante, W. Butylated hydroxyanisole stimulates heme oxygenase-1 gene expression and inhibits neointima formation in rat arteries. Cardiovasc. Res. 2007, 74, 169–174. [Google Scholar] [CrossRef]
- Oh, C.J.; Park, S.; Kim, Y.J.; Kim, H.J.; Jeong, N.H.; Choi, Y.K.; Go, Y.; Park, K.G.; Lee, I.K. Dimethylfumurate attenuates restenosis after acute vascular injury by cell-specific and Nrf2-dependent mechanisms. Redox Biol. 2014, 2, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Tulis, D.A.; Durante, W.; Liu, X.M.; Evan, A.J.; Peyton, K.J.; Schafer, A.I. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation 2001, 104, 2710–2715. [Google Scholar] [CrossRef] [Green Version]
- Exner, M.; Schillinger, M.; Minar, E.; Mlekusch, W.; Schlerka, G.; Haumer, M.; Mannhalter, C.; Wagner, O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J. Endovasc. Ther. 2001, 8, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Schillinger, M.; Exner, M.; Minar, E.; Mlekusch, W.; Mullner, M.; Mannhalter, C.; Bach, F.H.; Wagner, O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: A novel vascular protective factor. J. Am. Coll. Cardiol. 2004, 43, 950–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneda, H.; Ohno, J.; Tasguchi, J.; Togo, M.; Hashimoto, H.; Ogasawara, K.; Aizawa, T.; Ishizaka, N.; Nagai, R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1680–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-H.; Chau, L.-Y.; Lin, M.-W.; Chen, L.-C.; Yo, M.-H.; Chen, J.-W.; Lin, S.-J. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur. Heart J. 2004, 25, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tiroch, K.; Koch, W.; von Beckerath, N.; Kastrati, A.; Schomig, A. Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting. Eur. Heart J. 2007, 28, 968–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tulis, D.A.; Keswani, A.N.; Peyton, K.J.; Wang, H.; Schafer, A.I.; Durante, W. Local administration of carbon monoxide inhibits neointima formation in balloon-injured rat carotid arteries. Cell. Mol. Biol. 2005, 51, 441–446. [Google Scholar]
- Madigan, M.; Entabi, F.; Zuckerbraun, B.; Loughran, P.; Tzeng, E. Delayed inhaled carbon monoxide mediates the regression of established neointimal lesions. J. Vasc. Surg. 2015, 61, 1026–1033. [Google Scholar] [CrossRef] [Green Version]
- Bae, I.-H.; Park, D.S.; Lee, S.-Y.; Jang, E.-J.; Shim, J.-W.; Lim, K.-S.; Park, J.-K.; Kim, J.H.; Sim, D.S.; Jeong, M.H. Bilirubin coating attenuates the inflammatory response to everolimus-coated stents. J. Biomed. Mater. Res. 2018, 106B, 1486–1495. [Google Scholar] [CrossRef]
- Visner, G.A.; Lu, F.; Zhou, H.; Kazemfar, K.; Agarwal, A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: Implications in the anti-proliferative response to rapamycin. Circulation 2003, 107, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Durante, W.; Lin, C.-C. Homing in on arteriovenous fistula survival. Kidney Int. 2008, 74, 9–11. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-C.; Yang, W.-C.; Lin, S.-J.; Chen, T.-W.; Lee, W.-S.; Chang, C.-F.; Lee, P.-C.; Lee, S.-D.; Su, T.-S.; Fann, S.S.-Y.; et al. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006, 69, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Liu, X.-M.; Peyton, K.J.; Wang, H.; Yang, W.-C.; Lin, S.-J.; Durante, W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of HO-1. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 739–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Channg, C.F.; Lai, M.Y.; Chen, T.-W.; Lee, P.-C.; Yang, W.-C. Far-infrared therapy: A novel treatment to improve access blood flow and unassisted patency of arteriovenous fistula in hemodialysis patients. J. Am. Soc. Nephrol. 2007, 18, 985–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-C.; Chung, M.-Y.; Yang, W.-C.; Lin, S.-J.; Lee, P.-C. Length polymorphisms of heme oxygenase-1 determine the effect of far-infrared therapy on the function of arteriovenous fistula in hemodialysis patients: A novel physicogenomice study. Nephrol. Dial. Transplant. 2013, 28, 1284–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juncos, J.P.; Tracz, M.J.; Croatt, A.J.; Grande, J.P.; Ackerman, A.W.; Katusic, Z.S.; Nath, K.A. Genetic deficiency of heme oxygenase-1 impairs functionality and form of an arteriovenous fistula in the mouse. Kidney Int. 2008, 74, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, L.; Yamada, S.; Hernandez, M.C.; Croatt, A.J.; Grande, J.P.; Juncos, J.P.; Vercellotti, G.M.; Hebbel, R.P.; Katusic, Z.S.; Terzic, A.; et al. Regional and systemic hemodynamic responses following the creation of a murine arteriovenous fistula. Am. J. Physiol. Renal Physiol. 2011, 301, F845–F851. [Google Scholar] [CrossRef]
- Kang, L.; Grande, J.P.; Hillestad, M.L.; Croatt, A.J.; Barry, M.A.; Katusic, Z.S.; Nath, K.A. A new mouse model of an arteriovenous fistula in chronic kidney disease in the mouse: Beneficial effects of upregulated heme oxygenase-1. Am. J. Physiol. Renal Physiol. 2016, 310, F466–F476. [Google Scholar] [CrossRef] [Green Version]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R. Heart disease and stroke statistics-2017 update. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Tabas, I.; Garcia-Cardena, G.; Owens, G.K. Recent insight into the cellular biology of atherosclerosis. J. Cell. Biol. 2015, 209, 13–22. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Kondo, K.; Momiyama, Y. The protective role of heme oxygenase-1 in atherosclerotic diseases. Int. J. Mol. Med. 2019, 20, 3628. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.H.; Lin, S.Y.; Lin, M.W.; Tsai, H.L.; Kuo, S.S.; Chen, J.W.; Chang, M.J.; Wu, T.C.; Chen, L.C.; Ding, Y.A.; et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type II diabetic patients. Hum. Genet. 2002, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-H.; Chau, L.-Y.; Chen, J.-W.; Lin, S.-J. Serum bilirubin and ferritin levels link heme oxygenase-1 promoter polymorphisms and susceptibility to coronary artery disease in diabetic patients. Diabetes Care 2008, 31, 1615–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endler, G.; Exner, M.; Schillinger, M.; Marculescu, R.; Sunder-Plassmann, R.; Raith, M.; Jordanova, N.; Wojita, J.; Mannhalter, C.; Wagner, O.F.; et al. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb. Haemost. 2004, 91, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Sai, X.; Gai, L.; Huang, G.; Chen, X.; Tu, X.; Ding, Z. Association between heme oxygenase-1 gene polymorphisms and susceptibility to coronary artery disease: A HuGE review and meta-analysis. Am. J. Epidemiol. 2014, 179, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Yachie, A.; Niida, Y.; Wada, T.; Igarishi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahari, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of intimal smooth muscle cells to cholesterol accumulation and macrophage-like cells in human atherosclerosis. Circulation 2014, 129, 1551–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Dubland, J.A.; Allahverdian, S.; Asonye, E.; Sahin, B.; Jaw, J.E.; Sin, D.D.; Seidman, M.A.; Leeper, N.J.; Francis, G.A. Smooth muscle cells contribute to the majority of foam cells in ApoE (Apolipoprotein E)-deficient mouse atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 876–887. [Google Scholar] [CrossRef]
- Lin, T.-Z.; Tang, C.-H.; Hung, S.-Y.; Liu, S.-H.; Lin, Y.-M.; Fu, W.-M.; Yang, R.-S. Upregulation of heme oxygenase-1 inhibits the maturation and mineralization of osteoblasts. J. Cell Physiol. 2010, 222, 757–768. [Google Scholar] [CrossRef]
- Zarjou, A.; Jeney, V.; Arosio, P.; Poli, M.; Antal-Szalmas, P.; Agarwal, A.; Balla, G.; Jozsef, B. Ferritin prevents calcification and osteoblastic differentiation of vascular smooth muscle cells. J. Am. Soc. Nephrol. 2009, 20, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Vogel, M.E.; Idelman, G.; Konaniah, E.S.; Zucker, S.D. Bilirubin prevents atherosclerosis lesion formation in low-density lipoprotein receptor-deficient mice by inhibiting endothelial VCAM-1 and ICAM-1 signaling. J. Am. Heart Assoc. 2017, 6, e004820. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Noordeloos, A.M.; Jeney, V.; Soares, M.P.; Moll, F.; Pasterkamp, G.; Serruys, P.W.; Duckers, H.J. Heme oxygenase-1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation 2009, 119, 3017–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Tian, H.; Zhao, Y.; An, F.; Zhang, L.; Zhang, J.; Peng, J.; Zhang, Y.; Guo, Y. Heme oxygenase-1 inhibits progression and destabilization of vulnerable plaques in a rabbit model of atherosclerosis. Eur. J. Pharmacol. 2011, 672, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.; Bennett, M. The emerging role of vascular smooth muscle cell apoptosis in atherosclerosis and plaque stability. Am. J. Nephrol. 2006, 26, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Cruz, R.P.; Granville, D.J.; McManus, B.M. Allograft vasculopathy versus atherosclerosis. Circ. Res. 2006, 99, 801–815. [Google Scholar] [CrossRef] [PubMed]
- Bouche, D.; Chauveau, C.; Roussel, J.C.; Mathieu, P.; Bradeau, C.; Tesson, L.; Soulillou, J.P.; Iyer, S.; Buelow, R.; Anegon, I. Inhibition of graft arteriosclerosis development in rat aortas following heme oxygenase-1 gene transfer. Transp. Immunol. 2002, 9, 235–238. [Google Scholar] [CrossRef]
- Du, D.; Chang, S.; Chen, B.; Zhou, H.; Chen, Z.K. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transpl. Proc. 2007, 39, 3446–3468. [Google Scholar] [CrossRef]
- Chen, S.; Kapturczak, M.H.; Wasserfall, C.; Glushakova, O.; Campbell-Thompson, M.; Deshane, J.S.; Joseph, R.; Cruz, P.E.; Hauswirth, W.W.; Madsen, K.M.; et al. Interleukin-10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 7251–7256. [Google Scholar] [CrossRef] [Green Version]
- Chauveau, C.; Bouchet, D.; Roussel, J.-P.; Mathieu, P.; Braudeau, C.; Renaudin, K.; Tesson, L.; Soullilou, J.-P.; Iyer, S.; Buelow, R.; et al. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am. J. Transpl. 2002, 2, 581–592. [Google Scholar] [CrossRef]
- Yet, S.F.; Layne, M.D.; Liu, X.; Chen, Y.H.; Ith, B.; Sibinga, N.E.; Perrella, M.A. Absence of heme oxygenase-1 exacerbates atherosclerosis lesion formation and vascular remodeling. FASEB J. 2003, 17, 1759–1761. [Google Scholar] [CrossRef] [Green Version]
- Nakao, A.; Huang, C.-S.; Stolz, D.B.; Wang, Y.; Franks, J.M.; Tochigi, N.; Billiar, T.R.; Toyoda, Y.; Tzeng, E.; McCurry, K.R. Ex vivo carbon monoxide delivery inhibits intimal hyperplasia in arterialized vein grafts. Cardiovasc. Res. 2011, 89, 457–463. [Google Scholar] [CrossRef] [Green Version]
- Ramlawi, B.; Scott, J.R.; Feng, J.; Mieno, S.; Raman, K.G.; Gallo, D.; Csizmadia, E.; Chin, B.Y.; Bach, F.H.; Otterbein, L.E.; et al. Inhaled carbon monoxide prevents graft-induced intimal hyperplasia in swine. J. Surg. Res. 2007, 138, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.P.; Lin, Y.; Anrather, J.; Csizmadia, E.; Takigami, K.; Sato, K.; Grey, S.T.; Colvin, R.B.; Choi, A.M.; Poss, K.D.; et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat. Med. 1998, 4, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Hancock, W.W.; Buelow, R.; Sayegh, M.H.; Turka, L.A. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat. Med. 1998, 4, 1392–1396. [Google Scholar] [CrossRef]
- Musameh, M.D.; Green, C.J.; Mann, B.E.; Fuller, B.J.; Motterlini, R. Improved myocardial function after cold storage with preservation solution supplemented with carbon monoxide-releasing molecule (CORM3). J. Heart Lung Transpl. 2007, 26, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Loscalzo, J. Pathogenic mechanisms of pulmonary arterial hypertension. J. Mol. Cell. Cardiol. 2008, 44, 14–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabinovitch, M. Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Investig. 2012, 122, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Christou, H.; Morita, T.; Hsieh, C.-M.; Koike, H.; Arkonac, B.; Perrella, M.A.; Kourembanas, S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ. Res. 2000, 86, 1224–1229. [Google Scholar] [CrossRef] [Green Version]
- Zhen, G.; Zhang, Z.; Xu, Y. The role of endogenous carbon monoxide in the hypoxic vascular remodeling of rat model of hypoxic pulmonary hypertension. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2003, 23, 356–358. [Google Scholar] [PubMed]
- Shimzu, K.; Takahashi, T.; Iwasaki, T.; Shimzu, H.; Inoue, K.; Morimatsu, H.; Omori, E.; Matsumi, M.; Akagi, R.; Morita, K. Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med. Chem. 2008, 4, 572–576. [Google Scholar] [CrossRef] [Green Version]
- Minamino, T.; Christou, H.; Hsieh, C.M.; Liu, Y.; Dhawan, V.; Abraham, N.G.; Perrella, M.A.; Mitsialis, S.A.; Kourembanas, S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc. Natl. Acad. Sci. USA 2001, 98, 8798–8803. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Du, J.; Shi, L.; Shi, Y.; Tang, C. Effects of endogenous carbon monoxide on collagen synthesis in pulmonary artery in rats under hypoxia. Life Sci. 2004, 74, 1225–1241. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Okada, T.; Miyashita, H.; Nomoto, T.; Nonoka-Sarukawa, M.; Uchibori, R.; Maeda, Y.; Urabe, M.; Mizukami, H.; Kume, A.; et al. Interleukin-10 expression mediated by an adeno-associated virus vector prevents monocrotaline-induced pulmonary arterial hypertension in rats. Circ. Res. 2007, 101, 734–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitali, S.H.; Mitsialis, S.A.; Liang, O.D.; Liu, X.; Fernandez-Gonzalez, A.; Christou, H.; Wu, X.; McGowan, F.X.; Kourembanas, S. Divergent cardiopulmonary actions of heme oxygenase enzymatic products in chronic hypoxia. PLoS ONE 2009, 4, e5978. [Google Scholar] [CrossRef] [PubMed]
- DuBuis, E.; Potier, M.; Wang, R.; Vandier, C. Continuous inhalation of carbon monoxide attenuates hypoxic pulmonary hypertension development presumably through activation of BKCa channels. Cardiovasc. Res. 2005, 65, 751–761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuckerbraun, B.S.; Chin, B.Y.; Wegiel, B.; Billiar, T.R.; Czismadia, E.; Rao, J.; Shimoda, L.; Ifedigo, E.; Kanno, S.; Otterbein, L.E. Carbon monoxide reverses established pulmonary hypertension. J. Exp. Med. 2006, 203, 2109–2119. [Google Scholar] [CrossRef]
- Abid, S.; Houssaini, A.; Mouraret, N.; Marcos, E.; Amsellem, V.; Wan, F.; Dubois-Rande, J.L.; Derumeaux, G.; Boczkowski, J.; Motterlini, R.; et al. P21-dependent protective effects of a carbon monoxide-releasing molecule-3 in pulmonary hypertension. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, H.; Porvasnik, S.L.; Terada, N.; Agarwal, A.; Chen, Y.; Visner, G.A. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab. Investig. 2006, 86, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-H.; Zhang, Y.-J.; Liu, C.-P.; Yu, B.-X.; Lu, W.-X. Simvastatin protects against development of monocrotaline-induced pulmonary hypertension in rats via a heme oxygenase-1 dependent pathway. Exp. Lung Res. 2011, 37, 492–499. [Google Scholar] [CrossRef]
- Hsu, H.-H.; Ko, W.-J.; Hsu, J.-Y.; Chen, J.-S.; Lee, Y.-C.; Lai, I.-R.; Chen, C.-F. Simvastatin ameliorates established pulmonary hypertension through a heme oxygenase-1 dependent pathway in rats. Respir. Res. 2009, 10, 32. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, R.L.E.; Bartelds, B.; Wagener, F.A.D.T.G.; Affara, N.; Mohaupt, S.; Wijnberg, H.; Pennings, S.W.C.; Takens, J.; Berger, R.M.F. Erythropoietin attenuates pulmonary vascular remodeling in experimental pulmonary arterial hypertension through the interplay between endothelial progenitor cells and heme oxygenase. Front. Pediatr. 2015, 3, 71. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Rotenberg, M.O.; Hoshino, H.; Takaku, K.; Nakajima, O.; Muto, A.; Suzuki, H.; Tashiro, S.; Shibahara, S.; Alam, J.; et al. Hemoprotein Bach1 regulates enhancer availability of heme oxygenase-1 gene. EMBO J. 2002, 21, 5216–5224. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, Y.; Durante, W.; Lancaster, D.G.; Klattenhoff, J.; Tittel, F.K. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am. J. Physiol. Heart Circ. Physiol. 2001, 290, H483–H488. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Kulkarni, A.; Choi, K.M.; Camilleri, M.; Lempke, M.; Brunn, G.J.; Gibbons, S.J.; Zinsmeister, A.R.; Farrugia, G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin. Pharmacol. Ther. 2010, 87, 187–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreas, M.; Oeser, C.; Kainz, F.M.; Shabanian, S.; Aref, T.; Bilban, M.; Messner, B.; Heidtmann, J.; Laufer, G.; Kocher, A.; et al. Intravenous heme arginate induces HO-1 (heme oxygenase-1) in the human heart: Randomized, placebo-controlled, safety, and feasibility pharmacokinetic study. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2755–2762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bharucha, A.E.; Daley, S.L.; Low, P.A.; Gibbons, S.J.; Choi, K.M.; Camilleri, M.; Saw, J.J.; Farrugia, G.; Zinsmeister, A.R. Effect of hemin on heme oxygenase-1, gastric emptying, and symptoms in diabetic gastroparesis. Neurogastroenterol. Motil. 2016, 28, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Muhoberac, B.B.; Hanew, T.; Halter, S.; Schenker, S. A model of cytochrome-450-centerd hepatic dysfunction in drug metabolism induced by cobalt protoporphyrin administration. Biochem. Pharmacol. 1989, 38, 4103–4113. [Google Scholar] [CrossRef]
- Smith, T.J.; Drummond, G.S.; Kappas, A. Cobalt-prtoporphyrin suppresses thyroid and testicular hormone concentrations in rat serum: A novel action of this synthetic heme analogue. Pharmalogy 1987, 34, 9–16. [Google Scholar] [CrossRef]
- Bozza, M.T.; Jeney, V. Pro-inflammatory actions of heme and other hemoglobin-derived DAMPS. Front. Immunol. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Pamplona, A.; Ferreira, A.; Balla, J.; Jeney, V.; Balla, G.; Epiphanio, S.; Chora, A.; Rodrigues, C.D.; Gregoire, I.P.; Cunha-Rodrigues, M.; et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental malaria. Nat. Med. 2007, 13, 703–710. [Google Scholar] [CrossRef]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassu, A.M.; Bonaparte, D.; Marinho, M.; Cavalcante, M.M.; Chora, A.; et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010, 2, 51ra71. [Google Scholar] [CrossRef] [Green Version]
- Ogborne, R.M.; Rushworth, S.A.; Charalambos, C.A.; O’Connell, M.A. Haem oxygenase-1: A target for dietary antioxidants. Biochem. Soc. Trans. 2004, 32, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D”orazio, N.; Torella, M.; Gazzolo, D.; Li Volti, G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular disease. Antioxid. Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Raskin, P.; Toto, R.D.; Meyer, C.J.; Huff, J.W.; Grossman, E.B.; Krauth, M.; Ruiz, S.; Audhya, P.; Christ-Schmidt, H.; et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N. Eng. J. Med. 2011, 365, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Zeeuw, D.; Akizawa, T.; Audhya, P.; Bakris, G.L.; Chin, M.; Christ-Schmidt, H.; Goldsberry, A.; Houser, M.; Krauth, M.; Heerspiink, H.J.L.; et al. Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N. Eng. J. Med. 2013, 369, 2492–2503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, H.; Yamawaka, K. Bardoxolone methyl: Drug development for diabetic kidney disease. Clin. Exp. Nephrol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, R.S.; Abdel-Rahman, N. Dimethylfumarate ameliorates acetaminophen-induced hepatic injury in mice dependent on Nrf2/HO-1 pathway. Life Sci. 2019, 217, 251–260. [Google Scholar] [CrossRef]
- Behnammanesh, G.; Durante, G.L.; Khanna, Y.P.; Peyton, K.J.; Durante, W. Canagliflozin inhibits vascular smooth muscle cell proliferation and migration: Role of heme oxygenase-1. Redox Biol. 2020, 32, 101527. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Liu, X.M.; Peyton, K.J.; Wang, H.; Durante, W. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling mechanisms. Biochem. Pharmacol. 2012, 84, 1045–1054. [Google Scholar] [CrossRef] [Green Version]
- Bharucha, A.E.; Choi, K.M.; Saw, J.; Gibbons, S.J.; Farrugia, G.; Carlson, D.; Zinsmeister, A.R. Effects of aspirin & simvastatin, and aspirin, simvastatin & lipoic acid on heme oxygenase-1 in healthy human subjects. Neurogastroenterol. Motil. 2014, 26, 1437–1442. [Google Scholar]
- Mirjanic-Azaric, B.; Rizzo, M.; Jurgens, G.; Hallstroem, S.; Srdic, S.; Marc, J.; Cerne, D. Atorvastatin treatment increases plasma bilirubin but not HMOX1 expression in stable angina patients. Scand. J. Clin. Lab. Investig. 2015, 75, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C.P.; Meinel, L.; Stieger, C.; Otterbein, L.E. Where is the clinical breakthrough of HO-1/carbon monoxide therapeutics. Curr. Pharm. Des. 2018, 24, 2264–2282. [Google Scholar] [CrossRef] [PubMed]
- Wollborn, J.; Hermann, C.; Goebel, U.; Merget, B.; Wunder, C.; Maier, S.; Schafer, T.; Heuler, D.; Muller-Buschbaum, K.; Buerkle, H.; et al. Overcoming safety challenges in CO-therapy–extracorporeal CO delivery under precise feedback control of systemic carboxyhemoglobin levels. J. Control Release 2018, 279, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Motterlini, R. Interaction of carbon monoxide with transition metals: Evolutionary insights into drug development. Curr. Drug Targets 2010, 11, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Biological signaling by carbon monoxide and carbon monoxide-releasing molecules. Am. J. Physiol. Cell Physiol. 2017, 312, C302–C313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikam, A.; Ollivier, A.; Rivard, M.; Wilson, J.L.; Mebarki, K.; Martens, T.; Dubois, J.L.; Rande, R.; Motterlini, R.; Foresti, R. Diverse Nrf2 activators coordinated to cobalt carbonyls induce heme oxygenase-1 and release carbon monoxide in vitro and in vivo. J. Med. Chem. 2016, 59, 756–762. [Google Scholar] [CrossRef]
- Ali, Z.E.; Ollivier, A.; Manin, S.; Rivard, M.; Motterlini, R.; Foresti, R. Therapeutic effects of CO-releaser/Nrf2 activator hybrids (HYCOs) in the treatment of skin wound, psoriasis and multiple sclerosis. Redox Biol. 2020, 34, 101521. [Google Scholar] [CrossRef]
- Wang, D.; Viennois, E.; Ji, K.; Damera, K.; Draganov, A.; Zheng, Y.; Dai, C.; Merlin, D.; Wang, B. A click-and-release approach to CO prodrugs. Chem. Commun. 2014, 50, 1589015893. [Google Scholar] [CrossRef]
- Steiger, C.; Wollborn, J.; Gutmann, M.; Zehe, M.; Wunder, C. Controlled therapeutic gas delivery systems for quality-improved transplants. Eur. J. Pharmcol. Biopharm. 2015, 96, 95–106. [Google Scholar] [CrossRef]
- Belcher, J.D.; Gomperts, E.; Nguyen, J.; Chen, C.; Abdulla, F.; Kiser, Z.M.; Gallo, D.; Levy, H.; Otterbein, L.E. Oral carbon monoxide therapy in murine sickle cell disease: Beneficial effects on vaso-occlusion, inflammation, and anemia. PLoS ONE 2018, 13, e0205194. [Google Scholar] [CrossRef] [Green Version]
- Schwertner, H.A.; Jackson, W.G.; Tolan, G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin. Chem. 1994, 40, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin. Chem. 2000, 46, 1723–1727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwertner, H.A.; Vitek, L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: Possible protective effects and therapeutic applications of bilirubin. Atherosclerosis 2008, 198, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.Q.-H.; Carey, M.C. Therapeutic uses of animal biles in traditional Chinese medicine: An ethnopharmacological, biophysical chemical and medicinal review. World J. Gastroenterol. 2014, 20, 9952–9975. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, H.; Kang, S.; Lee, J.; Park, J.; Jon, S. Bilirubin nanoparticles as a nanomedicine for anti-inflammation. Angew. Chem. Int. Ed. 2016, 55, 7460–7463. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chando, T.; Everett, D.W.; Patten, C.J.; Dehal, S.S.; Humphreys, G. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab. Dispos. 2005, 33, 1729–1739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.-M.; Durante, Z.E.; Peyton, K.J.; Durante, W. Heme oxygenase-1-derived bilirubin counteracts HIV protease inhibitor-mediated endothelial cell dysfunction. Free Rad. Biol. Med. 2016, 94, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Dekker, D.; Dorresteijn, M.J.; Pijnenburg, M.; Heemskerk, S.; Rasing-Hoogveld, A.; Burger, D.M.; Wagener, F.A.D.T.G.; Smits, P. The bilirubin-increasing drug atazanavir improves endothelial function in patients with type 2 diabetes mellitus. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 458–463. [Google Scholar] [CrossRef] [Green Version]
- Pattanawongsa, A.; Chau, N.; Rowland, A.; Miner, J.O. Inhibition of human UDP-glucuronosyltransferase enzymes by canagliflozin and dapagliflozin: Implications for drug-drug interactions. Drug Metab. Dispos. 2015, 43, 1468–1476. [Google Scholar] [CrossRef]
- Cefalu, W.T.; Leiter, L.A.; Yoon, K.-H.; Arias, P.; Niskanen, L.; Xie, J.; Balis, D.A.; Canovatchel, W.; Meininger, G. Efficacy and safety of cangagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomized, double-blind, phase 3 non-inferiority trial. Lancet 2013, 382, 941–950. [Google Scholar] [CrossRef]
- Lavalle-Gonzalez, F.J.; Januszewicz, A.; Davidson, J.; Tong, C.; Qiu, R.; Canovatchel, W.; Meininger, G. Efficacy and safety of canagliflozin compared to placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: A randomized trial. Diabetologia 2013, 56, 2582–2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durante, W. Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease. Antioxidants 2020, 9, 829. https://doi.org/10.3390/antiox9090829
Durante W. Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease. Antioxidants. 2020; 9(9):829. https://doi.org/10.3390/antiox9090829
Chicago/Turabian StyleDurante, William. 2020. "Targeting Heme Oxygenase-1 in the Arterial Response to Injury and Disease" Antioxidants 9, no. 9: 829. https://doi.org/10.3390/antiox9090829