Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins
Abstract
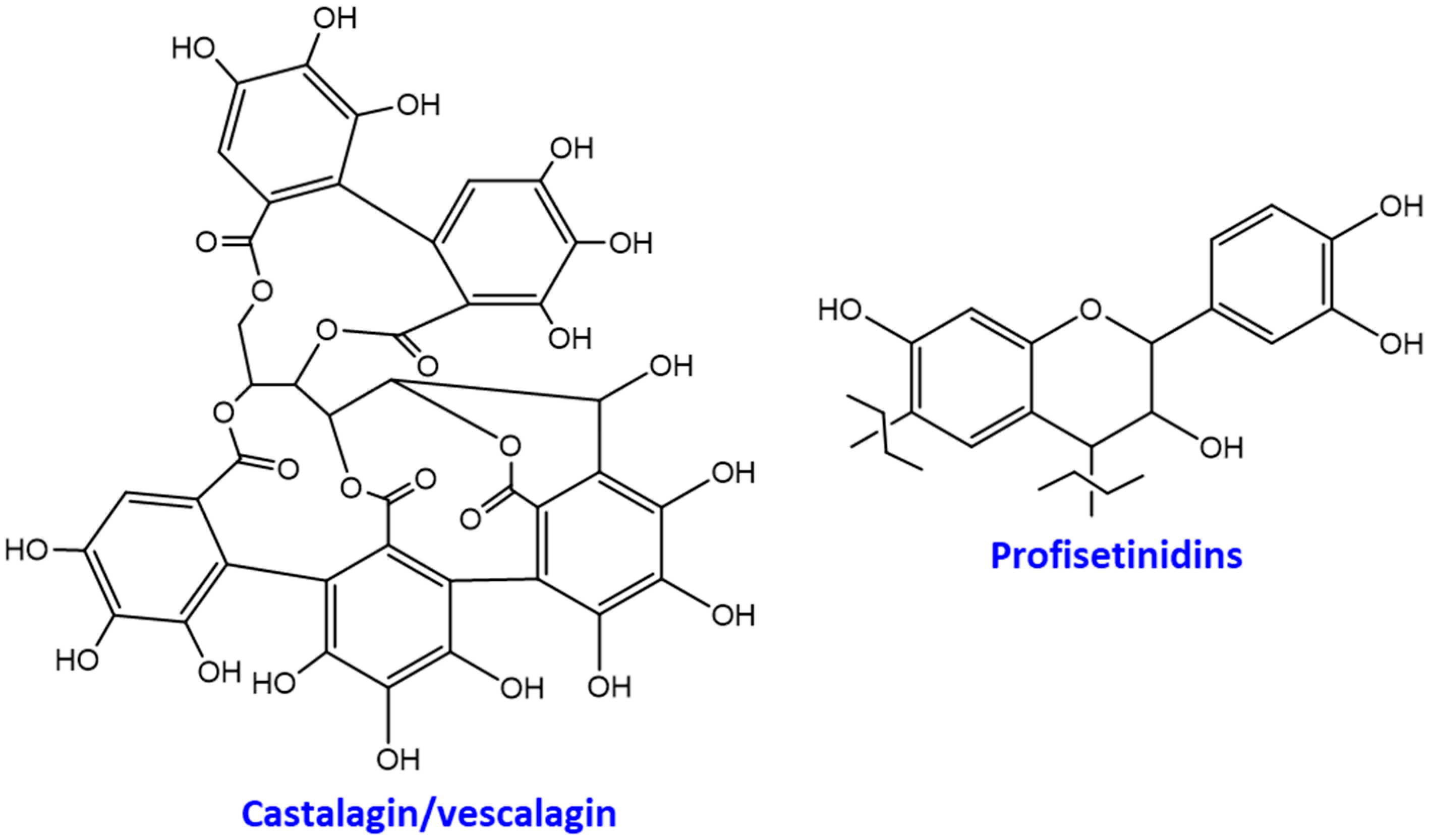
:1. Introduction
2. Materials and Methods
2.1. General Experimental Methods
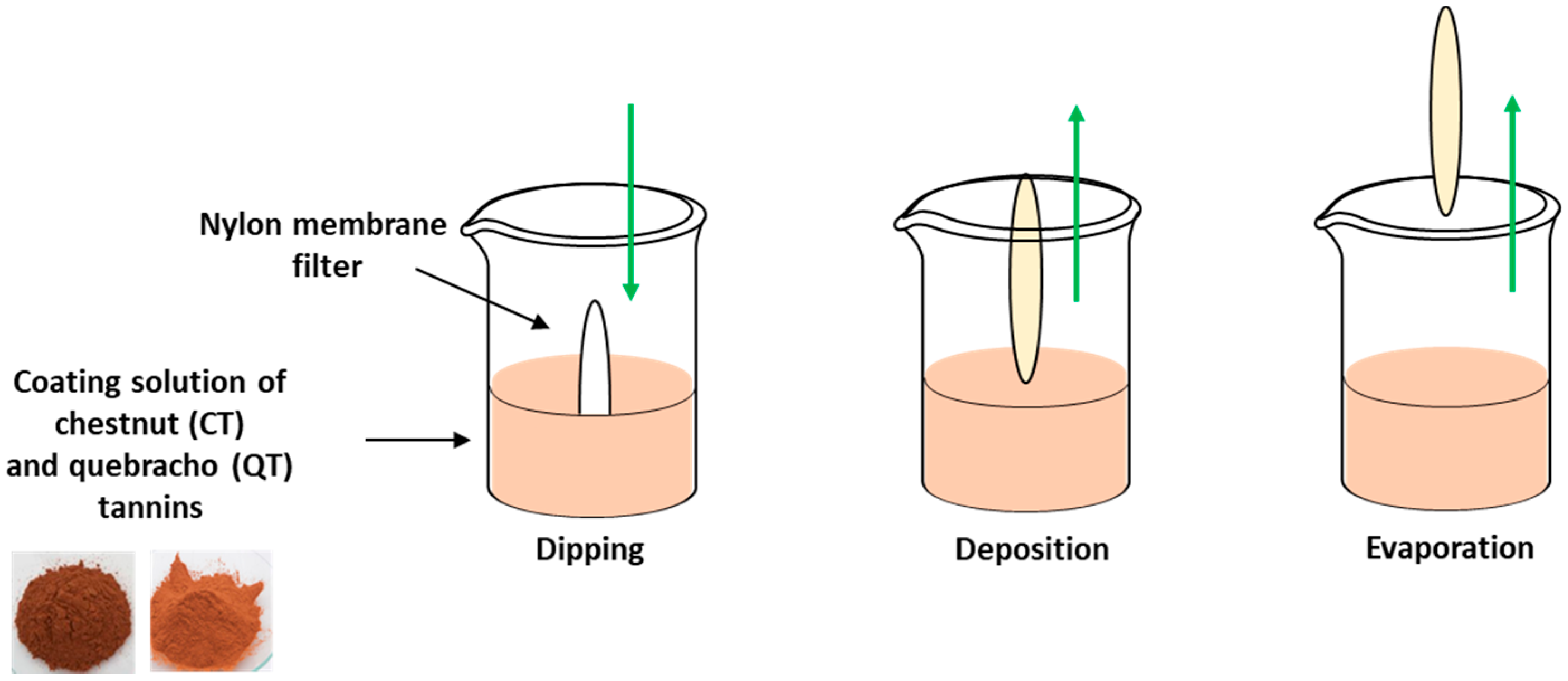
2.2. Coating of Nylon Membrane Filters with Tannins
- 7.5 mg of tannin dissolved in 15, 75 or 375 mL of distilled water
- 3.0 mg of tannin dissolved in 30 mL of distilled water containing 300 µL of a 1.7 U/mL laccase solution
- 3.0 mg of tannin dissolved in 30 mL of 0.05 M phosphate buffer (pH 6.0) containing 300 µL of a 1.7 U/mL laccase solution
- 3.0 mg of tannin dissolved in 30 mL of distilled water containing 3.5 mg of FeSO4
- 7.5 mg of tannin dissolved in 75 mL of 0.05 M carbonate buffer (pH 9.0)
- (1)
- addition of 6 M HCl until pH 2;
- (2)
- addition of NaBH4 followed or not by acidification with 6 M HCl;
- (3)
- addition of EDTA (sodium salt).
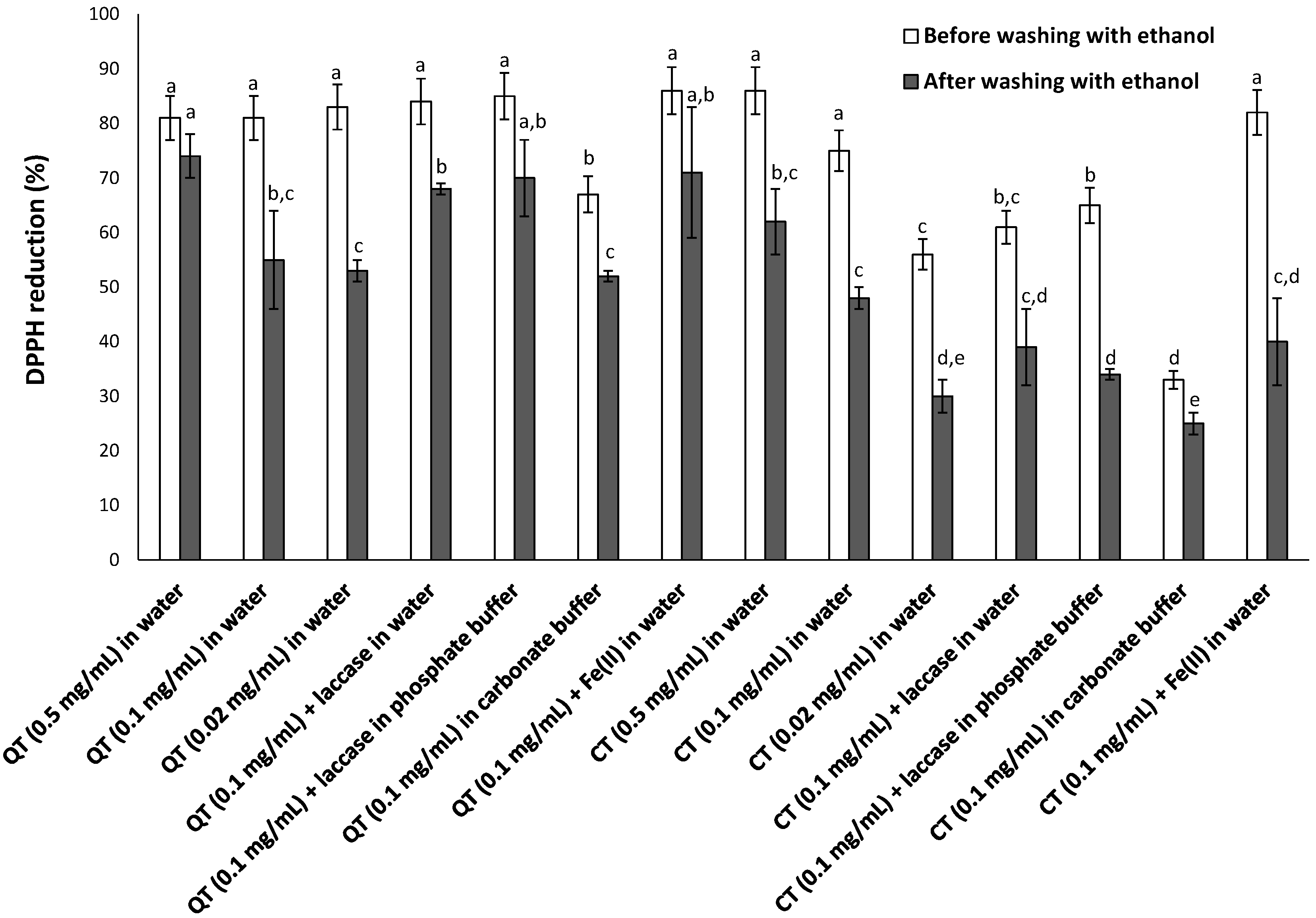
2.3. DPPH Assay
2.4. Ferric Reducing/Antioxidant Power (FRAP) Assay
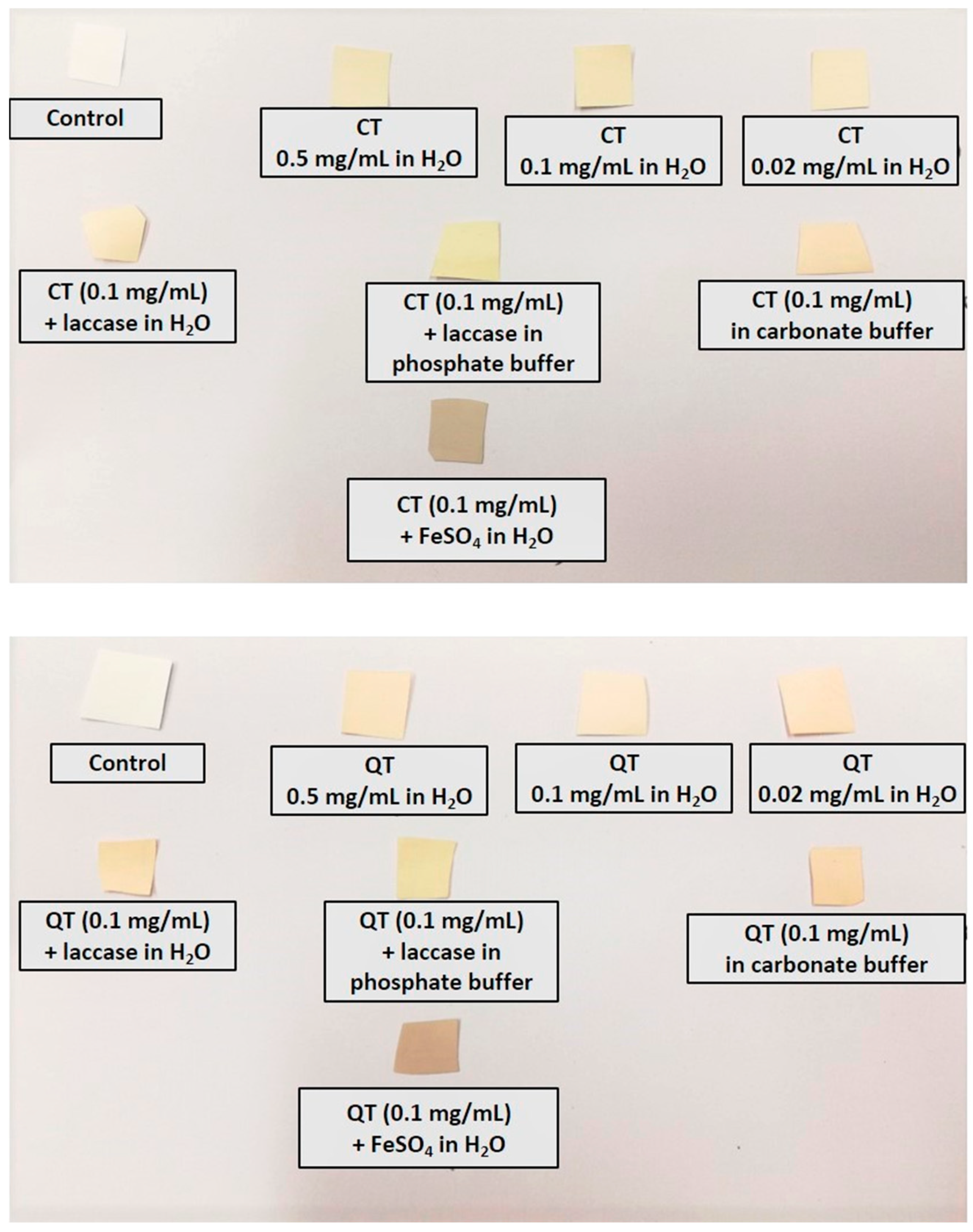
2.5. Quantification of Tannin Deposition on the Nylon Membrane Filters
- 0.5, 0.1, and 0.02 mg/mL QT or CT in distilled water
- 0.1 mg/mL QT or CT in distilled water containing 300 µL of a 1.7 U/mL laccase solution
- 0.1 mg/mL QT or CT in 0.05 M phosphate buffer (pH 6.0) containing 300 µL of a 1.7 U/mL laccase solution
- 0.1 mg/mL QT or CT in 0.05 M carbonate buffer (pH 9.0).
2.6. Statistical Analysis
3. Results and Discussion
3.1. Coating of Nylon Membrane Filters with Tannins
3.2. Antioxidant Properties of Tannin-Coated Nylon Membrane Filters
3.2.1. DPPH Assay
3.2.2. FRAP Assay
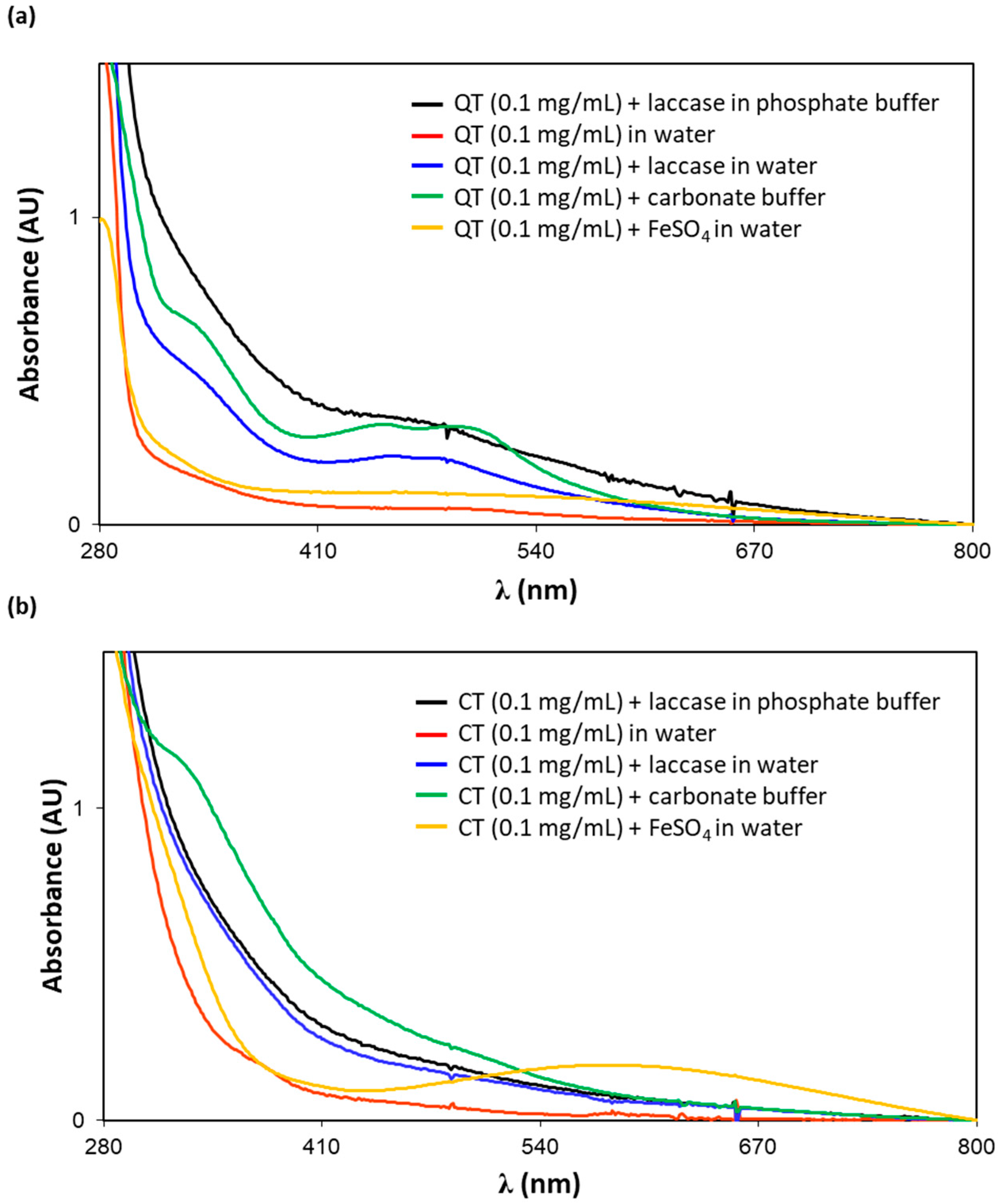
3.3. Spectrophotometric Analysis of Tannin Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Oomah, B.D.; Hernández-Arriaga, A.; Salazar-Lòpez, N.J.; Vázquez-Sánchez, K. Tannins. In Phenolic Compounds in Food: Characterization and Analysis; Nollet, L.M.L., Gutierrez-Uribe, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 211–258. [Google Scholar]
- Hussain, G.; Huang, J.; Rasul, A.; Anwar, H.; Imran, A.; Maqbool, J.; Razzaq, A.; Aziz, N.; Makhdoom, E.H.; Konuk, M.; et al. Putative roles of plant-derived tannins in neurodegenerative and neuropsychiatry disorders: An updated review. Molecules 2019, 24, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcão, L.; Araújo, M.E.M. Vegetable tannins used in the manufacture of historic leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.; Park, E.; Lee, H. Plant-inspired pyrogallol-containing functional materials. Adv. Funct. Mater. 2019, 29, 1903022. [Google Scholar] [CrossRef]
- Campo, M.; Pinelli, P.; Romani, A. Hydrolyzable tannins from sweet chestnut fractions obtained by a sustainable and eco-friendly industrial process. Nat. Prod. Commun. 2016, 11, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Karaseva, V.; Bergeret, A.; Lacoste, C.; Ferry, L.; Fulcrand, H. Influence of extraction conditions on chemical composition and thermal properties of chestnut wood extracts as tannin feedstock. ACS Sustain. Chem. Eng. 2019, 7, 17047–17054. [Google Scholar] [CrossRef]
- Reid, D.G.; Bonnet, S.L.; Kemp, G.; Van Der Westhuizen, J.H. Analysis of commercial proanthocyanidins. Part 4: Solid state 13C NMR as a tool for in situ analysis of proanthocyanidin tannins, in heartwood and bark of quebracho and acacia, and related species. Phytochemistry 2013, 94, 243–248. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Moccia, F.; Agustin-Salazar, S.; Verotta, L.; Caneva, E.; Giovando, S.; D’Errico, G.; Panzella, L.; d’Ischia, M.; Napolitano, A. Antioxidant properties of agri-food byproducts and specific boosting effects of hydrolytic treatments. Antioxidants 2020, 9, 438. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Daoud, S.; Noret, L.; Coelho, C.; Badet-Murat, M.L.; Schmitt-Kopplin, P.; Gougeon, R.D. Impact of oak wood barrel tannin potential and toasting on white wine antioxidant stability. J. Agric. Food Chem. 2019, 67, 8402–8410. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Teslić, N.; Kilmartin, P.A.; Versari, A. Suitability of the cyclic voltammetry measurements and DPPH• spectrophotometric assay to determine the antioxidant capacity of food-grade oenological tannins. Molecules 2019, 24, 2925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molino, S.; Casanova, N.A.; Rufián Henares, J.Á.; Fernandez Miyakawa, M.E. Natural tannin wood extracts as a potential food ingredient in the food industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Prospectives and actual industrial applications. Biomolecules 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sepperer, T.; Neubauer, J.; Eckardt, J.; Schnabel, T.; Petutschnigg, A.; Tondi, G. Pollutant absorption as a possible end-of-life solution for polyphenolic polymers. Polymers 2019, 11, 911. [Google Scholar] [CrossRef] [Green Version]
- Sepperer, T.; Tondi, G.; Petutschnigg, A.; Young, T.M.; Steiner, K. Mitigation of ammonia emissions from cattle manure slurry by tannins and tannin-based polymers. Biomolecules 2020, 10, 581. [Google Scholar] [CrossRef] [Green Version]
- Byrne, C.; Selmi, G.J.; D’Alessandro, O.; Deyá, C. Study of the anticorrosive properties of “quebracho colorado” extract and its use in a primer for aluminum1050. Prog. Org. Coat. 2020, 148, 105827. [Google Scholar] [CrossRef]
- Grigsby, W.; Steward, D. Applying the protective role of condensed tannins to acrylic-based surface coatings exposed to accelerated weathering. J. Polym. Environ. 2018, 26, 895–905. [Google Scholar] [CrossRef]
- Missio, A.L.; Mattos, B.D.; Ferreira, D.D.F.; Magalhães, W.L.E.; Bertuol, D.A.; Gatto, D.A.; Petutschnigg, A.; Tondi, G. Nanocellulose-tannin films: From trees to sustainable active packaging. J. Clean. Prod. 2018, 184, 143–151. [Google Scholar] [CrossRef]
- Li, P.; Sirviö, J.A.; Haapala, A.; Khakalo, A.; Liimatainen, H. Anti-oxidative and UV-absorbing biohybrid film of cellulose nanofibrils and tannin extract. Food Hydrocoll. 2019, 92, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Missio, A.L.; Mattos, B.D.; Otoni, C.G.; Gentil, M.; Coldebella, R.; Khakalo, A.; Gatto, D.A.; Rojas, O.J. Cogrinding wood fibers and tannins: Surfactant effects on the interactions and properties of functional films for sustainable packaging materials. Biomacromolecules 2020, 21, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L. Developing a bio-based packaging film from soya by-products incorporated with valonea tannin. J. Clean. Prod. 2017, 143, 624–633. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Peña-Rodriguez, C.; Martucci, J.F.; Neira, L.M.; Arbelaiz, A.; Eceiza, A.; Ruseckaite, R.A. Functional properties and in vitro antioxidant and antibacterial effectiveness of pigskin gelatin films incorporated with hydrolysable chestnut tannin. Food Sci. Technol. Int. 2015, 21, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M.G.C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Ribeiro, M.; de Sousa, T.; Poeta, P.; Bagulho, A.S.; Igrejas, G. Review of structural features and binding capacity of polyphenols to gluten proteins and peptides in vitro: Relevance to celiac disease. Antioxidants 2020, 9, 463. [Google Scholar] [CrossRef]
- Pezzella, C.; Giacobelli, V.G.; Lettera, V.; Olivieri, G.; Cicatiello, P.; Sannia, G.; Piscitelli, A. A step forward in laccase exploitation: Recombinant production and evaluation of techno-economic feasibility of the process. J. Biotechnol. 2017, 259, 175–181. [Google Scholar] [CrossRef]
- Goupy, P.; Dufour, C.; Loonis, M.; Dangles, O. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The “QUENCHER” approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Yoa, S.; Kim, J. A Study on the properties of silk and nylon 6 fabrics by tannic acid treatment. Fash. Bus. 2016, 20, 119–132. [Google Scholar] [CrossRef]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine surface chemistry: A decade of discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Ma, Y.; Zhou, F.; Hong, S.; Lee, H. Material-independent surface chemistry beyond polydopamine coating. Acc. Chem. Res. 2019, 52, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zeng, X.; Chen, H.; Li, Z.; Zeng, W.; Mei, L.; Zhao, Y. Versatile polydopamine platforms: Synthesis and promising applications for surface modification and advanced nanomedicine. ACS Nano 2019, 13, 8537–8565. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Alfieri, M.; Panzella, L.; Oscurato, S.; Salvatore, M.; Avolio, R.; Errico, M.; Maddalena, P.; Napolitano, A.; d’Ischia, M. The chemistry of polydopamine film formation: The amine-quinone interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Kim, W.I.; Youn, W.; Park, T.; Lee, S.; Kim, T.S.; Mano, J.F.; Choi, I.S. Iron gall ink revisited: In situ oxidation of Fe(II)–tannin complex for fluidic-interface engineering. Adv. Mater. 2018, 30, 1–8. [Google Scholar] [CrossRef]
- Han, S.Y.; Hong, S.P.; Kang, E.K.; Kim, B.J.; Lee, H.; Kim, W.I.; Choi, I.S. Iron gall ink revisited: Natural formulation for black hair-dyeing. Cosmetics 2019, 6, 23. [Google Scholar] [CrossRef] [Green Version]
- Andersen, A.; Chen, Y.; Birkedal, H. Bioinspired metal-polyphenol materials: Self-healing and beyond. Biomimetics 2019, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Panzella, L.; Moccia, F.; Toscanesi, M.; Trifuoggi, M.; Giovando, S.; Napolitano, A. Exhausted woods from tannin extraction as an unexplored waste biomass: Evaluation of the antioxidant and pollutant adsorption properties and activating e ects of hydrolytic treatments. Antioxidants 2019, 8, 84. [Google Scholar] [CrossRef] [Green Version]

| Sample | DPPH Reduced (%) (after 10 min) | DPPH Reduced (%) (after 2.5 h) |
|---|---|---|
| QT (0.5 mg/mL) in H2O | 85 ± 6 a | 81 ± 3 a |
| QT (0.1 mg/mL) in H2O | 83 ± 4 a | 81 ± 2 a |
| QT (0.02 mg/mL) in H2O | 56 ± 3 b | 83 ± 4 a |
| QT (0.1 mg/mL) + laccase in H2O | 79 ± 4 a | 84 ± 5 a |
| QT (0.1 mg/mL) + laccase in phosphate buffer (pH 6.0) | 61 ± 3 b | 85 ± 6 a |
| QT (0.1 mg/mL) in carbonate buffer (pH 9.0) | 41 ± 2 c | 67 ± 4 b |
| QT (0.1 mg/mL) + FeSO4 in H2O | 76 ± 4 a | 86 ± 6 a |
| CT (0.5 mg/mL) in H2O | 56 ± 3 b | 86 ± 6 a |
| CT (0.1 mg/mL) in H2O | 23 ± 1 d | 75 ± 3 a |
| CT (0.02 mg/mL) in H2O | 28 ± 1 e | 56 ± 3 c |
| CT (0.1 mg/mL) + laccase in H2O | 25 ± 1 d | 61 ± 3 b,c |
| CT (0.1 mg/mL) + laccase in phosphate buffer (pH 6.0) | 25 ± 1 d | 65 ± 4 b |
| CT (0.1 mg/mL) in carbonate buffer (pH 9.0) | 7 ± 1 f | 33 ± 2 d |
| CT (0.1 mg/mL) + FeSO4 in H2O | 40 ± 2 c | 82 ± 4 a |
| Sample | DPPH Reduced (%) 2 (after 2.5 h) | μmol Trolox/mg Sample (FRAP Assay) (after 2.5 h) |
|---|---|---|
| QT | 37 ± 2 a | 6.1 ± 0.3 a |
| CT | 47 ± 3 b | 7.0 ± 0.4 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moccia, F.; Piscitelli, A.; Giovando, S.; Giardina, P.; Panzella, L.; d’Ischia, M.; Napolitano, A. Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins. Antioxidants 2020, 9, 804. https://doi.org/10.3390/antiox9090804
Moccia F, Piscitelli A, Giovando S, Giardina P, Panzella L, d’Ischia M, Napolitano A. Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins. Antioxidants. 2020; 9(9):804. https://doi.org/10.3390/antiox9090804
Chicago/Turabian StyleMoccia, Federica, Alessandra Piscitelli, Samuele Giovando, Paola Giardina, Lucia Panzella, Marco d’Ischia, and Alessandra Napolitano. 2020. "Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins" Antioxidants 9, no. 9: 804. https://doi.org/10.3390/antiox9090804
APA StyleMoccia, F., Piscitelli, A., Giovando, S., Giardina, P., Panzella, L., d’Ischia, M., & Napolitano, A. (2020). Hydrolyzable vs. Condensed Wood Tannins for Bio-based Antioxidant Coatings: Superior Properties of Quebracho Tannins. Antioxidants, 9(9), 804. https://doi.org/10.3390/antiox9090804










