Advances on Food-Derived Peptidic Antioxidants—A Review
Abstract
1. Introduction
2. Preparation of PAs Derived from Food Proteins
2.1. Enzymatic Hydrolysis
2.2. Microbial Fermentation and Other Food Processing
3. Purification and Identification of PAs
3.1. Purification of PAs
3.2. PAs Identification and SAR
4. Strategies for Performance Evaluation of PAs
4.1. In Vitro Chemical Evaluation
4.2. In Vitro Biological Evaluation
4.3. In Vivo Evaluation
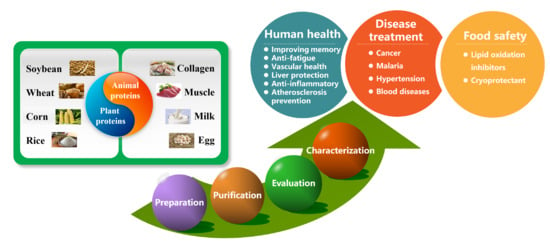
5. Potential Application of Food-Derived PAs
5.1. Functional Ingredients to Stabilize Food Quality
5.2. Human Health Promotion and Disease Treatment
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. Oxygen free radicals and iron in relation to biology and medicine: Some problems and concepts. Arch. Biochem. Biophys. 1986, 246, 501–514. [Google Scholar] [CrossRef]
- Weidinger, A.; Kozlov, A.V. Biological activities of reactive oxygen and nitrogen species: Oxidative stress versus signal transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Valencia, E.; Marin, A.; Hardy, G. Glutathione—Nutritional and pharmacological viewpoints: Part II. Nutrition 2001, 17, 485–486. [Google Scholar] [CrossRef]
- Poljšak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.W.; Zhang, J.; Townsend, D.M.; Tew, K.D. Oxidative stress, redox regulation and diseases of cellular differentiation. Biochim. Biophys. Acta 2014, 1850, 1607–1621. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Luca, M.; Luca, A.; Calandra, C. The role of oxidative damage in the pathogenesis and progression of alzheimer’s disease and vascular dementia. Oxidative Med. Cell. Longev. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Wu, J.; Huo, J.; Huang, M.; Zhao, M.; Luo, X.; Sun, B. Structural characterization of a tetrapeptide from sesame flavor-type baijiu and its preventive effects against AAPH-induced oxidative stress in HepG2 cells. J. Agric. Food Chem. 2017, 65, 10495–10504. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: Oxidants and antioxidants. Exp. Physiol. 1997, 82, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Boissonneault, G.A.; Hardwick, T.A.; Bogardus, S.L.; Chan, W.K.; Tatum, V.; Glauert, H.P.; Chow, C.K.; Decker, E.A. Interactions between carnosine and vitamin E in mammary cancer risk determination. Nutr. Res. 1998, 18, 723–733. [Google Scholar] [CrossRef]
- Rizzello, C.G.; De Angelis, M.; Di Cagno, R.; Camarca, A.; Silano, M.; Losito, I.; De Vincenzi, M.; De Bari, M.D.; Palmisano, F.; Maurano, F.; et al. Highly efficient gluten degradation by lactobacilli and fungal proteases during food processing: New perspectives for celiac disease. Appl. Environ. Microbiol. 2007, 73, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Chan, W.K.; Livisay, S.A.; Butterfield, D.A.; Faustman, C. Interactions between carnosine and the different redox states of myoglobin. J. Food Sci. 1995, 60, 1201–1204. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Nimse, S.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Yust, M.M.; Millan-Linares, M.D.; Alcaide-Hidalgo, J.M.; Millan, F.; Pedroche, J. Hypocholesterolemic and antioxidant activities of chickpea (Cicer arietinum L.) protein hydrolysates. J. Sci. Food Agric. 2012, 92, 1994–2001. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Jahandideh, F.; Wu, J. Food-derived bioactive peptides on inflammation and oxidative stress. BioMed Res. Int. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Park, S.Y.; Je, J.Y.; Ahn, C.B. Protein hydrolysates and ultrafiltration fractions obtained from krill (Euphausia superba): Nutritional, functional, antioxidant, and ACE-inhibitory characterization. J. Aquat. Food Prod. Technol. 2016, 25, 1266–1277. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Hu, F.; Ci, A.T.; Wang, H.; Zhang, Y.Y.; Zhang, J.G.; Thakur, K.; Wei, Z. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Lemes, A.C.; Sala, L.; Ores, J.D.; Braga, A.R.C.; Egea, M.B.; Fernandes, K.F. A review of the latest advances in encrypted bioactive peptides from protein-rich waste. Int. J. Mol. Sci. 2016, 17, 950. [Google Scholar] [CrossRef] [PubMed]
- Monari, S.; Ferri, M.; Russo, C.; Prandi, B.; Tedeschi, T.; Bellucci, P.; Zambrini, A.V.; Donati, E.; Tassoni, A. Enzymatic production of bioactive peptides from scotta, an exhausted by-product of ricotta cheese processing. PLoS ONE 2019, 14, e0226834. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Power, O.; Jakeman, P.M.; Fitzgerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2012, 44, 797–820. [Google Scholar] [CrossRef]
- Stefanucci, A.; Mollica, A.; Macedonio, G.; Dall’Acqua, S.; Ahmed, A.A.; Novellino, E. Exogenous opioid peptides derived from food proteins and their possible uses as dietary supplements: A critical review. Food Rev. Int. 2016, 34, 70–86. [Google Scholar] [CrossRef]
- Bamdad, F.; Wu, J.; Chen, L. Effects of enzymatic hydrolysis on molecular structure and antioxidant activity of barley hordein. J. Cereal Sci. 2011, 54, 20–28. [Google Scholar] [CrossRef]
- Shazly, A.B.; He, Z.; El-Aziz, M.A.; Zeng, M.; Zhang, S.; Qin, F.; Chen, J. Fractionation and identification of novel antioxidant peptides from buffalo and bovine casein hydrolysates. Food Chem. 2017, 232, 753–762. [Google Scholar] [CrossRef]
- Wang, B.; Gong, Y.D.; Li, Z.R.; Yu, D.; Chi, C.F.; Ma, J.Y. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Li, X.F.; Lai, F.; Xiao, X. Purification and characterization of high antioxidant peptides from duck egg white protein hydrolysates. Biochem. Biophys. Res. Commun. 2014, 452, 888–894. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Gao, J.; Xue, Z.; Zhang, Z.; Wang, H.; Wang, X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT Food Sci. Technol. 2013, 50, 591–598. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Alaiz, M.; Vioque, J. Chickpea chelating peptides inhibit copper-mediated lipid peroxidation. J. Sci. Food Agric. 2014, 94, 3181–3188. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jimenez-Aliaga, K.L.; Zavaleta, A.I.; Arnao, I.; Peña, C.; Chavez-Hidalgo, E.L.; Hernández-Ledesma, B. Erythrina edulis (pajuro) seed protein: A new source of antioxidant peptides. Nat. Prod. Commun. 2016, 11, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Chen, J.R. Isolation and characterization of peptides with antioxidant activity derived from wheat gluten. Food Sci. Technol. Res. 2002, 8, 227–230. [Google Scholar] [CrossRef]
- Zhu, B.; He, H.; Hou, T. A comprehensive review of corn protein-derived bioactive peptides: Production, characterization, bioactivities, and transport pathways. Compr. Rev. Food Sci. Food Saf. 2018, 18, 329–345. [Google Scholar] [CrossRef]
- Piu’, L.D.; Tassoni, A.; Serrazanetti, D.I.; Ferri, M.; Babini, E.; Tagliazucchi, D.; Gianotti, A. Exploitation of starch industry liquid by-product to produce bioactive peptides from rice hydrolyzed proteins. Food Chem. 2014, 155, 199–206. [Google Scholar] [CrossRef]
- Di Bernardini, R.; Harnedy, P.A.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Liu, R.; Xing, L.; Fu, Q.; Zhou, G.; Zhang, W. A review of antioxidant peptides derived from meat muscle and by-products. Antioxidants 2016, 5, 32. [Google Scholar] [CrossRef]
- Yang, J.I.; Liang, W.S.; Chow, C.J.; Siebert, K.J. Process for the production of tilapia retorted skin gelatin hydrolysates with optimized antioxidative properties. Process. Biochem. 2009, 44, 1152–1157. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Benedé, S.; Molina, E. Chicken egg proteins and derived peptides with antioxidant properties. Foods 2020, 9, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Influence of the degree of hydrolysis (DH) on antioxidant properties and radical-scavenging activities of peanut peptides prepared from fermented peanut meal. Eur. Food Res. Technol. 2011, 232, 941–950. [Google Scholar] [CrossRef]
- Kleekayai, T.; Le Gouic, A.V.; Deracinois, B.; Cudennec, B.; FitzGerald, R.J. In vitro characterisation of the antioxidative properties of whey protein hydrolysates generated under pH- and Non pH-controlled onditions. Foods 2020, 9, 582. [Google Scholar] [CrossRef]
- El-Fattah, A.M.A.; Sakr, S.S.; El-Dieb, S.M.; Elkashef, H.A.S. Bioactive peptides with ACE-I and antioxidant activity produced from milk proteolysis. Int. J. Food Prop. 2016, 20, 3033–3042. [Google Scholar] [CrossRef]
- Harnedy, P.A.; O’Keeffe, M.B.; Fitzgerald, R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017, 100, 416–422. [Google Scholar] [CrossRef]
- Zhao, Q.; Xiong, H.; Selomulya, C.; Chen, X.D.; Zhong, H.; Wang, S.; Sun, W.; Zhou, Q. Enzymatic hydrolysis of rice dreg protein: Effects of enzyme type on the functional properties and antioxidant activities of recovered proteins. Food Chem. 2012, 134, 1360–1367. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef]
- Darewicz, M.; Borawska, J.; Pliszka, M. Carp proteins as a source of bioactive peptides—An in Silico approach. Czech. J. Food Sci. 2016, 34, 111–117. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef]
- Esfandi, R.; Willmore, W.G.; Tsopmo, A. Peptidomic analysis of hydrolyzed oat bran proteins, and their in vitro antioxidant and metal chelating properties. Food Chem. 2018, 279, 49–57. [Google Scholar] [CrossRef]
- Shang, W.-H.; Tang, Y.; Su, S.-Y.; Han, J.-R.; Yan, J.-N.; Wu, H.-T.; Zhu, B.-W. In silico assessment and structural characterization of antioxidant peptides from major yolk protein of sea urchin Strongylocentrotus nudus. Food Funct. 2018, 9, 6435–6443. [Google Scholar] [CrossRef] [PubMed]
- Iwaniak, A.; Darewicz, M.; Mogut, D.; Minkiewicz, P. Elucidation of the role of in silico methodologies in approaches to studying bioactive peptides derived from foods. J. Funct. Foods 2019, 61, 103486. [Google Scholar] [CrossRef]
- Liu, J.; Jin, Y.; Lin, S.; Jones, G.S.; Chen, F. Purification and identification of novel antioxidant peptides from egg white protein and their antioxidant activities. Food Chem. 2015, 175, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Huang, J.; Chen, Y.L.; Huang, M.; Zhou, G.H. Identification and characterization of antioxidant peptides from enzymatic hydrolysates of duck meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef]
- Torres-Fuentes, C.; Contreras-Gámez, M.; Recio, I.; Alaiz, M.; Vioque, J. Identification and characterization of antioxidant peptides from chickpea protein hydrolysates. Food Chem. 2015, 180, 194–202. [Google Scholar] [CrossRef]
- Yan, Q.; Huang, L.H.; Sun, Q.; Jiang, Z.; Wu, X. Isolation, identification and synthesis of four novel antioxidant peptides from rice residue protein hydrolyzed by multiple proteases. Food Chem. 2015, 179, 290–295. [Google Scholar] [CrossRef]
- Ngoh, Y.Y.; Gan, C.Y. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Isolation, purification and characterization of antioxidative peptide of pearl millet (Pennisetum glaucum) protein hydrolysate. Food Chem. 2016, 204, 365–372. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, M.; Beta, T.; Dong, T.; Bao, X.; Li, Z. Purification and structural identification of glutelin peptides derived from oats. Cyta-J. Food 2017, 15, 508–515. [Google Scholar] [CrossRef][Green Version]
- Zhang, J.; Liu, S.; Zhang, Y.; Lu, Y.; Wang, M.; Wang, G.; Liu, X. Purification and antioxidant ability of peptide from egg in sea cucumber apostichopus japonicus. Int. J. Food Prop. 2016, 20, 306–317. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Liu, B.; Aisa, H.A.; Yili, A. Isolation and identification of two potential antioxidant peptides from sheep abomasum protein hydrolysates. Eur. Food Res. Technol. 2018, 244, 1615–1625. [Google Scholar] [CrossRef]
- Intiquilla, A.; Jimenez-Aliaga, K.L.; Guzmán, F.; Alvarez, C.A.; Zavaleta, A.I.; Izaguirre, V.; Hernández-Ledesma, B. Novel antioxidant peptides obtained by alcalase hydrolysis of Erythrina edulis (pajuro) protein. J. Sci. Food Agric. 2018, 99, 2420–2427. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2018, 120, 697–707. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, W.; Lee, J.H.; Sanjeewa, K.; Fernando, I.; Ko, S.C.; Lee, S.H.; Kim, Y.T.; Jeon, Y.J. Purification and identification of an antioxidative peptide from digestive enzyme hydrolysis of cutlassfish muscle. J. Aquat. Food Prod. Technol. 2018, 27, 934–944. [Google Scholar] [CrossRef]
- Kim, S.S.; Ahn, C.B.; Moon, S.W.; Je, J.Y. Purification and antioxidant activities of peptides from sea squirt (Halocynthia roretzi) protein hydrolysates using pepsin hydrolysis. Food Biosci. 2018, 25, 128–133. [Google Scholar] [CrossRef]
- Bordbar, S.; Ebrahimpour, A.; Zarei, M.; Hamid, A.A.; Saari, N. Alcalase-generated proteolysates of stone fish (Actinopyga lecanora) flesh as a new source of antioxidant peptides. Int. J. Food Prop. 2018, 21, 1541–1559. [Google Scholar] [CrossRef]
- Karami, Z.; Peighambardoust, S.H.; Hesari, J.; Akbari-Adergani, B. Response surface methodology to optimize hydrolysis parameters in production of antioxidant peptides from wheat germ protein by Alcalase digestion and identification of antioxidant peptides by LC-MS/MS. J. Agric. Sci. Tech. Iran. 2019, 21, 829–844. [Google Scholar]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2018, 116, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.X.; Xu, H.P.; Li, Y.; Zhang, Q.W.; Xie, H. Preparation and evaluation of peptides with potential antioxidant activity by microwave assisted enzymatic hydrolysis of collagen from sea cucumber acaudina molpadioides obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef]
- Luo, X.; Fei, Y.; Xu, Q.; Lei, T.; Mo, X.; Wang, Z.; Zhang, L.; Mou, X.; Li, H. Isolation and identification of antioxidant peptides from tartary buckwheat albumin (Fagopyrum tataricum Gaertn) and their antioxidant activities. J. Food Sci. 2020, 85, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 319, 126534. [Google Scholar] [CrossRef] [PubMed]
- Vanvi, A.; Tsopmo, A. Pepsin digested oat bran proteins: Separation, antioxidant activity, and identification of new peptides. J. Chem. 2016, 2016, 8216378. [Google Scholar] [CrossRef]
- Ran, L.Y.; Su, H.N.; Zhao, G.Y.; Gao, X.; Zhou, M.Y.; Wang, P.; Zhao, H.L.; Xie, B.B.; Zhang, X.Y.; Chen, X.L.; et al. Structural and mechanistic insights into collagen degradation by a bacterial collagenolytic serine protease in the subtilisin family. Mol. Microbiol. 2013, 90, 997–1010. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Liu, D.; Wu, R.; Wu, C.; Zhang, J.; Huang, J.; Liao, B.; He, H. Optimization of collagenase production by pseudoalteromonas sp. SJN2 and application of collagenases in the preparation of antioxidative hydrolysates. Mar. Drugs 2017, 15, 377. [Google Scholar] [CrossRef]
- Neto, Y.A.A.H.; Rosa, J.C.; Cabral, H. Peptides with antioxidant properties identified from casein, whey, and egg albumin hydrolysates generated by two novel fungal proteases. Prep. Biochem. Biotechnol. 2019, 49, 639–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef]
- Ren, Y.; Wu, H.; Li, X.; Lai, F.; Zhao, G.; Xiao, X. A two-step, one-pot enzymatic method for preparation of duck egg white protein hydrolysates with high antioxidant activity. Appl. Biochem. Biotechnol. 2013, 172, 1227–1240. [Google Scholar] [CrossRef]
- Rios, G.; Belleville, M.P.; Paolucci, D.; Marcano, J.S. Progress in enzymatic membrane reactors—A review. J. Membr. Sci. 2004, 242, 189–196. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Lv, Z.; Liu, Y.; Lu, F. Preparing oligopeptides from broken rice protein by ultrafiltration-coupled enzymatic hydrolysis. Eur. Food Res. Technol. 2013, 236, 419–424. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Claaßen, W.; Appel, D.; Rabe, S.; Stressler, T.; Fischer, L. Continuous long-term hydrolysis of wheat gluten using a principally food-grade enzyme membrane reactor system. Biochem. Eng. J. 2015, 99, 114–123. [Google Scholar] [CrossRef]
- Martin-Orue, C.; Henry, G.; Bouhallab, S. Tryptic hydrolysis of kappa-caseinomacropepitide: Control of the enzymatic reaction in a continuous membrane reactor. Enzyme Microb. Tech. 1999, 24, 173–180. [Google Scholar] [CrossRef]
- Giorno, L.; Drioli, E. Biocatalytic membrane reactors: Applications and perspectives. Trends Biotechnol. 2000, 18, 339–349. [Google Scholar] [CrossRef]
- Ewert, J.; Claaßen, W.; Stressler, T.; Fischer, L. An innovative two-step enzymatic membrane bioreactor approach for the continuous production of antioxidative casein hydrolysates with reduced bitterness. Biochem. Eng. J. 2019, 150, 107261. [Google Scholar] [CrossRef]
- Tanasković, S.J.; Luković, N.; Grbavčić, S.; Stefanović, A.; Jovanovic, J.R.; Bugarski, B.; Knežević-Jugović, Z.D. Production of egg white protein hydrolysates with improved antioxidant capacity in a continuous enzymatic membrane reactor: Optimization of operating parameters by statistical design. J. Food Sci. Technol. 2017, 55, 128–137. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, H.; Wang, J.; Xing, L. Preparation of active corn peptides from zein through double enzymes immobilized with calcium alginate–chitosan beads. Process. Biochem. 2014, 49, 1682–1690. [Google Scholar] [CrossRef]
- Neto, J.M.D.; Maciel, J.D.C.; Campos, J.F.; Junior, L.B.D.C.; Marques, D.D.A.V.; Lima, C.D.A.; Porto, A.L.F. Optimization ofPenicillium aurantiogriseumprotease immobilization on magnetic nanoparticles for antioxidant peptides’ obtainment. Prep. Biochem. Biotechnol. 2017, 47, 644–654. [Google Scholar] [CrossRef]
- Siddiqui, I.; Husain, Q.; Azam, A. Exploring the antioxidant effects of peptides from almond proteins using PAni-Ag-GONC conjugated trypsin by improving enzyme stability & applications. Int. J. Biol. Macromol. 2020, 158, 150–158. [Google Scholar] [CrossRef]
- Uluko, H.; Zhang, S.; Liu, L.; Tsakama, M.; Lu, J.; Lv, J. Effects of thermal, microwave, and ultrasound pretreatments on antioxidative capacity of enzymatic milk protein concentrate hydrolysates. J. Funct. Foods 2015, 18, 1138–1146. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, T.S.; Mu, T.H. Production and in vitro gastrointestinal digestion of antioxidant peptides from enzymatic hydrolysates of sweet potato protein affected by pretreatment. Plant. Foods Hum. Nutr. 2019, 74, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bruno, S.F.; Kudre, T.G.; Narayan, B. Effects of different pretreatments and proteases on recovery, umami taste compound contents and antioxidant potentials of Labeo rohita head protein hydrolysates. J. Food Sci. Technol. 2019, 56, 1966–1977. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S. Production and characterisation of duck albumen hydrolysate using enzymatic process. Int. J. Food Sci. Technol. 2019, 54, 3015–3023. [Google Scholar] [CrossRef]
- Zhu, K.X.; Su, C.Y.; Guo, X.N.; Peng, W.; Zhou, H.M. Influence of ultrasound during wheat gluten hydrolysis on the antioxidant activities of the resulting hydrolysate. Int. J. Food Sci. Technol. 2011, 46, 1053–1059. [Google Scholar] [CrossRef]
- Girgih, A.T.; Chao, D.; Lin, L.; He, R.; Jung, S.; Aluko, R.E. Enzymatic protein hydrolysates from high pressure-pretreated isolated pea proteins have better antioxidant properties than similar hydrolysates produced from heat pretreatment. Food Chem. 2015, 188, 510–516. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Lin, S.; Liu, X.; Yang, S.; Jones, G.S. Analysis of DPPH inhibition and structure change of corn peptides treated by pulsed electric field technology. J. Food Sci. Technol. 2014, 52, 4342–4350. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, G.H.; Qin, Z.; Li, H.; Zheng, Y. Antioxidant activity measurement and potential antioxidant peptides exploration from hydrolysates of novel continuous microwave-assisted enzymolysis of the Scomberomorus niphonius protein. Food Chem. 2017, 223, 89–95. [Google Scholar] [CrossRef]
- Ketnawa, S.; Liceaga, A.M. Effect of microwave treatments on antioxidant activity and antigenicity of fish frame protein hydrolysates. Food Bioprocess. Technol. 2016, 10, 582–591. [Google Scholar] [CrossRef]
- Kangsanant, S.; Murkovic, M.; Thongraung, C. Antioxidant and nitric oxide inhibitory activities of tilapia (Oreochromis niloticus) protein hydrolysate: Effect of ultrasonic pretreatment and ultrasonic-assisted enzymatic hydrolysis. Int. J. Food Sci. Technol. 2014, 49, 1932–1938. [Google Scholar] [CrossRef]
- Abadía-García, L.; Castaño-Tostado, E.; Ozimek, L.; Romero-Gómez, S.; Ozuna, C.; Amaya-Llano, S.L. Impact of ultrasound pretreatment on whey protein hydrolysis by vegetable proteases. Innov. Food Sci. Emerg. Technol. 2016, 37, 84–90. [Google Scholar] [CrossRef]
- Li, X.; Da, S.; Li, C.; Xue, F.; Zang, T. Effects of high-intensity ultrasound pretreatment with different levels of power output on the antioxidant properties of alcalase hydrolyzates from Quinoa (Chenopodium quinoa Willd) protein isolate. Cereal Chem. J. 2018, 95, 518–526. [Google Scholar] [CrossRef]
- Walters, M.E.; Willmore, W.G.; Tsopmo, A. Antioxidant, physicochemical, and cellular secretion of glucagon-like peptide-1 properties of oat bran protein hydrolysates. Antioxidants 2020, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Zhang, H.; Ahmed, M.S.; Wang, J. Ultrasonic pretreatment improved the antioxidant potential of enzymatic protein hydrolysates from highland barley brewer’s spent grain (BSG). J. Food Sci. 2020, 85, 1045–1059. [Google Scholar] [CrossRef]
- Liang, Q.; Ren, X.; Ma, H.; Li, S.; Xu, K.; Oladejo, A.O. Effect of low-frequency ultrasonic-assisted enzymolysis on the physicochemical and antioxidant properties of corn protein hydrolysates. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Hemker, A.K.; Nguyen, L.T.; Karwe, M.; Salvi, D. Effects of pressure-assisted enzymatic hydrolysis on functional and bioactive properties of tilapia (Oreochroniis niloticus) by-product protein hydrolysates. LWT Food Sci. Technol. 2020, 122, 109003. [Google Scholar] [CrossRef]
- Zhang, T.; Jiang, B.; Miao, M.; Mu, W.; Li, Y. Combined effects of high-pressure and enzymatic treatments on the hydrolysis of chickpea protein isolates and antioxidant activity of the hydrolysates. Food Chem. 2012, 135, 904–912. [Google Scholar] [CrossRef]
- Al-Ruwaih, N.; Ahmed, J.; Mulla, M.Z.; Arfat, Y.A. High-pressure assisted enzymatic proteolysis of kidney beans protein isolates and characterization of hydrolysates by functional, structural, rheological and antioxidant properties. LWT Food Sci. Technol. 2019, 100, 231–236. [Google Scholar] [CrossRef]
- Dong, X.H.; Li, J.; Jiang, G.X.; Li, H.Y.; Zhao, M.M.; Jiang, Y. Effects of combined high pressure and enzymatic treatments on physicochemical and antioxidant properties of peanut proteins. Food Sci. Nutr. 2019, 7, 1417–1425. [Google Scholar] [CrossRef]
- Bamdad, F.; Bark, S.; Kwon, C.H.; Suh, J.W.; Sunwoo, H. Anti-Inflammatory and antioxidant properties of peptides released from beta-Lactoglobulin by high hydrostatic pressure-assisted enzymatic hydrolysis. Molecules 2017, 22, 949. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Franck, M.; Perreault, V.; Suwal, S.; Marciniak, A.; Bazinet, L.; Doyen, A. High hydrostatic pressure-assisted enzymatic hydrolysis improved protein digestion of flaxseed protein isolate and generation of peptides with antioxidant activity. Food Res. Int. 2019, 115, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, K.; Lin, S.; Zhao, P.; Jones, G.; Trang, H.; Liu, J.; Ye, H. Improvement of antioxidant activity of peptides with molecular weights ranging from 1 to 10kDa by PEF technology. Int. J. Boil. Macromol. 2012, 51, 244–249. [Google Scholar] [CrossRef]
- Lin, S.; Guo, Y.; You, Q.; Yin, Y.; Liu, J. Preparation of antioxidant peptide from egg white protein and improvement of its activities assisted by high-intensity pulsed electric field. J. Sci. Food Agric. 2011, 92, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Jin, Y.; Liu, M.; Yang, Y.; Zhang, M.; Guo, Y.; Jones, G.; Liu, J.; Yin, Y. Research on the preparation of antioxidant peptides derived from egg white with assisting of high-intensity pulsed electric field. Food Chem. 2013, 139, 300–306. [Google Scholar] [CrossRef]
- Lin, S.; Liang, R.; Li, X.; Xing, J.; Yuan, Y. Effect of pulsed electric field (PEF) on structures and antioxidant activity of soybean source peptides-SHCMN. Food Chem. 2016, 213, 588–594. [Google Scholar] [CrossRef]
- Lin, S.; Liang, R.; Xue, P.; Zhang, S.; Liu, Z.; Dong, X. Antioxidant activity improvement of identified pine nut peptides by pulsed electric field (PEF) and the mechanism exploration. LWT Food Sci. Technol. 2017, 75, 366–372. [Google Scholar] [CrossRef]
- Zhou, Q.C.; Liu, N.; Feng, C.X. Research on the effect of papain co-extrusion on pea protein and enzymolysis antioxidant peptides. J. Food Process. Preserv. 2017, 41, e13301. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, T.; Mu, T. Production and characterisation of antioxidant peptides from sweet potato protein by enzymatic hydrolysis with radio frequency pretreatment. Int. J. Food Sci. Technol. 2019, 55, 2352–2358. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Chen, Z.; Li, Y.; Luo, X.; Li, Y. Effect of high energy electron beam on proteolysis and antioxidant activity of rice proteins. Food Funct. 2020, 11, 871–882. [Google Scholar] [CrossRef]
- Kim, J.; Whang, J.; Kim, K.; Koh, J.; Suh, H.J. Preparation of corn gluten hydrolysate with angiotensin I converting enzyme inhibitory activity and its solubility and moisture sorption. Process. Biochem. 2004, 39, 989–994. [Google Scholar] [CrossRef]
- Moreno-Montoro, M.; Olalla-Herrera, M.; Rufián-Henares, J.A.; Martínez, R.G.; Miralles, B.; Bergillos, T.; Navarro-Alarcón, M.; Jauregi, P. Antioxidant, ACE-inhibitory and antimicrobial activity of fermented goat milk: Activity and physicochemical property relationship of the peptide components. Food Funct. 2017, 8, 2783–2791. [Google Scholar] [CrossRef] [PubMed]
- Marti-Quijal, F.J.; Tornos, A.; Príncep, A.; Luz, C.; Meca, G.; Tedeschi, P.; Ruiz, M.J.; Barba, F.J. Impact of fermentation on the recovery of antioxidant bioactive compounds from sea bass byproducts. Antioxidants 2020, 9, 239. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Rajapakse, N.; Kim, S.K. Antioxidative activity of a low molecular weight peptide derived from the sauce of fermented blue mussel, Mytilus edulis. Eur. Food Res. Technol. 2004, 220, 535–539. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Chang, X.; Saito, M.; Li, Z. Changes in the radical scavenging activity of bacterial-type douchi, a traditional fermented soybean product, during the primary fermentation process. Biosci. Biotechnol. Biochem. 2009, 73, 2749–2753. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.D.F.; Da Silva, R.A.; Silva, M.F.; Da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian kefir-fermented sheep’s milk, a source of antimicrobial and antioxidant peptides. Probiotics Antimicrob. Proteins 2017, 10, 446–455. [Google Scholar] [CrossRef]
- Aspri, M.; Leni, G.; Galaverna, G.; Papademas, P. Bioactive properties of fermented donkey milk, before and after in vitro simulated gastrointestinal digestion. Food Chem. 2018, 268, 476–484. [Google Scholar] [CrossRef]
- Padghan, P.; Mann, B.; Hati, S. Purification and characterization of antioxidative peptides derived from fermented milk (lassi) by lactic cultures. Int. J. Pept. Res. Ther. 2017, 24, 235–249. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of new peptides from fermented milk showing antioxidant properties: Mechanism of action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Purification and identification of antioxidant peptides from fermented fish sauce (budu). J. Aquat. Food Prod. Technol. 2018, 28, 14–24. [Google Scholar] [CrossRef]
- Ohata, M.; Uchida, S.; Zhou, L.; Arihara, K. Antioxidant activity of fermented meat sauce and isolation of an associated antioxidant peptide. Food Chem. 2016, 194, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Kleekayai, T.; Harnedy, P.A.; O’Keeffe, M.B.; Poyarkov, A.A.; CunhaNeves, A.; Suntornsuk, W.; Fitzgerald, R.J. Extraction of antioxidant and ACE inhibitory peptides from Thai traditional fermented shrimp pastes. Food Chem. 2015, 176, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Fractionation and identification of novel antioxidant peptides from fermented fish (pekasam). J. Food Meas. Charact. 2018, 12, 2174–2183. [Google Scholar] [CrossRef]
- He, R.; Ju, X.; Yuan, J.; Wang, L.; Girgih, A.T.; Aluko, R.E. Antioxidant activities of rapeseed peptides produced by solid state fermentation. Food Res. Int. 2012, 49, 432–438. [Google Scholar] [CrossRef]
- Moayedi, A.; Hashemi, M.; Safari, M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: Optimization of fermentation conditions. J. Food Sci. Technol. 2015, 53, 391–400. [Google Scholar] [CrossRef]
- Mejri, L.; Vásquez-Villanueva, R.; Hassouna, M.; Marina, M.L.; García, M.C. Identification of peptides with antioxidant and antihypertensive capacities by RP-HPLC-Q-TOF-MS in dry fermented camel sausages inoculated with different starter cultures and ripening times. Food Res. Int. 2017, 100, 708–716. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Lu, X.; Zhang, H.; Wang, L.; Guo, X.; Qi, X.; Qian, H. Isolation and identification of an antioxidant peptide prepared from fermented peanut meal using bacillus subtilis fermentation. Int. J. Food Prop. 2014, 17, 1237–1253. [Google Scholar] [CrossRef]
- Jemil, I.; Mora, L.; Nasri, R.; Abdelhedi, O.; Aristoy, M.C.; Hajji, M.; Nasri, M.; Toldrá, F.; Mora, L. A peptidomic approach for the identification of antioxidant and ACE-inhibitory peptides in sardinelle protein hydrolysates fermented by Bacillus subtilis A26 and Bacillus amyloliquefaciens An6. Food Res. Int. 2016, 89, 347–358. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Appl. Environ. Microbiol. 2011, 78, 1087–1096. [Google Scholar] [CrossRef]
- Moslehishad, M.; Ehsani, M.R.; Salami, M.; Mirdamadi, S.; Ezzatpanah, H.; Naslaji, A.N.; Moosavi-Movahedi, A.A. The comparative assessment of ACE-inhibitory and antioxidant activities of peptide fractions obtained from fermented camel and bovine milk by Lactobacillus rhamnosus PTCC 1637. Int. Dairy J. 2013, 29, 82–87. [Google Scholar] [CrossRef]
- Raveschot, C.; Cudennec, B.; Coutte, F.; Flahaut, C.; Fremont, M.; Drider, D.; Dhulster, P. Production of bioactive peptides by lactobacillus species: From gene to application. Front. Microbiol. 2018, 9, 2354. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Mirdamadi, S.; Ehsani, M.R.; Aminlari, M.; Hosseini, E. Purification and identification of antioxidant and ACE-inhibitory peptide from Saccharomyces cerevisiae protein hydrolysate. J. Funct. Foods 2015, 19, 259–268. [Google Scholar] [CrossRef]
- Xu, F.Z.; Peng, H.H.; Li, Y.; Li, L.M.; Qian, K.; Ding, X.L. Separation and purification of small peptides from fermented sesame meal and their antioxidant activities. Protein Peptide Lett. 2014, 21, 966–974. [Google Scholar]
- Ruan, S.; Li, Y.; Wang, Y.; Huang, S.; Luo, J.; Ma, H.L. Analysis in protein profile, antioxidant activity and structure-activity relationship based on ultrasound-assisted liquid-state fermentation of soybean meal with Bacillus subtilis. Ultrason. Sonochem. 2020, 64, 104846. [Google Scholar] [CrossRef]
- Setti, K.; Kachouri, F.; Hamdi, M. Improvement of the antioxidant activity of fenugreek protein isolates by lactococcus lactis fermentation. Int. J. Pept. Res. Ther. 2017, 24, 499–509. [Google Scholar] [CrossRef]
- Toldrá, F.; Reig, M.; Aristoy, M.C.; Mora, L. Generation of bioactive peptides during food processing. Food Chem. 2018, 267, 395–404. [Google Scholar] [CrossRef]
- Minelli, A.; Conte, C.; Grottelli, S.; Bellezza, I.; Cacciatore, I.; Bolaños, J.P. Cyclo (His-Pro) promotes cytoprotection by activating Nrf2-mediated up-regulation of antioxidant defence. J. Cell. Mol. Med. 2008, 13, 1149–1161. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Zhu, C.Z.; Zhang, W.G.; Zhou, G.; Xu, X.L.; Kang, Z.L.; Yin, Y. Isolation and identification of antioxidant peptides from jinhua ham. J. Agric. Food Chem. 2013, 61, 1265–1271. [Google Scholar] [CrossRef]
- Escudero, E.; Aristoy, M.C.; Nishimura, H.; Arihara, K.; Toldrá, F. Antihypertensive effect and antioxidant activity of peptide fractions extracted from Spanish dry-cured ham. Meat Sci. 2012, 91, 306–311. [Google Scholar] [CrossRef]
- Mora, L.; Escudero, E.; Fraser, P.D.; Aristoy, M.C.; Toldrá, F.; Mora, L. Proteomic identification of antioxidant peptides from 400 to 2500 Da generated in Spanish dry-cured ham contained in a size-exclusion chromatography fraction. Food Res. Int. 2014, 56, 68–76. [Google Scholar] [CrossRef]
- Gallego, M.; Mora, L.; Toldrá, F. Characterisation of the antioxidant peptide AEEEYPDL and its quantification in Spanish dry-cured ham. Food Chem. 2018, 258, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Garem, A.; Daufin, G.; Maubois, J.L. Selective separation of amino acids with a charged inorganic nanofiltration membrane:Effect of physicochemical parameters on selectivity. Biotechnol. Bioeng. 1997, 54, 291–302. [Google Scholar] [CrossRef]
- Jemil, I.; Abdelhedi, O.; Nasri, R.; Mora, L.; Jridi, M.; Toldrá, F.; Nasri, M.; Aristoy, M.C. Novel bioactive peptides from enzymatic hydrolysate of Sardinelle (Sardinella aurita) muscle proteins hydrolysed by Bacillus subtilis A26 proteases. Food Res. Int. 2017, 100, 121–133. [Google Scholar] [CrossRef]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; Piovesana, S.; Samperi, R.; Chiozzi, R.Z.; Laganà, A. Development of an analytical strategy for the identification of potential bioactive peptides generated by in vitro tryptic digestion of fish muscle proteins. Anal. Bioanal. Chem. 2014, 407, 845–854. [Google Scholar] [CrossRef]
- Holder, A.; Merath, C.; Kulozik, U.; Hinrichs, J. Impact of diffusion, transmembrane pressure and the electrical field on peptide fractionation using cross-flow electro membrane filtration. Int. Dairy J. 2015, 46, 31–38. [Google Scholar] [CrossRef]
- Suwal, S.; Ketnawa, S.; Liceaga, A.M.; Huang, J.Y. Electro-membrane fractionation of antioxidant peptides from protein hydrolysates of rainbow trout (Oncorhynchus mykiss) byproducts. Innov. Food Sci. Emerg. Technol. 2018, 45, 122–131. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar] [CrossRef]
- Sudhakar, S.; Nazeer, R.A. Preparation of potent antioxidant peptide from edible part of shortclub cuttlefish against radical mediated lipid and DNA damage. LWT Food Sci. Technol. 2015, 64, 593–601. [Google Scholar] [CrossRef]
- Ranathunga, S.; Rajapakse, N.; Kim, S.K. Purification and characterization of antioxidative peptide derived from muscle of conger eel (Conger myriaster). Eur. Food Res. Technol. 2005, 222, 310–315. [Google Scholar] [CrossRef]
- Galli, V.; Mazzoli, L.; Luti, S.; Venturi, M.; Guerrini, S.; Paoli, P.; Vincenzini, M.; Granchi, L.; Pazzagli, L. Effect of selected strains of lactobacilli on the antioxidant and anti-inflammatory properties of sourdough. Int. J. Food Microbiol. 2018, 286, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolysates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Kurzatkowska, K.; Mielecki, M.; Grzelak, K.; Verwilst, P.; Dehaen, W.; Radecki, J.; Radecka, H. Immobilization of His-tagged kinase JAK2 onto the surface of a plasmon resonce gold disc modified with different copper (II) complexes. Talanta 2014, 130, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Canabady-Rochelle, L.L.; Selmeczi, K.; Collin, S.; Pasc, A.; Muhr, L.; Boschi-Muller, S. SPR screening of metal chelating peptides in a hydrolysate for their antioxidant properties. Food Chem. 2018, 239, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Malumba, P.; Vanderghem, C.; Deroanne, C.; Béra, F. Influence of drying temperature on the solubility, the purity of isolates and the electrophoretic patterns of corn proteins. Food Chem. 2008, 111, 564–572. [Google Scholar] [CrossRef]
- Min, S.G.; Jo, Y.J.; Park, S.H. Potential application of static hydrothermal processing to produce the protein hydrolysates from porcine skin by-products. LWT Food Sci. Technol. 2017, 83, 18–25. [Google Scholar] [CrossRef]
- Duan, X.; Ocen, D.; Wu, F.; Li, M.; Yang, N.; Xu, J.; Chen, H.; Huang, L.; Jin, Z.; Xu, X. Purification and characterization of a natural antioxidant peptide from fertilized eggs. Food Res. Int. 2014, 56, 18–24. [Google Scholar] [CrossRef]
- Zhang, M.; Mu, T.; Sun, M. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative properties of histidine-containing peptides designed from peptide fragments found in the digests of a soybean protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Yuan, H.; Lv, J.; Gong, J.; Xiao, G.; Zhu, R.; Li, L.; Qiu, J. Secondary structures and their effects on antioxidant capacity of antioxidant peptides in yogurt. Int. J. Food Prop. 2018, 21, 2167–2180. [Google Scholar] [CrossRef]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun-Waterhouse, D.; Zhao, M.; Sun, W. Beyond antioxidant actions: Insights into the antioxidant activities of tyr-containing dipeptides in aqueous solution systems and liposomal systems. Int. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process. Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Bougatef, A.; Nedjar-Arroume, N.; Manni, L.; Ravallec, R.; Barkia, A.; Guillochon, D.; Nasri, M. Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 2010, 118, 559–565. [Google Scholar] [CrossRef]
- Pouzo, L.; Descalzo, A.; Zaritzky, N.; Rossetti, L.; Pavan, E. Antioxidant status, lipid and color stability of aged beef from grazing steers supplemented with corn grain and increasing levels of flaxseed. Meat Sci. 2016, 111, 1–8. [Google Scholar] [CrossRef]
- Freitas, A.C.; Andrade, J.C.; Silva, F.; Rocha-Santos, T.; Duarte, A.C.; Gomes, A. Antioxidative peptides: Trends and perspectives for future research. Curr. Med. Chem. 2013, 20, 4575–4594. [Google Scholar] [CrossRef]
- Eftekharzadeh, B.; Khodagholi, F.; Abdi, A.; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohydr. Polym. 2010, 79, 1063–1072. [Google Scholar] [CrossRef]
- Najafian, L.; Babji, A.S. Isolation, purification and identification of three novel antioxidative peptides from patin (Pangasius sutchi) myofibrillar protein hydrolysates. LWT Food Sci. Technol. 2015, 60, 452–461. [Google Scholar] [CrossRef]
- Bamdad, F.; Chen, L.Y. Antioxidant capacities of fractionated barley hordein hydrolysates in relation to peptide structures. Mol Nutr Food Res. 2013, 57, 493–503. [Google Scholar] [CrossRef]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Dziuba, J.; Iwaniak, A.; Dziuba, M.; Darewicz, M. BIOPEP database and other programs for processing bioactive peptide sequences. J. AOAC Int. 2008, 91, 965–980. [Google Scholar] [CrossRef]
- Tian, M.; Fang, B.; Jiang, L.; Guo, H.Y.; Cui, J.Y.; Ren, F.Z. Structure-activity relationship of a series of antioxidant tripeptides derived from beta-Lactoglobulin using QSAR modeling. Dairy Sci. Technol. 2015, 95, 451–463. [Google Scholar] [CrossRef]
- Huang, B.-B.; Lin, H.C.; Chang, Y.-W. Analysis of proteins and potential bioactive peptides from tilapia (Oreochromis spp.) processing co-products using proteomic techniques coupled with BIOPEP database. J. Funct. Foods 2015, 19, 629–640. [Google Scholar] [CrossRef]
- Deng, B.C.; Long, H.; Tang, T.; Ni, X.; Chen, J.; Yang, G.; Zhang, F.; Cao, R.; Cao, D.S.; Zeng, M.; et al. Quantitative structure-activity relationship study of antioxidant tripeptides based on model population analysis. Int. J. Mol. Sci. 2019, 20, 995. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; He, Q.; Zhang, Y.; Hu, Y.; Wang, Y.; Lin, Z. In silico rational design and virtual screening of antixoidant tripeptides based on 3D-QSAR modeling. J. Mol. Struct. 2019, 1193, 223–230. [Google Scholar] [CrossRef]
- Leung, R.; Venus, C.; Zeng, T.; Tsopmo, A. Structure-function relationships of hydroxyl radical scavenging and chromium-VI reducing cysteine-tripeptides derived from rye secalin. Food Chem. 2018, 254, 165–169. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, E.; Simpson, B.K. Generation of antioxidative peptides from Atlantic sea cucumber using alcalase versus trypsin: In vitro activity, de novo sequencing, and in silico docking for in vivo function prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Wu, R.; Wu, C.; Liu, D.; Yang, X.; Huang, J.; Zhang, J.; Liao, B.; He, H.; Li, H. Overview of antioxidant peptides derived from marine resources: The sources, characteristic, purification, and evaluation methods. Appl. Biochem. Biotechnol. 2015, 176, 1815–1833. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Y.; Zhu, D.Y.; Thakur, K.; Li, L.; Zhang, J.-G.; Wei, Z. Thermal and antioxidant properties of polysaccharides sequentially extracted from mulberry leaves (morus alba L.). Molecules 2017, 22, 2271. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A.; Welch, B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990, 38, 674–677. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfafa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Baron, C.P.; Nielsen, N.S.; Jacobsen, C. Antioxidant activity of yoghurt peptides: Part 1-in vitro assays and evaluation in w3 enriched milk. Food Chem. 2010, 123, 1081–1089. [Google Scholar] [CrossRef]
- Jin, D.X.; Liu, X.L.; Zheng, X.Q.; Wang, X.J.; He, J. Preparation of antioxidative corn protein hydrolysates, purification and evaluation of three novel corn antioxidant peptides. Food Chem. 2016, 204, 427–436. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Marina, M.L.; García, M.C. Identification by hydrophilic interaction and reversed-phase liquid chromatography–tandem mass spectrometry of peptides with antioxidant capacity in food residues. J. Chromatogr. A 2016, 1428, 185–192. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Becerra, L.; Cervantes, E.L. Antioxidant activity of hydrolysates and peptide fractions of glutelin from cocoa (Theobroma cacao L) seed. Cyta-J. Food 2017, 15, 489–496. [Google Scholar] [CrossRef]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef] [PubMed]
- Cheli, F.; Baldi, A. Nutrition-based health: Cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef]
- Liu, R.H.; Finley, J. Potential cell culture models for antioxidant research. J. Agric. Food Chem. 2005, 53, 4311–4314. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of Antioxidant Capacity in vitro and in vivo. Free. Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, M.; Huang, L.; Wang, J.; Gong, L.; Liu, J.; Sun, B. Evaluation of the cellular and animal models for the study of antioxidant activity: A review. J. Food Sci. 2017, 82, 278–288. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and metabolism of peptide WDHHAPQLR derived from rapeseed protein and inhibition of huvec apoptosis under oxidative stress. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, T.; Jiang, B.; Miao, M.; Mu, W. The effects of an antioxidative pentapeptide derived from chickpea protein hydrolysates on oxidative stress in Caco-2 and HT-29 cell lines. J. Funct. Foods 2014, 7, 719–726. [Google Scholar] [CrossRef]
- Yi, G.; Din, J.U.; Zhao, F.; Liu, X. Effect of soybean peptides against hydrogen peroxide induced oxidative stress in HepG2 cells via Nrf2 signaling. Food Funct. 2020, 11, 2725–2737. [Google Scholar] [CrossRef]
- Wang, J.; Liao, W.; Nimalaratne, C.; Chakrabarti, S.; Wu, J. Purification and characterization of antioxidant peptides from cooked eggs using a dynamic in vitro gastrointestinal model in vascular smooth muscle A7r5 cells. NPJ Sci. Food 2018, 2, 7. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.R.; Jang, A. Anti-proliferative effect of a novel anti-oxidative peptide in hanwoo beef on human colorectal carcinoma cells. Food Sci. Anim. Resour. 2018, 38, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.X.; Jin, M.M.; Zhang, L.; Yu, H.X.; Sun, Z.; Lu, R.R. Hydrophobicity of whey protein hydrolysates enhances the protective effect against oxidative damage on PC 12 cells. J. Dairy Res. 2014, 82, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, S.; Nazeer, R.A. Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods 2015, 14, 502–512. [Google Scholar] [CrossRef]
- Ma, H.; Liu, R.; Zhao, Z.; Zhang, Z.; Cao, Y.; Ma, Y.; Guo, Y.; Xu, L. A novel peptide from soybean protein isolate significantly enhances resistance of the organism under oxidative stress. PLoS ONE 2016, 11, e0159938. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Lin, S.; Ye, H.; Chen, F. In vitroantioxidant activities of the novel pentapeptides Ser-His-Glu-Cys-Asn and Leu-Pro-Phe-Ala-Met and the relationship between activity and peptide secondary structure. J. Sci. Food Agric. 2016, 97, 1945–1952. [Google Scholar] [CrossRef]
- Yang, R.; Li, X.; Lin, S.; Zhang, Z.; Chen, F. Identification of novel peptides from 3 to 10kDa pine nut (Pinus koraiensis) meal protein, with an exploration of the relationship between their antioxidant activities and secondary structure. Food Chem. 2017, 219, 311–320. [Google Scholar] [CrossRef]
- Liang, R.; Zhang, Z.; Lin, S. Effects of pulsed electric field on intracellular antioxidant activity and antioxidant enzyme regulating capacities of pine nut (Pinus koraiensis) peptide QDHCH in HepG2 cells. Food Chem. 2017, 237, 793–802. [Google Scholar] [CrossRef]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef]
- Yang, J.; Hu, L.; Cai, T.; Chen, Q.; Ma, Q.; Yang, J.; Meng, C.; Hong, J. Purification and identification of two novel antioxidant peptides from perilla (Perilla frutescens L. Britton) seed protein hydrolysates. PLoS ONE 2018, 13, e0200021. [Google Scholar] [CrossRef]
- Guo, X.; Shang, W.; Strappe, P.M.; Zhou, Z.; Blanchard, C. Peptides derived from lupin proteins confer potent protection against oxidative stress. J. Sci. Food Agric. 2018, 98, 5225–5234. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Li, Y.; Wang, H.; Wang, Z.; Qi, B.; Sui, X.; Jiang, L. Purification and characterization of antioxidant peptides from alcalase-hydrolyzed soybean (Glycine max L.) hydrolysate and their cytoprotective effects in human intestinal Caco-2 cells. J. Agric. Food Chem. 2019, 67, 5772–5781. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, D.; Sun, J.; Luo, X.; Li, H.; Sun, X.; Zheng, F. Analysis of antioxidant effect of two tripeptides isolated from fermented grains (Jiupei) and the antioxidative interaction with 4-methylguaiacol, 4-ethylguaiacol, and vanillin. Food Sci. Nutr. 2019, 7, 2391–2403. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative effects and mechanism study of bioactive peptides from defatted walnut (Juglans regia L.) meal hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Tang, X.; Ren, Y.; Wang, E.; Shi, L.; Wu, X.Y.; Wu, H. Novel antioxidant peptides purified from mulberry (Morus Atropurpurea Roxb.) leaf protein hydrolysates with hemolysis inhibition ability and cellular antioxidant activity. J. Agric. Food Chem. 2019, 67, 7650–7659. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Wang, Y.; Yu, Y.; Liu, J.; Liu, B.; Zhang, T. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H2O2 -induced cell damage. Int. J. Food Sci. Technol. 2019, 54, 2219–2227. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Lin, S.J.; Yang, Z.; Jin, H.X. Preparation of antioxidant peptide by microwave- assisted hydrolysis of collagen and its protective effect against H2O2-induced damage of RAW264.7 cells. Mar. Drugs 2019, 17, 642. [Google Scholar] [CrossRef]
- Wang, W.Y.; Sun, K.L.; Zhao, G.X.; Chi, C.F.; Wang, B. Antioxidant peptides from collagen hydrolysate of redlip croaker (Pseudosciaena polyactis) scales: Preparation, characterization, and cytoprotective effects on H2O2-damaged HepG2 cells. Mar. Drugs 2020, 18, 156. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Bettiol, M.D.R.; Braber, N.L.V.; Aminahuel, C.A.; Rossi, Y.E.; Petroselli, G.; Erra-Balsells, R.; Cavaglieri, L.R.; Montenegro, M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020, 319, 126472. [Google Scholar] [CrossRef]
- Lin, X.; Yao, M.; Lu, J.H.; Wang, Y.; Yin, X.; Liang, M.; Yuan, E.; Ren, J. Identification of novel oligopeptides from the simulated digestion of sea cucumber (Stichopus japonicus) to alleviate Aβ aggregation progression. J. Funct. Foods 2019, 60, 103412. [Google Scholar] [CrossRef]
- Vilcacundo, R.; Martínez-Villaluenga, C.; Miralles, B.; Hernández-Ledesma, B. Release of multifunctional peptides from kiwicha (Amaranthus caudatus) protein under in vitro gastrointestinal digestion. J. Sci. Food Agric. 2018, 99, 1225–1232. [Google Scholar] [CrossRef]
- Zhang, J.; Du, H.; Zhang, G.; Kong, F.; Hu, Y.; Xiong, S.; Zhao, S. Identification and characterization of novel antioxidant peptides from crucian carp (Carassius auratus) cooking juice released in simulated gastrointestinal digestion by UPLC-MS/MS and in silico analysis. J. Chromatogr. B 2020, 1136, 121893. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Marina, M.L.; García, M.C. Isolation and identification of antioxidant peptides from commercial soybean-based infant formulas. Food Chem. 2014, 148, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Li, C.; Duan, Y.; Zhang, H.; Ma, H. Antioxidant peptide fractions isolated from wheat germ protein with subcritical water extraction and its transport across Caco-2 cells. J. Food Sci. 2019, 84, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Xie, N.; Wang, B.; Jiang, L.; Liu, C.; Li, B. Hydrophobicity exerts different effects on bioavailability and stability of antioxidant peptide fractions from casein during simulated gastrointestinal digestion and Caco-2 cell absorption. Food Res. Int. 2015, 76, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, T.; Muraoka, I. Exercise-induced oxidative stress and the effects of antioxidant intake from a physiological viewpoint. Antioxidants 2018, 7, 119. [Google Scholar] [CrossRef] [PubMed]
- Ben Khaled, H.; Ghlissi, Z.; Chtourou, Y.; Hakim, A.; Ktari, N.; Fatma, M.A.; Barkia, A.; Sahnoun, Z.; Nasri, M. Effect of protein hydrolysates from sardinelle (Sardinella aurita) on the oxidative status and blood lipid profile of cholesterol-fed rats. Food Res. Int. 2012, 45, 60–68. [Google Scholar] [CrossRef]
- Ben Khaled, H.; Ktari, N.; Ghorbel-Bellaaj, O.; Jridi, M.; Lassoued, I.; Nasri, M. Composition, functional properties and in vitro antioxidant activity of protein hydrolysates prepared from sardinelle (Sardinella aurita) muscle. J. Food Sci. Technol. 2011, 51, 622–633. [Google Scholar] [CrossRef]
- Ding, J.F.; Li, Y.Y.; Xu, J.J.; Su, X.; Gao, X.; Yue, F.P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidation. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, D.; Guo, J.; Zhang, Y.; Zheng, B. In vitro antioxidant activity and in vivo anti-fatigue effect of sea horse (Hippocampus) peptides. Molecules 2017, 22, 482. [Google Scholar] [CrossRef]
- Baakdah, M.M.; Tsopmo, A. Identification of peptides, metal binding and lipid peroxidation activities of HPLC fractions of hydrolyzed oat bran proteins. J. Food Sci. Technol. 2016, 53, 3593–3601. [Google Scholar] [CrossRef]
- Coelho, M.S.; Aquino, S.D.; Latorres, J.M.; Salas-Mellado, M.D. In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll. 2019, 91, 19–25. [Google Scholar] [CrossRef]
- Lin, C.C.; Liang, J.H. Effect of antioxidants on the oxidative stability of chicken breast meat in a dispersion system. J. Food Sci. 2002, 67, 530–533. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Qian, Z.J.; Lee, S.H.; Choi, S.Y.; Sung, N.J.; Byun, H.G.; Kim, S.K. Free radical scavenging activity of a novel antioxidative peptide isolated from in vitro gastrointestinal digests of mytilus coruscus. J. Med. Food 2007, 10, 197–202. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.K. Marine bioactive peptides as potential antioxidants. Curr. Protein Pept. Sci. 2013, 14, 189–198. [Google Scholar] [CrossRef]
- Chi, C.F.; Hu, F.Y.; Wang, B.; Li, T.; Ding, G.F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Sivaraman, B.; Shakila, R.J.; Jeyasekaran, G.; Sukumar, D.; Manimaran, U.; Sumathi, G. Antioxidant activities of squid protein hydrolysates prepared with papain using response surface methodology. Food Sci. Biotechnol. 2016, 25, 665–672. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, R.; Li, Y. Antioxidant and emulsifying activities of corn gluten meal hydrolysates in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2019, 97, 175–185. [Google Scholar] [CrossRef]
- Li, Y.; Kong, B.; Liu, Q.; Xia, X.; Chen, H. Improvement of the emulsifying and oxidative stability of myofibrillar protein prepared oil-in-water emulsions by addition of zein hydrolysates. Process. Biochem. 2017, 53, 116–124. [Google Scholar] [CrossRef]
- Jie, Y.; Zhao, H.; Sun, X.; Lv, X.; Zhang, Z.; Zhang, B. Isolation of antioxidative peptide from the protein hydrolysate of Caragana ambigua seeds and its mechanism for retarding lipid auto-oxidation. J. Sci. Food Agric. 2019, 99, 3078–3085. [Google Scholar] [CrossRef]
- Rossini, K.; Noreña, C.P.Z.; Cladera-Olivera, F.; Brandelli, A. Casein peptides with inhibitory activity on lipid oxidation in beef homogenates and mechanically deboned poultry meat. LWT Food Sci. Technol. 2009, 42, 862–867. [Google Scholar] [CrossRef]
- Singh, P.; Singh, T.P.; Gandhi, N. Prevention of lipid oxidation in muscle foods by milk proteins and peptides: A review. Food Rev. Int. 2016, 34, 226–247. [Google Scholar] [CrossRef]
- Sakanaka, S.; Tachibana, Y.; Ishihara, N.; Juneja, L.R. Antioxidant properties of casein calcium peptides and their effects on lipid oxidation in beef homogenates. J. Agric. Food Chem. 2005, 53, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, S.; Guo, X.; Li, R.; Huang, L. Effect of crude peptide extract from mutton ham on antioxidant properties and quality of mutton patties. J. Food Process. Preserv. 2020, 44, e14436. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Benjakul, S. Cryoprotective and antioxidative effects of gelatin hydrolysate from unicorn leatherjacket skin. Int. J. Refrig. 2015, 49, 69–78. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Ehsani, A.; Li, J.; Wu, F.; Yang, N.; Xu, B.; Jin, Z.; Xu, X. Antioxidant and cryoprotective effects of a tetrapeptide isolated from Amur sturgeon skin gelatin. J. Funct. Foods 2014, 7, 609–620. [Google Scholar] [CrossRef]
- Qiu, X.; Chen, S.; Dong, S. Effects of silver carp antioxidant peptide on the lipid oxidation of sierra fish fillets (Scomberomorus Niphonius) during frozen storage. J. Food Biochem. 2013, 38, 167–174. [Google Scholar] [CrossRef]
- Zhang, L.; Shan, Y.; Hong, H.; Luo, Y.; Hong, X.; Ye, W. Prevention of protein and lipid oxidation in freeze-thawed bighead carp (Hypophthalmichthys nobilis) fillets using silver carp (Hypophthalmichthys molitrix) fin hydrolysates. LWT Food Sci Technol. 2020, 123, 109050. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S. Potential application of seafood-derived peptides as bifunctional ingredients, antioxidant–cryoprotectant: A review. J. Funct. Foods 2015, 19, 753–764. [Google Scholar] [CrossRef]
- Jridi, M.; Lassoued, I.; Nasri, R.; Ayadi, M.A.; Nasri, M.; Souissi, N. Characterization and potential use of cuttlefish skin gelatin hydrolysates prepared by different microbial proteases. BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.S.; Dora, K.C. Antioxidative activity of protein hydrolysate produced by alcalase hydrolysis from shrimp waste (Penaeus monodon and Penaeus indicus). J. Food Sci. Technol. 2011, 51, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, H.; Krichen, F.; Kobbi, S.; Martínez-Álvarez, Ó.; Nedjar, N.; Bougatef, A.; Sila, A. Physicochemical and biological properties of eel by-products protein hydrolysates: Potential application to meat product preservation. Waste Biomass Valori. 2018, 11, 931–942. [Google Scholar] [CrossRef]
- Jiang, W.; Hu, S.; Li, S.; Liu, Y. Evaluation of the preservation effect of gelatin-water soluble chitosan film incorporated with maillard peptides on bluefin tuna (Thunnus thynnus) slices packaging. LWT Food Sci. Technol. 2019, 113, 108294. [Google Scholar] [CrossRef]
- Li, X.F.; Yang, R.W.; Lin, S.Y.; Ye, H.Q.; Chen, F. Identification of key volatiles responsible for aroma changes of egg white antioxidant peptides during storage by HS-SPMEGC-MS and sensory evaluation. J. Food Meas. Charact. 2017, 11, 1118–1127. [Google Scholar] [CrossRef]
- Mada, S.B.; Ugwu, C.P.; Abarshi, M.M. Health promoting effects of food-derived bioactive peptides: A review. Int. J. Pept. Res. Ther. 2019, 26, 831–848. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, M.; Lin, L.; Wang, J.; Sun-Waterhouse, D.X.; Dong, Y.; Zhuang, M.; Su, G. Identification of antioxidative peptides from defatted walnut meal hydrolysate with potential for improving learning and memory. Food Res. Int. 2015, 78, 216–223. [Google Scholar] [CrossRef]
- Han, B.K.; Park, Y.; Choi, H.S.; Suh, H. Hepatoprotective effects of soluble rice protein in primary hepatocytes and in mice. J. Sci. Food Agric. 2015, 96, 685–694. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Liao, W.; Davidge, S.T.; Wu, J. Milk-derived tripeptides IPP (Ile-Pro-Pro) and VPP (Val-Pro-Pro) differentially modulate angiotensin II effects on vascular smooth muscle cells. J. Funct. Foods 2017, 30, 151–158. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Lu, Y.L.; Han, C.H.; Hou, W.C.; Aluko, R.E. Flaxseed protein-derived peptide fractions: Antioxidant properties and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Food Chem. 2009, 116, 277–284. [Google Scholar] [CrossRef]
- Pan, M.; Huo, Y.; Wang, C.; Zhang, Y.; Dai, Z.; Li, B. Positively charged peptides from casein hydrolysate show strong inhibitory effects on LDL oxidation and cellular lipid accumulation in Raw264.7 cells. Int. Dairy J. 2019, 91, 119–128. [Google Scholar] [CrossRef]
- Oh, Y.; Ahn, C.B.; Nam, K.H.; Kim, Y.A.; Yoon, N.Y.; Je, J.Y. Amino acid composition, antioxidant, and cytoprotective effect of blue Mussel (Mytilus edulis) hydrolysate through the inhibition of caspase-3 activation in oxidative stress-mediated endothelial cell injury. Mar. Drugs 2019, 17, 135. [Google Scholar] [CrossRef] [PubMed]
- Selamassakul, O.; Laohakunjit, N.; Kerdchoechuen, O.; Yang, L.; Maier, C.S. Bioactive peptides from brown rice protein hydrolyzed by bromelain: Relationship between biofunctional activities and flavor characteristics. J. Food Sci. 2020, 85, 707–717. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubzadeh, Z.; Ghadikolaii, F.P.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant activity and anticancer effect of bioactive peptides from rainbow trout (oncorhynchus mykiss) skin hydrolysate. Int. J. Pept. Res. Ther. 2019, 26, 625–632. [Google Scholar] [CrossRef]
- Adewole, K.E.; Adebayo, J.O. Antioxidant defense system induced by cysteine-stabilized peptide fraction of aqueous extract of Morinda lucida leaf in selected tissues of Plasmodium berghei -infected mice. J. Integr. Med. 2017, 15, 388–397. [Google Scholar] [CrossRef]
- Chalé, F.G.H.; Ruiz, J.C.R.; Fernández, J.J.A.; Ancona, D.A.B.; Campos, M.R.S. ACE inhibitory, hypotensive and antioxidant peptide fractions from Mucuna pruriens proteins. Process. Biochem. 2014, 49, 1691–1698. [Google Scholar] [CrossRef]
- Indiano-Romacho, P.; Fernández-Tomé, S.; Amigo, L.; Hernández-Ledesma, B. Multifunctionality of lunasin and peptides released during its simulated gastrointestinal digestion. Food Res. Int. 2019, 125, 108513. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Borczak, B.; Piątkowska, E.; Kapusta-Duch, J.; Morawska, M.; Czech, T. Effect of protein hydrolysates from carp (Cyprinus carpio) skin gelatine on oxidative stress biomarkers and other blood parameters in healthy rats. J. Funct. Foods 2019, 60, 103411. [Google Scholar] [CrossRef]
- Kamdem, J.P.; Tsopmo, A. Reactivity of peptides within the food matrix. J. Food Biochem. 2017, 43, e12489. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zheng, Y.; Wu, Y.; Chen, H.; Tong, P.; Gao, J. Double enzyme hydrolysis for producing antioxidant peptide from egg white: Optimization, evaluation, and potential allergenicity. J. Food Biochem. 2019, 44, 13113. [Google Scholar] [CrossRef] [PubMed]
| Source | Enzyme and Purification Methods | In Vitro Chemical Evaluation | IC50 or Scavenging Activity 2 | Amino Acid Sequence or Molecular Weight | Ref |
|---|---|---|---|---|---|
| Egg white protein powder | Alcalase; UF (30, 10, 1 kDa), GFC (Sephadex), RP-HPLC | Reducing power assay DPPH radical scavenging activity ABTS radical scavenging activity ORAC assay | ABTS (92.21 ± 0.5% at 5 mg/mL) ORAC (1238.56 ± 0.6 μmol TE/g) DPPH (FFGFN IC50 = 80 mM; MPDAHL IC50 = 60 mM) | DHTKE, FFGFN, MPDAHL | [55] |
| Duck (Anas platyrhynchos) breasts | Protamex; UF (30, 10 kDa), GFC (Sephadex G-25), IEC | DPPH radical scavenging activity •OH scavenging activity Fe2+ chelating activity | DPPH (93.63 ± 0.13% at 1.0 mg/mL) | LQAEVEELRAALE, IEDPFDQDDWGAWKK, AGRALTAYLMKIL, GYDLGEAEFARIM | [56] |
| Chickpea seeds | Pepsin, Pancreatin; AC, GFC, nanofiltration | Reducing power assay DPPH radical scavenging activity | - | ALEPDHR, TETWNPNHPEL, FVPH, SAEHGSLH | [57] |
| Rice residue protein | Papain, Flavourzyme, Protamex; GFC (Sephadex G-15), RP-FPLC | DPPH radical scavenging activity ABTS radical scavenging activity FRAP-Fe3+ reducing capacity assay | DPPH (77.6% at 0.5 mg/mL, IC50 = 0.25 mg/mL) | RPNYTDA, TSQLLSDQ, TRTGDPFF, NFHPQ | [58] |
| Rice bran protein | Trypsin; RP-HPLC | ORAC assay | ORAC (4.07 μmol TE/g) | 800–2100 Da | [49] |
| Pinto bean protein isolate | Protamex; UF (100, 50, 30, 10, 3 kDa) | ABTS radical scavenging activity FRAP assay | ABTS (42.2% at 7mM); FRAP (0.81 mM) | PPHMLP, PPMHLP, PLPPHMLP, PLPLHMLP, ACSNHSPLGWRGH, LSSLEMGSLGALFVCM | [59] |
| Pearl millet | Trypsin; GFC (Sephadex G-25), RP-UFLC | DPPH radical scavenging activity ABTS radical scavenging activity Fe2+ chelating activity Reducing power assay •OH scavenging activity | DPPH (67.66% at 1 mg/mL); ABTS (78.81% at 1 mg/mL) | SDRDLLGPNNQYLPK | [60] |
| Palmaria palmata protein | Corolase® PP; SPE, SP-RP-HPLC | ORAC assay, FRAP assay | ORAC (4380.75 ± 66.44 μmol TE/g dw); FRAP (51.86 ± 1.85 μmol TE/g dw) | SDITRPGGQM | [47] |
| Oat glutelin | Alcalase; IEC, RP-HPLC | •OH scavenging activity DPPH radical scavenging activity | •OH (IC50 = 0.68 mg/mL) | HYNAPAL | [61] |
| Egg in fresh Apostichopus japonicus | Papain, Protamex; UF, HSCCC; GFC (Sephadex G-100, G-50) | •OH scavenging activity O2•− scavenging activity | •OH (93.26, 70.04, and 89.82 U/mL, respectively) | 30 kDa (3 kinds of peptides) | [62] |
| Hazelnut protein | Alcalase; GFC (Sephadex G-25, G-15), RP-HPLC | DPPH radical scavenging activity ABTS radical scavenging activity | DPPH (69.2 ± 1.2%); ABTS (92.9 ± 1.0%) | ADGF, AGGF, AWDPE, DWDPK, ETTL, SGAF | [63] |
| Pecan protein isolate | Alcalase; UF (10, 5, 3 kDa), IEC, GFC (Sephadex G-50) | DPPH radical scavenging activity ABTS radical scavenging activity •OH scavenging activity Reducing power assay Fe2+ chelating activity | DPPH, ABTS, •OH (LAYLQYTDFETR: 56.25%, 67.67%, 47.42% at 0.1 mg/mL) | LAYLQYTDFETR | [23] |
| Sheep abomasum protein | Papain; UF (10, 3 kDa), IEC; GFC (Sephadex G-50), RP-HPLC | DPPH radical scavenging activity ABTS radical scavenging activity •OH scavenging activity | DPPH (LEDGLK: IC50 = 0.63 mg/mL; IDDVLK: IC50 = 0.58 mg/mL) | LEDGLK, IDDVLK | [64] |
| Erythrina edulis (pajuro) protein | Alcalase; Polyamide SPE, RP-HPLC | ABTS radical scavenging activity ORAC assay | ABTS (1.37 ± 0.09 μmol TE/mg); ORAC (2.83 ± 0.07 μmol TE/mg) | DGLGYY, CCGDYY, YDLHGY | [65] |
| Finger millet protein | Trypsin; UF, GFC, RP-UFLC | DPPH radical scavenging activity ABTS radical scavenging activity Fe2+ chelating activity •OH scavenging activity | DPPH, ABTS, Fe2+ chelating, •OH (61.79%, 78.61%, 51.20%, 66.66% at 1.0 mg/mL) | TSSSLNMAVRGGLTR STTVGLGISMRSASVR | [66] |
| Cutlassfish muscle | Pepsin; UF, GFC (Sephadex G-25), RP-UFLC | DPPH radical scavenging activity Peroxyl radical scavenging activity | DPPH (IC50 = 0.03 mg/mL); Peroxyl (IC50 = 0.02 mg/mL) | FSGE | [67] |
| Sea squirt protein | Pepsin; GFC, RP-HPLC | DPPH radical scavenging activity ABTS radical scavenging activity ORAC assay Reducing power assay Fe2+ chelating activity | DPPH (LEW: IC50 = 1.29 mM); Fe2+ (LEW, MTTL, YYPYQL: 9.20–12.5% at 1 mM) | MTTL, LEW, YYPYQL | [68] |
| Freeze-dried stone fish flesh | Alcalase; UF, SDS-PAGE, RP-HPLC, Isoelectric point focusing fractionation | DPPH radical scavenging activity ABTS radical scavenging activity FRAP | DPPH (62.5% at 0.1 mg/mL) | GVSGLHID | [69] |
| Wheat germ protein | Alcalase; RP-HPLC | ABTS | - | TVGGAPAGRIVME, GNPIPREPGQVPAY | [70] |
| Sesame protein | Alcalase, Trypsin; UF (3, 5, 8, 10 kDa), prep-HPLC | DPPH radical scavenging activity ABTS radical scavenging activity | DPPH (IC50 = 2.793 mg/mL); ABTS (IC50 = 2.949 mg/mL) | 1008.2–1402.7 Da (7 kinds of peptide) | [71] |
| Sea cucumber collagen | Neutrase; UF (5, 1 kDa) GFC (Sephadex G-15) | DPPH radical scavenging activity ABTS radical scavenging activity | DPPH (35% at 0.2 mg/mL) DPPH (FLAP EC50 = 0.385 mg/mL) | FLAP | [72] |
| Tartary buckwheat albumin | Alkaline Protease; UF (3, 10 kDa), IEC, GFC (Sephadex G-15), RP-HPLC | DPPH radical scavenging activity •OH scavenging activity Reducing power assay Lipid peroxidation inhibition | GEVPW, YMENF, AFYRW: DPPH (IC50 = 1.20, 2.91, 0.64 mM); •OH (IC50 = 2.21, 1.56, 0.65 mM); Reducing power (0.702, 0.554, 0.927 at 4 mg/mL) | GEVPW, YMENF, AFYRW | [73] |
| Duck plasma powder | Alcalase; UF (10, 3 kDa), GFC (Sephadex G-25), RP-HPLC | O2•− scavenging activity DPPH radical scavenging activity ABTS radical scavenging activity Fe2+ chelating activity Reducing capacity | DPPH (88.36% at 1.0 mg/mL) O2•− (64.47% at 1.0 mg/mL), ABTS (149.67 mM TE/mg at 1.0 mg/mL) | LDGP, TGVGTK, EVGK, RCLQ, LHDVK, KLGA, AGGVPAG | [74] |
| Source | Enzyme | Processing Method | Processing Conditions | Advantages | Ref |
|---|---|---|---|---|---|
| Fish frame protein | Alcalase | Microwave | T = 90 °C, t = 5 min | Improved protein solubility, protein recovery, DH, and ABTS radical scavenging activity. | [99] |
| Barley beer waste protein | Alcalase | Ultrasound | Frequency = 50 Hz, t = 4 h | Improved metal-chelating activity (54%); improved DPPH radical, O2•− scavenging, and •OH scavenging activity (28%, 18%, 25%) | [104] |
| Tilapia by-product protein | Alcalase | High pressure-assisted | Pressure = 250 MPa, t = 35 min | Facilitated the release of low Mw peptides and essential amino acids; improved soluble protein content (5.7 mg/mL), RP (44 μg AAE/g), and solubility (71%) of hydrolysates; decreased IC50 (DPPH) values from 653 μg/mL to 304 μg/mL | [106] |
| Soybean protein isolate | Corolase PP | High hydrostatic pressure | Pressure = 200 MPa, t = 4 h | Enhanced the efficiency of enzymolysis; decreased surface hydrophobicity of hydrolysates; increased the production of small peptides (< 3 kDa); increased RP, ABTS radical scavenging activity | [111] |
| Egg white protein | Alcalase | Pulsed electric field | Strength = 10 kV cm−1, pulsed number = 300, frequency = 3000 Hz | Increased RP ability; broke down larger peptides into smaller peptides | [114] |
| Pea protein | Papain | Protease co-extrusion | E = 12.0%, T = 60.2 °C, pH = 6.5, S = 7.1% | Enhanced the efficiency of enzymolysis and DPPH radical scavenging activity (98.1%) of enzymatic hydrolysate | [118] |
| Sweet potato protein | Alcalase, Protease | Radio frequency | T = 80 °C/90 °C | Increased Mw <3 kDa peptide fraction and its antioxidant capacity | [119] |
| Rice protein | Alcalase | High-energy electron beam | Irradiation doses = 30 kGy | Increased ratio of antioxidative amino acids; produced smaller peptides; increased DPPH and ABTS radical scavenging activity (32.06% and 79.11%) of hydrolysates | [120] |
| Source | Peptide | Cellular Model | Cellular Effect | Ref |
|---|---|---|---|---|
| Whey protein | Hydrophobic peptide | H2O2-treated PC12 cells | Increased cell survival rate (19.3%); decreased cell death (28.6%) | [212] |
| Indian squid protein | WCTSVS | H2O2-treated breast cancer cells (MCF7) | Decreased intracellular ROS | [213] |
| Soybean protein | FDPAL | H2O2-treated HeLa cells | Increased cell viability under oxidative stress | [214] |
| Soybean protein | SHECN | AAPH-treated HepG2 cells | Possessed CAA (776.22 μmol QE/ 100 g) | [215] |
| Pine nut meal protein | KWFCT, Ac-QWFCT | AAPH-treated HepG2 cells | Possessed CAA (612.8, 916.3 μmol QE/ 100 g) | [216] |
| Pine nut protein | QDHCH | AAPH-treated/H2O2-treated HepG2 cells | Possessed CAA (3051.84 μmol QE/100 g); increased SOD, GSH-Px, CAT, GR activities; decreased MDA content increased cell viability under oxidative stress | [217] |
| Hanwoo beef protein | CCCCSVQK | Human colorectal carcinoma cells (HCT116) | Inhibits the proliferation of HCT116 cells | [211] |
| Chinese Baijiu | PHP | AAPH-treated HepG2 cells | Increased SOD, GSH-Px, CAT activities; increased GSH content; decreased MDA, GSSG content; decreased intracellular ROS levels | [218] |
| Rapeseed protein | WDHHAPQLR | H2O2-treated HUVECs cells | Reduced cell apoptosis | [207] |
| Perilla seed protein | YL, FY | H2O2-treated HepG2 cells | Reduced cell apoptosis | [219] |
| Lupin protein confer | Peptides with Mw < 3 kDa | H2O2-treated HepG2 cells | Increased cell survival rate; decreased intracellular ROS levels; increased SOD, GSH-Px | [220] |
| Soybean protein | IYVVDLR; IYVFVR, VVFVDRL, VIYVVDLR | H2O2-treated Caco-2 cells | Increased CAT, GR activity (IYVVDLR, IYVFVR); increased GSH content (IYVVDLR, IYVFVR, VVFVDRL); increased cell viability under oxidative stress (IYVVDLR, IYVFVR, VVFVDRL); decreased MDA content; decreased intracellular ROS levels | [221] |
| Fermented grain (Jiupei) | VNP, YGD | AAPH-treated HepG2 cells | Increased SOD, GSH-Px, CAT activities; decreased intracellular ROS levels; decreased MDA, GSSG content; increased GSH content | [222] |
| Defatted walnut meal | VEGNLQVLRPR, LAGNPHQQQQN, HNLDTQTESDV, AGNDGFEYVTLK, QQRQQQGL, AELQVVDHLGQTV, EQEEEESTGRMK, WSVWEQELEDR | H2O2-treated SHSY5Y cells | Decreased intracellular ROS levels (ex WSVWEQELEDR); increased cell viability under oxidative stress | [223] |
| Mulberry leaf protein | SVL, EAVQ, RDY | AAPH-treated HepG2 cells | Possessed CAA (1706, 1501, 2204 μmol QE/ 100 g); inhibited oxidant-induced hemolysis (RDY: 92%) | [224] |
| Egg white protein | VYLPR | H2O2-treated HEK-293 cells | Increased cell viability under oxidative stress (97.45%); increased SOD, GSH-Px activities; decreased MDA; inhibit LDH activity | [225] |
| Collagen from sea cucumber | Peptides with Mw < 1 kDa | H2O2-treated RAW264.7 cells | Promote cell proliferation; decreased intracellular ROS levels; decreased intracellular ROS levels; increased SOD, GSH-Px activities; decreased MDA | [226] |
| Collagen of Redlip Croaker | GPEGPMGLE, EGPFGPEG, GFIGPTE | H2O2-treated HepG2 cells | Decreased intracellular ROS levels; decreased MDA; increased SOD, GSH-Px, CAT activities | [227] |
| Fermented milk | NTVPAKSCQAQPTTM, EDELQDKIHPF, QGPIVLNPWDQVKR, APSFSDIPNPIGSENSE | T-BHP-treated Caco-2 cells | Increased cell viability under oxidative stress; decreased intracellular ROS levels | [129] |
| Whey protein | Peptides with Mw ≤ 3 kDa | Menadione-treated IEC-18 cells | Increased cell viability under oxidative stress (88%) | [228] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Liu, K.; Yang, J.; Liu, S.; Wang, S.; Wang, S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants 2020, 9, 799. https://doi.org/10.3390/antiox9090799
Pan M, Liu K, Yang J, Liu S, Wang S, Wang S. Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants. 2020; 9(9):799. https://doi.org/10.3390/antiox9090799
Chicago/Turabian StylePan, Mingfei, Kaixin Liu, Jingying Yang, Shengmiao Liu, Shan Wang, and Shuo Wang. 2020. "Advances on Food-Derived Peptidic Antioxidants—A Review" Antioxidants 9, no. 9: 799. https://doi.org/10.3390/antiox9090799
APA StylePan, M., Liu, K., Yang, J., Liu, S., Wang, S., & Wang, S. (2020). Advances on Food-Derived Peptidic Antioxidants—A Review. Antioxidants, 9(9), 799. https://doi.org/10.3390/antiox9090799