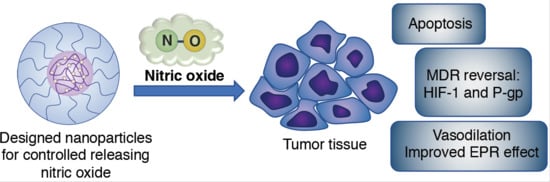
Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges
Abstract
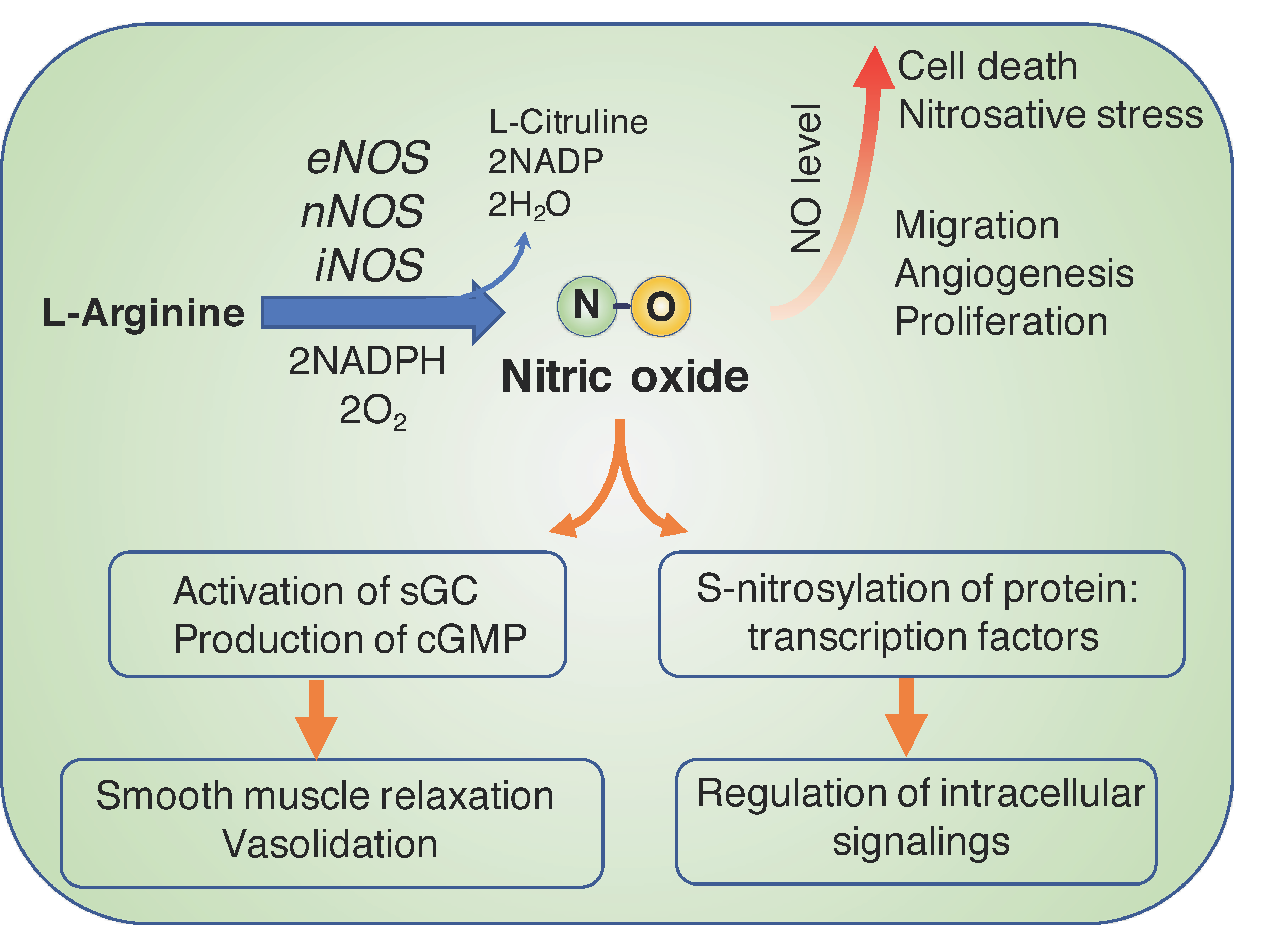
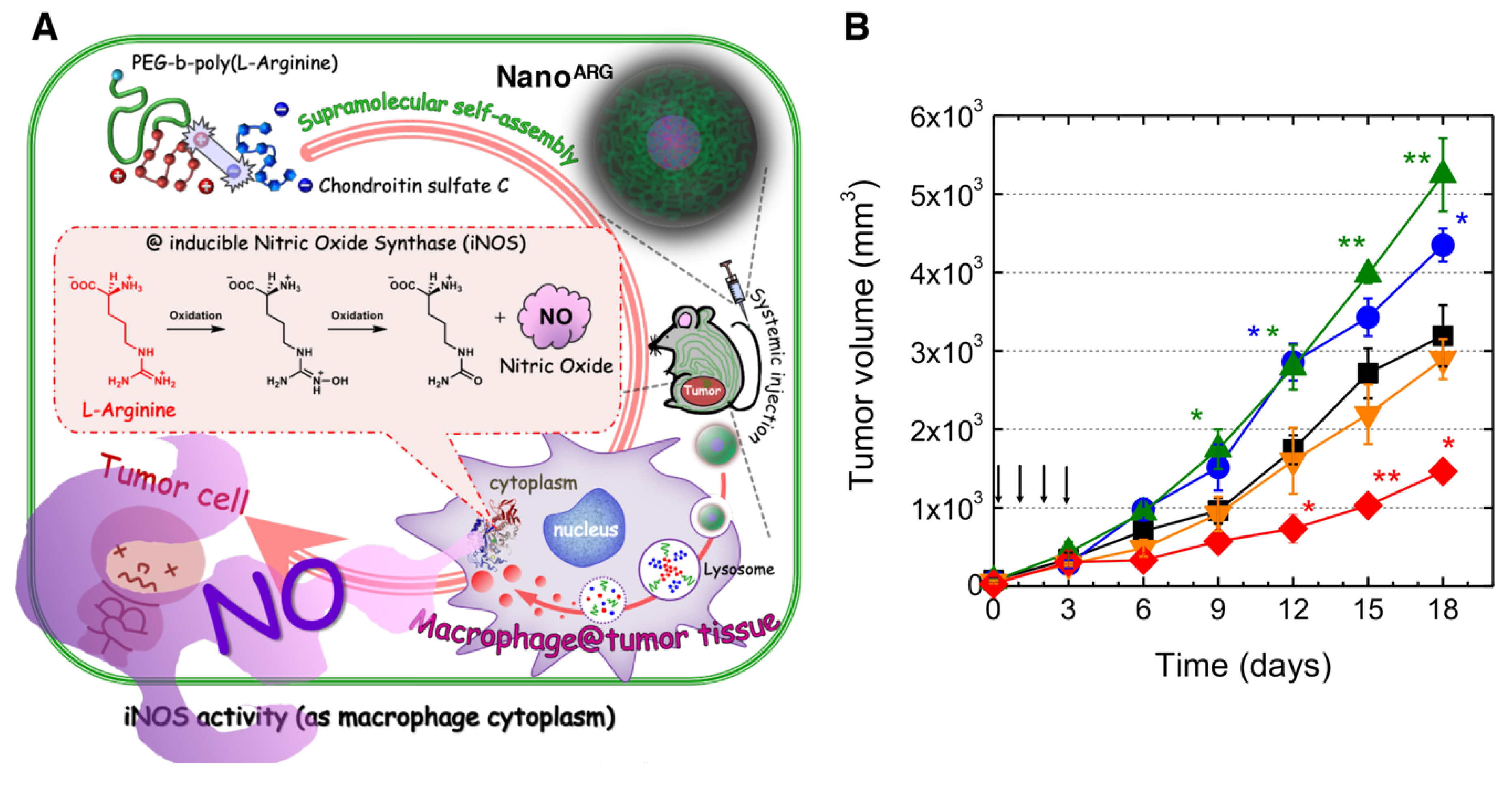
:1. Biological Functions of Nitric Oxide and Therapeutics in Cancer
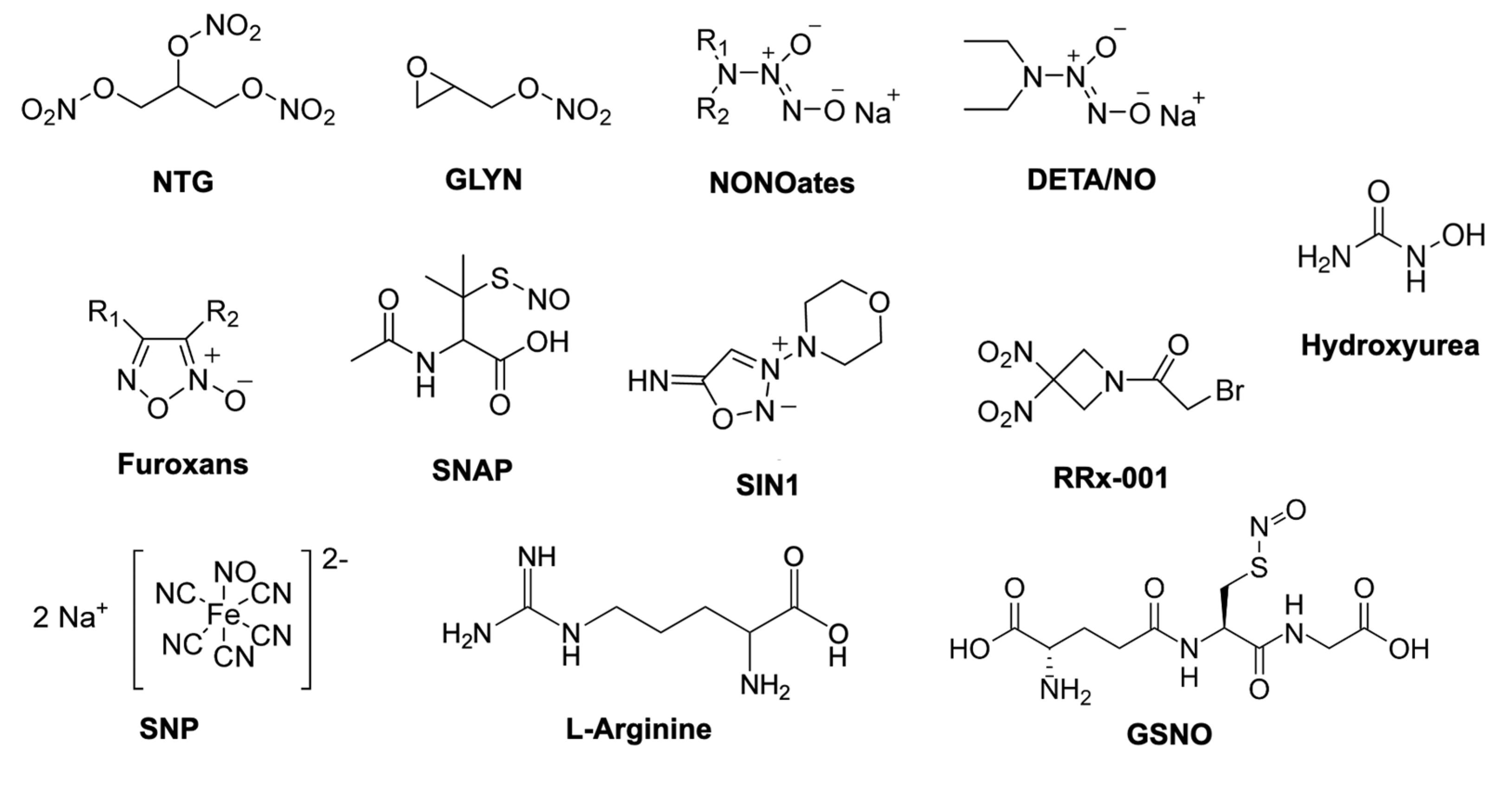
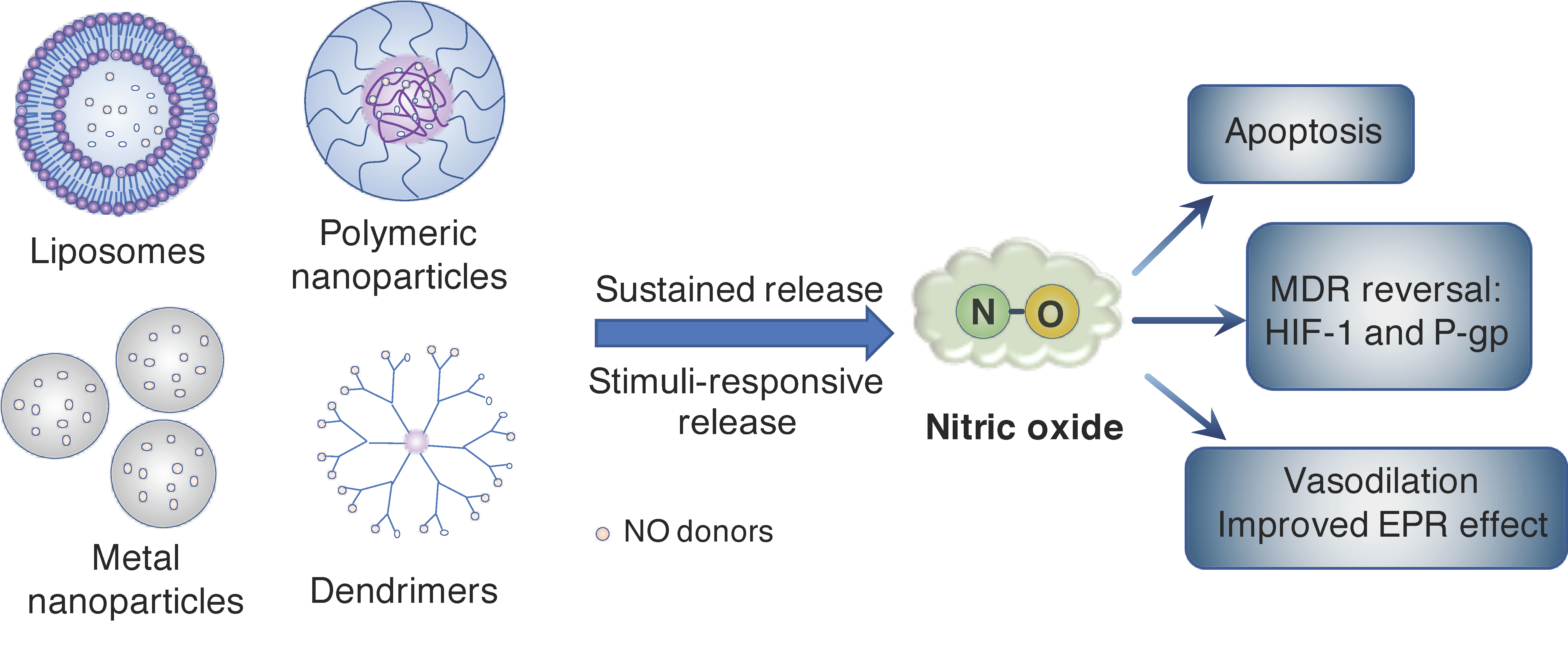
2. Nanoparticle Systems for Controlled Release of NO Molecule in Cancer
3. NO-Delivery Nanocarriers Overcome Multidrug Resistance in Cancer
4. NO Improves the EPR Effect in Nano-DDS
5. Improvement in NO Bioavailability
6. Conclusions
Funding
Conflicts of Interest
References
- Chen, K.; Pittman, R.N.; Popel, A.S. Nitric oxide in the vasculature: Where does it come from and where does it go? A quantitative perspective. Antioxid. Redox Signal. 2008, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luiking, Y.C.; Engelen, M.P.K.J.; Deutz, N.E.P. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncada, S.; Bolaños, J.P. Nitric oxide, cell bioenergetics and neurodegeneration. J. Neurochem. 2006, 97, 1676–1689. [Google Scholar] [CrossRef]
- Vincent, S.R. Nitric oxide neurons and neurotransmission. Prog. Neurobiol. 2010, 90, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on immune cells and its role in diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanini, F.; Kashfi, K.; Nath, N. The dual role of iNOS in cancer. Redox Biol. 2015, 6, 334–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schairer, D.O.; Chouake, J.S.; Nosanchuk, J.D.; Friedman, A.J. The potential of nitric oxide releasing therapies as antimicrobial agents. Virulence 2012, 3, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.C. Perspectives series: Host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. J. Clin. Investig. 1997, 99, 2818–2825. [Google Scholar] [CrossRef] [Green Version]
- Coleman, J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001, 1, 1397–1406. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Choudhari, S.K.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, A.J.; Sullivan, F.J.; Giles, F.J.; Glynn, S.A. The yin and yang of nitric oxide in cancer progression. Carcinogenesis 2013, 34, 503–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vahora, H.; Khan, M.A.; Alalami, U.; Hussain, A. The potential role of Nitric Oxide in halting cancer progression through chemoprevention. J. Cancer Prev. 2016, 21, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ying, L.; Hofseth, L.J. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007, 67, 1407–1410. [Google Scholar] [CrossRef] [Green Version]
- MacLauchlan, S.; Yu, J.; Parrish, M.; Asoulin, T.A.; Schleicher, M.; Krady, M.M.; Zeng, J.; Huang, P.L.; Sessa, W.C.; Kyriakides, T.R. Endothelial nitric oxide synthase controls the expression of the angiogenesis inhibitor thrombospondin 2. Proc. Natl. Acad. Sci. USA 2011, 108, E1137–E1145. [Google Scholar] [CrossRef] [Green Version]
- Ridnour, L.A.; Thomas, D.D.; Switzer, C.; Flores-Santana, W.; Isenberg, J.S.; Ambs, S.; Roberts, D.D.; Wink, D.A. Molecular mechanisms for discrete nitric oxide levels in cancer. Nitric Oxide 2008, 19, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Silkstone, R.S.; Mason, M.G.; Nicholls, P.; Cooper, C.E. Nitrogen dioxide oxidizes mitochondrial cytochrome c. Free Radic. Biol. Med. 2012, 52, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Heinrich, T.A.; da Silva, R.S.; Miranda, K.M.; Switzer, C.H.; Wink, D.A.; Fukuto, J.M. Biological nitric oxide signalling: Chemistry and terminology. Br. J. Pharmacol. 2013, 169, 1417–1429. [Google Scholar] [CrossRef] [Green Version]
- Hickok, J.; Thomas, D. Nitric Oxide and cancer therapy: The emperor has no clothes. Curr. Pharm. Des. 2010, 16, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef]
- Thomas, D.D. The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. USA 2001, 98, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sha, Y.; Marshall, H.E. S-nitrosylation in the regulation of gene transcription. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 701–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Singh, R.K.; Bhardwaj, T.R. Therapeutic role of nitric oxide as emerging molecule. Biomed. Pharmacother. 2017, 85, 182–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.G.; Xian, M.; Tang, X.; Wu, X.; Wen, Z.; Cai, T.; Janczuk, A.J. Nitric oxide donors: Chemical activities and biological applications. Chem. Rev. 2002, 102, 1091–1134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric Oxide donor-based cancer therapy: Advances and prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Delivering nitric oxide with nanoparticles. J. Control. Release 2015, 205, 190–205. [Google Scholar] [CrossRef]
- Carpenter, A.; Schoenfisch, M. Nitric Oxide release part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef]
- Suchyta, D.J.; Schoenfisch, M.H. Controlled release of Nitric Oxide from liposomes. ACS Biomater. Sci. Eng. 2017, 3, 2136–2143. [Google Scholar] [CrossRef]
- Lee, S.Y.; Rim, Y.; McPherson, D.D.; Huang, S.L.; Kim, H. A novel liposomal nanomedicine for nitric oxide delivery and breast cancer treatment. Biomed. Mater. Eng. 2014, 24, 61–67. [Google Scholar] [CrossRef]
- Butler, A.R.; Megson, I.L.; Wright, P.G. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim. Biophys. Acta Gen. Subj. 1998, 1425, 168–176. [Google Scholar] [CrossRef]
- Kanayama, N.; Yamaguchi, K.; Nagasaki, Y. PEGylated polymer micelle-based nitric oxide (NO) photodonor with NO-mediated antitumor activity. Chem. Lett. 2010, 39, 1008–1009. [Google Scholar] [CrossRef]
- Choi, H.W.; Kim, J.; Kim, J.; Kim, Y.; Song, H.B.; Kim, J.H.; Kim, K.; Kim, W.J. Light-induced acid generation on a gatekeeper for smart Nitric Oxide delivery. ACS Nano 2016, 10, 4199–4208. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, X.; Tang, Y.; Zou, J.; Wang, P.; Zhang, Y.; Si, W.; Huang, W.; Dong, X. A light-induced nitric oxide controllable release nano-platform based on diketopyrrolopyrrole derivatives for pH-responsive photodynamic/photothermal synergistic cancer therapy. Chem. Sci. 2018, 9, 8103–8109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Du, J.; Guo, Z.; Yu, J.; Gao, Q.; Yin, W.; Zhu, S.; Gu, Z.; Zhao, Y. Efficient near infrared light triggered Nitric Oxide release nanocomposites for sensitizing mild photothermal therapy. Adv. Sci. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Ren, H.; Liu, J.; Wang, Y.; Meng, Z.; He, Z.; Miao, W.; Chen, G.; Li, X. A switchable NO-releasing nanomedicine for enhanced cancer therapy and inhibition of metastasis. Nanoscale 2019, 11, 5474–5488. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Z.; Liu, J.; Tian, G.; Chen, K.; Yu, S.; Gu, Z. Near infrared light triggered nitric oxide releasing platform based on upconversion nanoparticles for synergistic therapy of cancer stem-like cells. Sci. Bull. 2017, 62, 985–996. [Google Scholar] [CrossRef] [Green Version]
- Jin, Z.; Wen, Y.; Hu, Y.; Chen, W.; Zheng, X.; Guo, W.; Wang, T.; Qian, Z.; Su, B.L.; He, Q. MRI-guided and ultrasound-triggered release of NO by advanced nanomedicine. Nanoscale 2017, 9, 3637–3645. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J.; Park, J.; Lee, Y.M.; Saravanakumar, G.; Park, K.M.; Choi, W.; Kim, K.; Lee, E.; Kim, C.; et al. Tumor vasodilation by N-Heterocyclic carbene-based nitric oxide delivery triggered by high-intensity focused ultrasound and enhanced drug homing to tumor sites for anti-cancer therapy. Biomaterials 2019, 217, 119297. [Google Scholar] [CrossRef]
- Zhang, K.; Xu, H.; Jia, X.; Chen, Y.; Ma, M.; Sun, L.; Chen, H. Ultrasound-triggered Nitric Oxide release platform based on energy transformation for targeted inhibition of pancreatic tumor. ACS Nano 2016, 10, 10816–10828. [Google Scholar] [CrossRef]
- Nagase, S.; Takemura, K.; Ueda, A.; Hirayama, A.; Aoyagi, K.; Kondoh, M.; Koyama, A. A novel nonenzymatic pathway for the generation of Nitric Oxide by the reaction of hydrogen peroxide and D- or L-Arginine. Biochem. Biophys. Res. Commun. 1997, 233, 150–153. [Google Scholar] [CrossRef]
- Mijatović, S.; Savić-Radojević, A.; Plješa-Ercegovac, M.; Simić, T.; Nicoletti, F.; Maksimović-Ivanić, D. The double-faced role of Nitric Oxide and reactive oxygen species in solid tumors. Antioxidants 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed]
- Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as tools to target redox homeostasis in cancer cells. Antioxidants 2020, 9, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Lu, N.; Huang, P.; Liu, Y.; Yang, Z.; Wang, S.; Yu, G.; Liu, Y.; Hu, J.; He, Q.; et al. Glucose-responsive sequential generation of hydrogen peroxide and Nitric Oxide for synergistic cancer starving-like/gas therapy. Angew. Chem. 2017, 129, 1249–1253. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, M.; Cheng, J.; Yin, J.; Huang, C.; Cui, H.; Zhang, X.; Zhao, G. Acidity-triggered tumor-targeted nanosystem for synergistic therapy via a cascade of ROS generation and NO release. ACS Appl. Mater. Interfaces 2020, 12, 28975–28984. [Google Scholar] [CrossRef]
- Jiang, D.; Yue, T.; Wang, G.; Wang, C.; Chen, C.; Cao, H.; Gao, Y. Peroxynitrite (ONOO−) generation from the HA-TPP@NORM nanoparticles based on synergistic interactions between nitric oxide and photodynamic therapies for elevating anticancer efficiency. New J. Chem. 2020, 44, 162–170. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, C.W.; Lee, H.J.; Min, K.H.; Kwack, K.H.; Lee, H.-W.; Bang, J.; Chang, K.; Lee, S.C. Intracellular NO-releasing hyaluronic acid-based nanocarriers: A potential chemosensitizing agent for cancer chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 26870–26881. [Google Scholar] [CrossRef]
- Sun, F.; Wang, Y.; Luo, X.; Ma, Z.; Xu, Y.; Zhang, X.; Lv, T.; Zhang, Y.; Wang, M.; Huang, Z.; et al. Anti-CD24 antibody–nitric oxide conjugate selectively and potently suppresses hepatic carcinoma. Cancer Res. 2019, 79, 3395–3405. [Google Scholar] [CrossRef] [Green Version]
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Kuang, Y.; Balakrishnan, K.; Gandhi, V.; Peng, X. Hydrogen peroxide inducible DNA cross-linking agents: Targeted anticancer prodrugs. J. Am. Chem. Soc. 2011, 133, 19278–19281. [Google Scholar] [CrossRef] [Green Version]
- Gotte, G.; Amelio, E.; Russo, S.; Marlinghaus, E.; Musci, G.; Suzuki, H. Short-time non-enzymatic nitric oxide synthesis from L -arginine and hydrogen peroxide induced by shock waves treatment. FEBS Lett. 2002, 520, 153–155. [Google Scholar] [CrossRef]
- Mukherjee, M.; Ray, A.R. Biomimetic oxidation of l-arginine with hydrogen peroxide catalyzed by the resin-supported iron (III) porphyrin. J. Mol. Catal. A Chem. 2007, 266, 207–214. [Google Scholar] [CrossRef]
- Sun, H.; Chen, L.; Cao, S.; Liang, Y.; Xu, Y. Warburg effects in cancer and normal proliferating cells: Two tales of the same name. Genom. Proteom. Bioinform. 2019, 17, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Fadaka, A.; Ajiboye, B.; Ojo, O.; Adewale, O.; Olayide, I.; Emuowhochere, R. Biology of glucose metabolization in cancer cells. J. Oncol. Sci. 2017, 3, 45–51. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An update on calcium carbonate nanoparticles as cancer drug/gene delivery system. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, D.E.; Park, D.J.; Choi, G.H.; Yang, D.N.; Heo, J.S.; Lee, S.C. PH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf. B Biointerfaces 2016, 146, 1–8. [Google Scholar] [CrossRef]
- Zhang, J.; Song, H.; Ji, S.; Wang, X.; Huang, P.; Zhang, C.; Wang, W.; Kong, D. NO prodrug-conjugated, self-assembled, pH-responsive and galactose receptor targeted nanoparticles for co-delivery of nitric oxide and doxorubicin. Nanoscale 2018, 10, 4179–4188. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Kudo, S.; Nagasaki, Y. Facile and quantitative synthesis of a poly(ethylene glycol)-b-poly(l-arginine) block copolymer and its use for the preparation of polyion complex micelles with polyanions for biomedical applications. Macromol. Rapid Commun. 2015, 36, 1916–1922. [Google Scholar] [CrossRef]
- Kudo, S.; Nagasaki, Y. A novel nitric oxide-based anticancer therapeutics by macrophage-targeted poly(l-arginine)-based nanoparticles. J. Control. Release 2015, 217, 256–262. [Google Scholar] [CrossRef]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar]
- Fang, J.; Nakamura, H.; Maeda, H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011, 63, 136–151. [Google Scholar] [CrossRef]
- Vong, L.B.; Trinh, N.-T.; Nagasaki, Y. Design of amino acid-based self-assembled nano-drugs for therapeutic applications. J. Control. Release 2020, 326, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Ibayashi, Y.; Lee, Y.; Ngo, D.-N.; Nishikawa, Y.; Nagasaki, Y. Poly(ornithine)-based self-assembling drug for recovery of hyperammonemia and damage in acute liver injury. J. Control. Release 2019, 310, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Vong, L.B.; Sato, Y.; Chonpathompikunlert, P.; Tanasawet, S.; Hutamekalin, P.; Nagasaki, Y. Self-assembled polydopamine nanoparticles improve treatment in Parkinson’s disease model mice and suppress dopamine-induced dyskinesia. Acta Biomater. 2020, 109, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.S.; van der Vlies, A.; Gantz, J.; Thacher, T.N.; Antonijevic, S.; Cavadini, S.; Demurtas, D.; Stergiopulos, N.; Hubbell, J.A. Micelles for delivery of nitric oxide. J. Am. Chem. Soc. 2009, 131, 14413–14418. [Google Scholar] [CrossRef]
- Gillet, J.-P.; Gottesman, M.M. Mechanisms of Multidrug Resistance in Cancer BT-Multi-Drug Resistance in Cancer; Zhou, J., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 47–76. ISBN 978-1-60761-416-6. [Google Scholar]
- Riganti, C.; Miraglia, E.; Viarisio, D.; Costamagna, C.; Pescarmona, G.; Ghigo, D.; Bosia, A. Nitric oxide reverts the resistance to doxorubicin in human colon cancer cells by inhibiting the drug efflux. Cancer Res. 2005, 65, 516–525. [Google Scholar]
- Sinha, B.K.; Bortner, C.D.; Mason, R.P.; Cannon, R.E. Nitric oxide reverses drug resistance by inhibiting ATPase activity of p-glycoprotein in human multi-drug resistant cancer cells. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 2806–2814. [Google Scholar] [CrossRef]
- Fan, J.; He, Q.; Liu, Y.; Zhang, F.; Yang, X.; Wang, Z.; Lu, N.; Fan, W.; Lin, L.; Niu, G.; et al. Light-responsive biodegradable nanomedicine overcomes multidrug resistance via NO-enhanced chemosensitization. ACS Appl. Mater. Interfaces 2016, 8, 13804–13811. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.F.; Liu, H.Y.; Lin, K.J.; Chia, W.T.; Sung, H.W. A pH-responsive carrier system that generates NO bubbles to trigger drug release and reverse P-Glycoprotein-Mediated multidrug resistance. Angew. Chem. Int. Ed. 2015, 54, 9890–9893. [Google Scholar] [CrossRef]
- Niu, X.; Cao, J.; Zhang, Y.; Gao, X.; Cheng, M.; Liu, Y.; Wang, W.; Yuan, Z. A glutathione responsive nitric oxide release system based on charge-reversal chitosan nanoparticles for enhancing synergistic effect against multidrug resistance tumor. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102015. [Google Scholar] [CrossRef]
- Sullivan, R.; Paré, G.C.; Frederiksen, L.J.; Semenza, G.L.; Graham, C.H. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol. Cancer Ther. 2008, 7, 1961–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callapina, M.; Zhou, J.; Schmid, T.; Köhl, R.; Brüne, B. NO restores HIF-1α hydroxylation during hypoxia: Role of reactive oxygen species. Free Radic. Biol. Med. 2005, 39, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Sogawa, K.; Numayama-Tsuruta, K.; Ema, M.; Abe, M.; Abe, H.; Fujii-Kuriyama, Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc. Natl. Acad. Sci. USA 1998, 95, 7368–7373. [Google Scholar] [CrossRef] [Green Version]
- Guo, R.; Tian, Y.; Wang, Y.; Yang, W. Near-infrared laser-triggered Nitric Oxide nanogenerators for the reversal of multidrug resistance in cancer. Adv. Funct. Mater. 2017, 27, 1606398. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug delivery: Is the enhanced permeability and retention effect sufficient for curing cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef]
- Cabral, H.; Matsumoto, Y.; Mizuno, K.; Chen, Q.; Murakami, M.; Kimura, M.; Terada, Y.; Kano, M.R.; Miyazono, K.; Uesaka, M.; et al. Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size. Nat. Nanotechnol. 2011, 6, 815–823. [Google Scholar] [CrossRef]
- Wu, J.; Akaike, T.; Maeda, H. Modulation of enhanced vascular permeability in tumors by a bradykinin antagonist, a cyclooxygenase inhibitor, and a nitric oxide scavenger. Cancer Res. 1998, 58, 159–165. [Google Scholar]
- Islam, W.; Fang, J.; Imamura, T.; Etrych, T.; Subr, V.; Ulbrich, K.; Maeda, H. Augmentation of the enhanced permeability and retention effect with nitric oxide–generating agents improves the therapeutic effects of nanomedicines. Mol. Cancer Ther. 2018, 17, 2643–2653. [Google Scholar] [CrossRef] [Green Version]
- Weidensteiner, C.; Reichardt, W.; Shami, P.J.; Saavedra, J.E.; Keefer, L.K.; Baumer, B.; Werres, A.; Jasinski, R.; Osterberg, N.; Weyerbrock, A. Effects of the nitric oxide donor JS-K on the blood-tumor barrier and on orthotopic U87 rat gliomas assessed by MRI. Nitric Oxide 2013, 30, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Tahara, Y.; Yoshikawa, T.; Sato, H.; Mori, Y.; Zahangir, M.H.; Kishimura, A.; Mori, T.; Katayama, Y. Encapsulation of a nitric oxide donor into a liposome to boost the enhanced permeation and retention (EPR) effect. Medchemcomm 2017, 8, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Šírová, M.; Horková, V.; Etrych, T.; Chytil, P.; Říhová, B.; Studenovský, M. Polymer donors of nitric oxide improve the treatment of experimental solid tumours with nanosized polymer therapeutics. J. Drug Target. 2017, 25, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H. Solid tumor physiology and hypoxia-induced chemo/radio-resistance: Novel strategy for cancer therapy: Nitric oxide donor as a therapeutic enhancer. Nitric Oxide 2008, 19, 205–216. [Google Scholar] [CrossRef]
- Yasuda, H.; Yamaya, M.; Nakayama, K.; Sasaki, T.; Ebihara, S.; Kanda, A.; Asada, M.; Inoue, D.; Suzuki, T.; Okazaki, T.; et al. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J. Clin. Oncol. 2006, 24, 688–694. [Google Scholar] [CrossRef]
- Davidson, A.; Veillard, A.-S.; Tognela, A.; Chan, M.M.K.; Hughes, B.G.M.; Boyer, M.; Briscoe, K.; Begbie, S.; Abdi, E.; Crombie, C.; et al. A phase III randomized trial of adding topical nitroglycerin to first-line chemotherapy for advanced nonsmall-cell lung cancer: The Australasian lung cancer trials group NITRO trial. Ann. Oncol. 2015, 26, 2280–2286. [Google Scholar] [CrossRef]
- de Jong, E.E.C.; van Elmpt, W.; Leijenaar, R.T.H.; Hoekstra, O.S.; Groen, H.J.M.; Smit, E.F.; Boellaard, R.; van der Noort, V.; Troost, E.G.C.; Lambin, P.; et al. [18F]FDG PET/CT-based response assessment of stage IV non-small cell lung cancer treated with paclitaxel-carboplatin-bevacizumab with or without nitroglycerin patches. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 8–16. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Cun, X.; Ruan, S.; Liu, R.; Xiao, W.; Yang, X.; Yang, Y.; Yang, C.; Gao, H. Enzyme-triggered size shrink and laser-enhanced NO release nanoparticles for deep tumor penetration and combination therapy. Biomaterials 2018, 168, 64–75. [Google Scholar] [CrossRef]
- Deepagan, V.G.; Ko, H.; Kwon, S.; Rao, N.V.; Kim, S.K.; Um, W.; Lee, S.; Min, J.; Lee, J.; Choi, K.Y.; et al. Intracellularly activatable nanovasodilators to enhance passive cancer targeting regime. Nano Lett. 2018, 18, 2637–2644. [Google Scholar] [CrossRef]
- Maeda, H.; Tsukigawa, K.; Fang, J. A retrospective 30 years after discovery of the enhanced permeability and retention effect of solid tumors: Next-generation chemotherapeutics and photodynamic therapy—problems, solutions, and prospects. Microcirculation 2016, 23, 173–182. [Google Scholar] [CrossRef]
- Fang, J.; Islam, W.; Maeda, H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Seki, T.; Fang, J.; Maeda, H. Enhanced delivery of macromolecular antitumor drugs to tumors by nitroglycerin application. Cancer Sci. 2009, 100, 2426–2430. [Google Scholar] [CrossRef]
- Kwon, I.K.; Lee, S.C.; Han, B.; Park, K. Analysis on the current status of targeted drug delivery to tumors. J. Control. Release 2012, 164, 108–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef]
- Lee, D.I.; Zhu, G.; Sasaki, T.; Cho, G.S.; Hamdani, N.; Holewinski, R.; Jo, S.H.; Danner, T.; Zhang, M.; Rainer, P.P.; et al. Phosphodiesterase 9A controls nitric-oxide-independent cGMP and hypertrophic heart disease. Nature 2015, 519, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol. Asp. Med. 2005, 26, 33–65. [Google Scholar] [CrossRef]
- Thomas, D.D. Breathing new life into nitric oxide signaling: A brief overview of the interplay between oxygen and nitric oxide. Redox Biol. 2015, 5, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Rodríguez-Iturbe, B. Mechanisms of disease: Oxidative stress and inflammation in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2006, 2, 582–593. [Google Scholar] [CrossRef]
- Beckman, J.S.; Beckman, T.W.; Chen, J.; Marshall, P.A.; Freeman, B.A. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc. Natl. Acad. Sci. USA 1990, 87, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; LLeonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [Green Version]
- Tratsiakovich, Y.; Gonon, A.T.; Krook, A.; Yang, J.; Shemyakin, A.; Sjöquist, P.O.; Pernow, J. Arginase inhibition reduces infarct size via nitric oxide, protein kinase C epsilon and mitochondrial ATP-dependent K+ channels. Eur. J. Pharmacol. 2013, 712, 16–21. [Google Scholar] [CrossRef]
- Drummond, G.R.; Selemidis, S.; Griendling, K.K.; Sobey, C.G. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 453–471. [Google Scholar] [CrossRef] [Green Version]
- Anter, E.; Thomas, S.R.; Schulz, E.; Shapira, O.M.; Vita, J.A.; Keaney, J.F. Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. J. Biol. Chem. 2004, 279, 46637–46643. [Google Scholar] [CrossRef] [Green Version]
- Jackson, T.S.; Xu, A.; Vita, J.A.; Keaney, J.F. Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ. Res. 1998, 83, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Huang, A.; Vita, J.A.; Venema, R.C.; Keaney, J.F. Ascorbic acid enhances endothelial nitric-oxide synthase activity by increasing intracellular tetrahydrobiopterin. J. Biol. Chem. 2000, 275, 17399–17406. [Google Scholar] [CrossRef] [Green Version]
- Prasad, A.; Andrews, N.P.; Padder, F.A.; Husain, M.; Quyyumi, A.A. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J. Am. Coll. Cardiol. 1999, 34, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Vong, L.B.; Bui, T.Q.; Tomita, T.; Sakamoto, H.; Hiramatsu, Y.; Nagasaki, Y. Novel angiogenesis therapeutics by redox injectable hydrogel—Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials 2018, 167, 143–152. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Redox polyion complex micelle-based injectable hydrogel as local reactive oxygen species scavenging therapeutics. ACS Symp. Ser. 2019, 1309, 287–307. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vong, L.B.; Nagasaki, Y. Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants 2020, 9, 791. https://doi.org/10.3390/antiox9090791
Vong LB, Nagasaki Y. Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants. 2020; 9(9):791. https://doi.org/10.3390/antiox9090791
Chicago/Turabian StyleVong, Long Binh, and Yukio Nagasaki. 2020. "Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges" Antioxidants 9, no. 9: 791. https://doi.org/10.3390/antiox9090791
APA StyleVong, L. B., & Nagasaki, Y. (2020). Nitric Oxide Nano-Delivery Systems for Cancer Therapeutics: Advances and Challenges. Antioxidants, 9(9), 791. https://doi.org/10.3390/antiox9090791