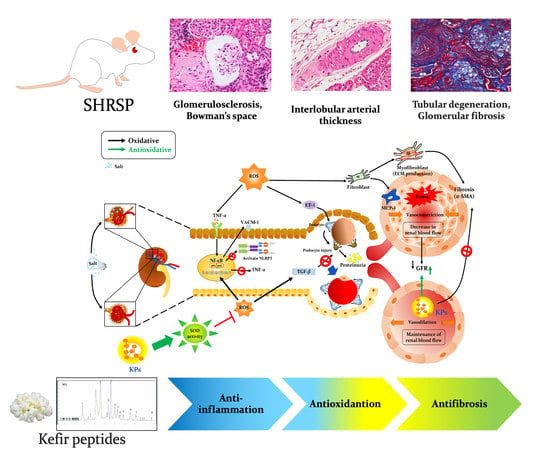
Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Kefir Peptides
2.2. Animal Experiment
2.3. Determination of Serum and Urine Biochemical Markers
2.4. Measurement of the Glomerular Filtration Rate (GFR) of Plasma FITC-Inulin
2.5. Histopathological Examination of the Kidneys
2.6. Immunohistochemical Staining
2.7. Oxidative Stress and Inflammatory Activity Assays
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of KPs on the Body Weight, Organ Index, and Biochemical Profiles of Salt-Treated SHRSP Rats
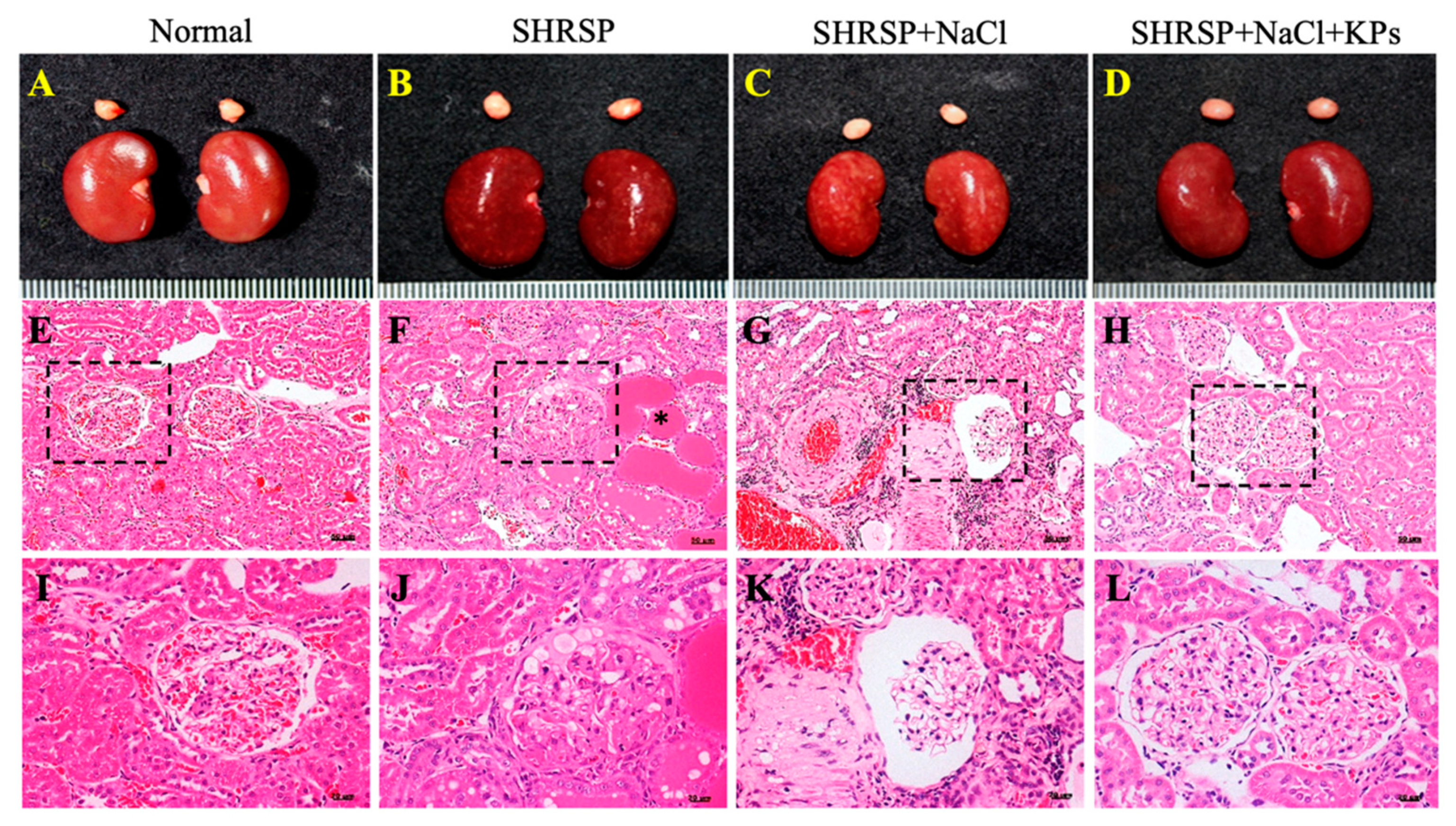
3.2. KPs Attenuated Salt-Induced Glomerulosclerosis and Tubular Damage in SHRSP Rats
3.3. KPs Ameliorated Salt-Induced Interlobular Arterial Thickness in SHRSP Rats
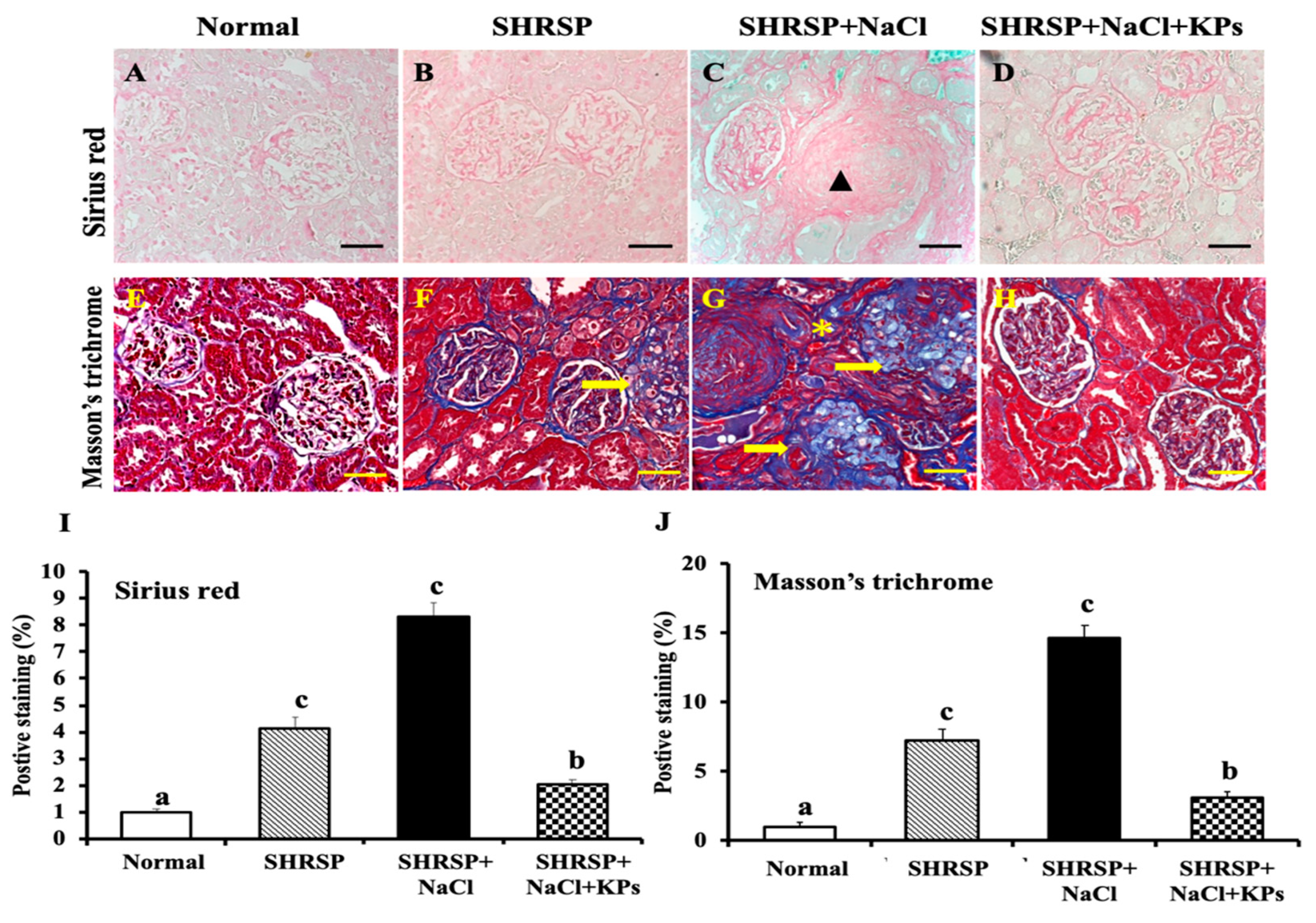
3.4. KPs Improved Salt-Induced Renal Interstitial Collagen Deposition in SHRSP Rats
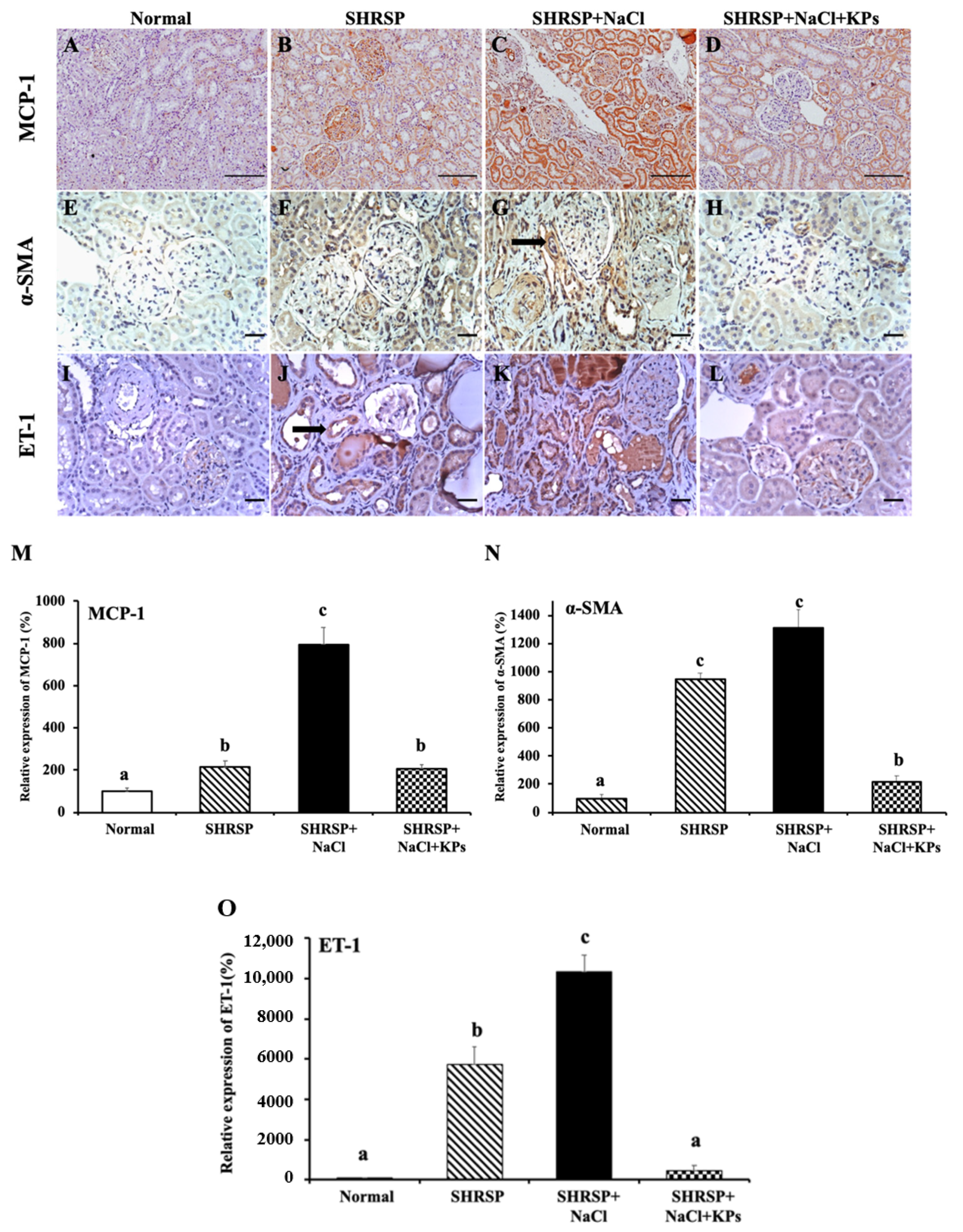
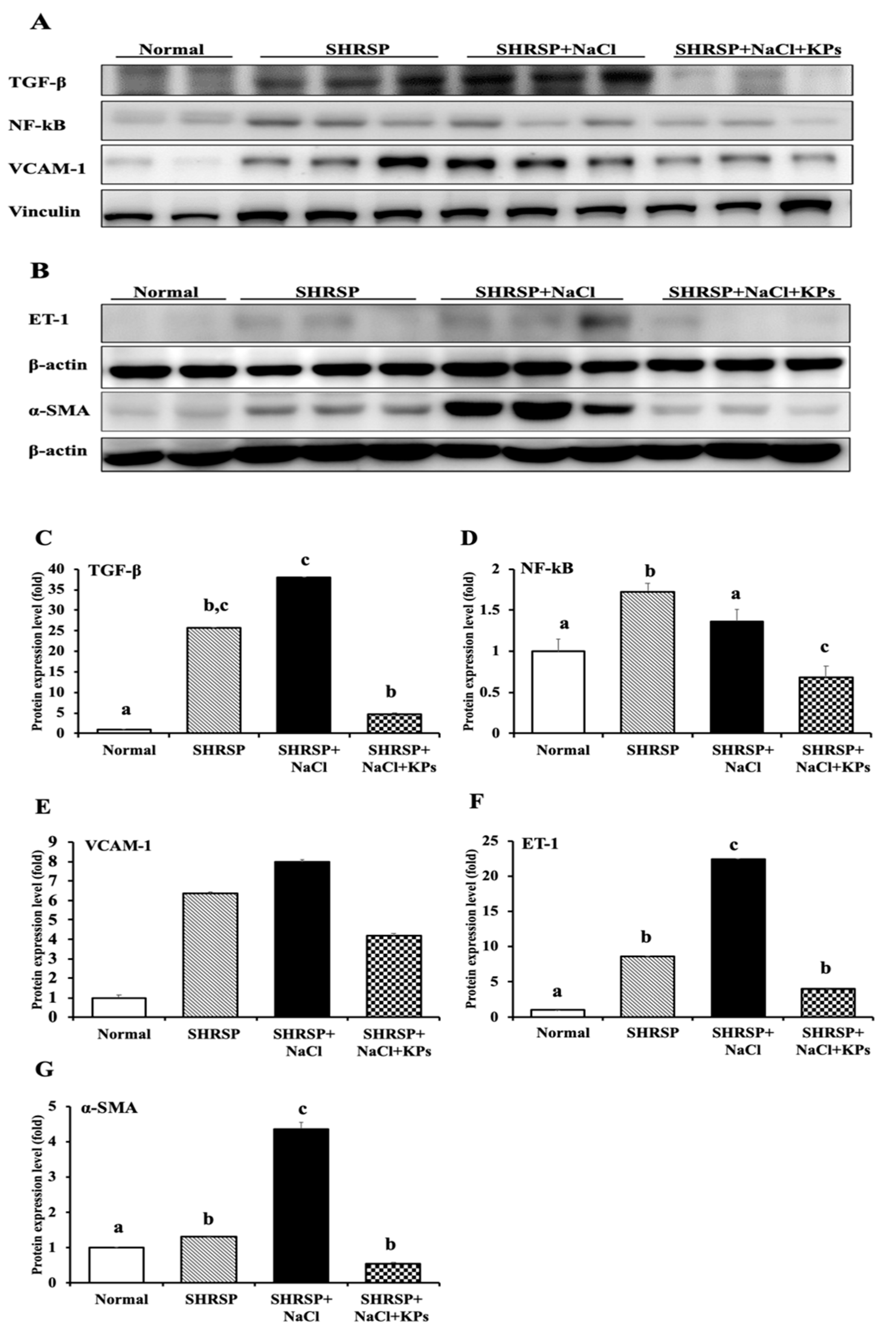
3.5. KPs Decreased Salt-Induced Renal MCP-1, α-SMA, and ET-1 Expression in SHRSP Rats
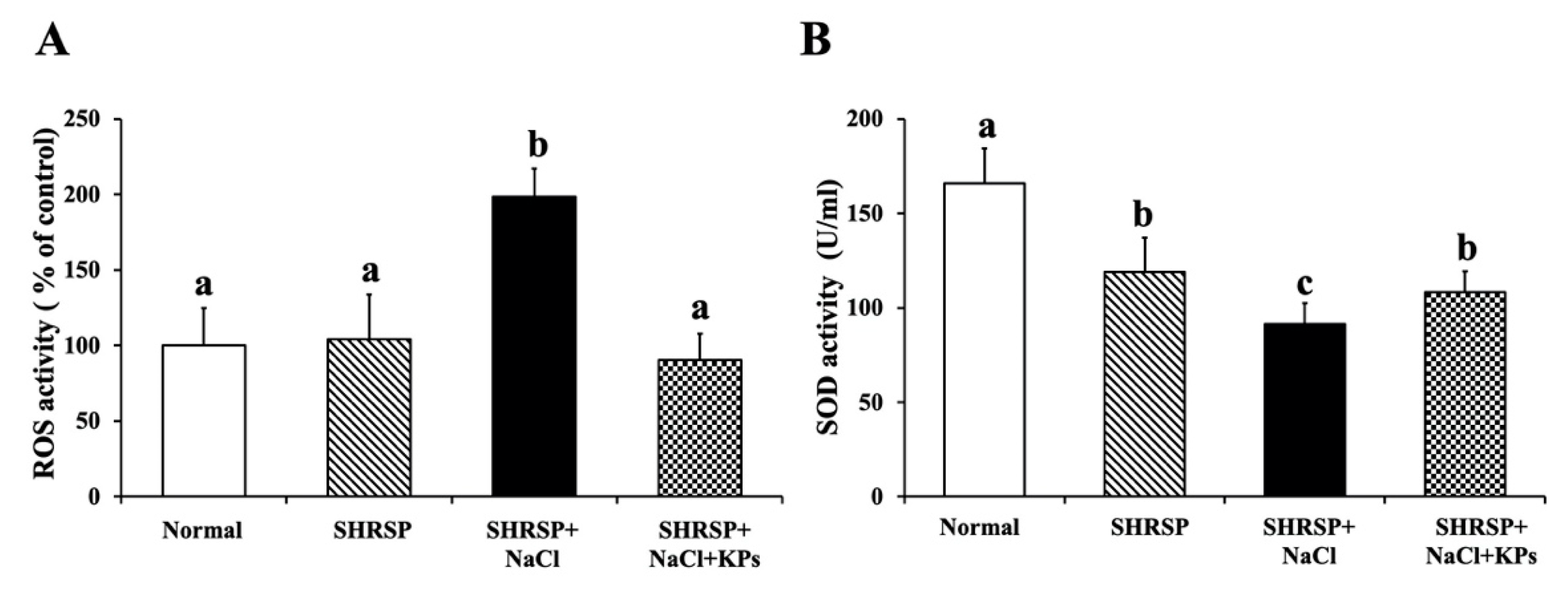
3.6. Effect of KPs on Oxidative Stress and Antioxidative SOD Activity in the Kidneys of Salt-Treated SHRSP Rats
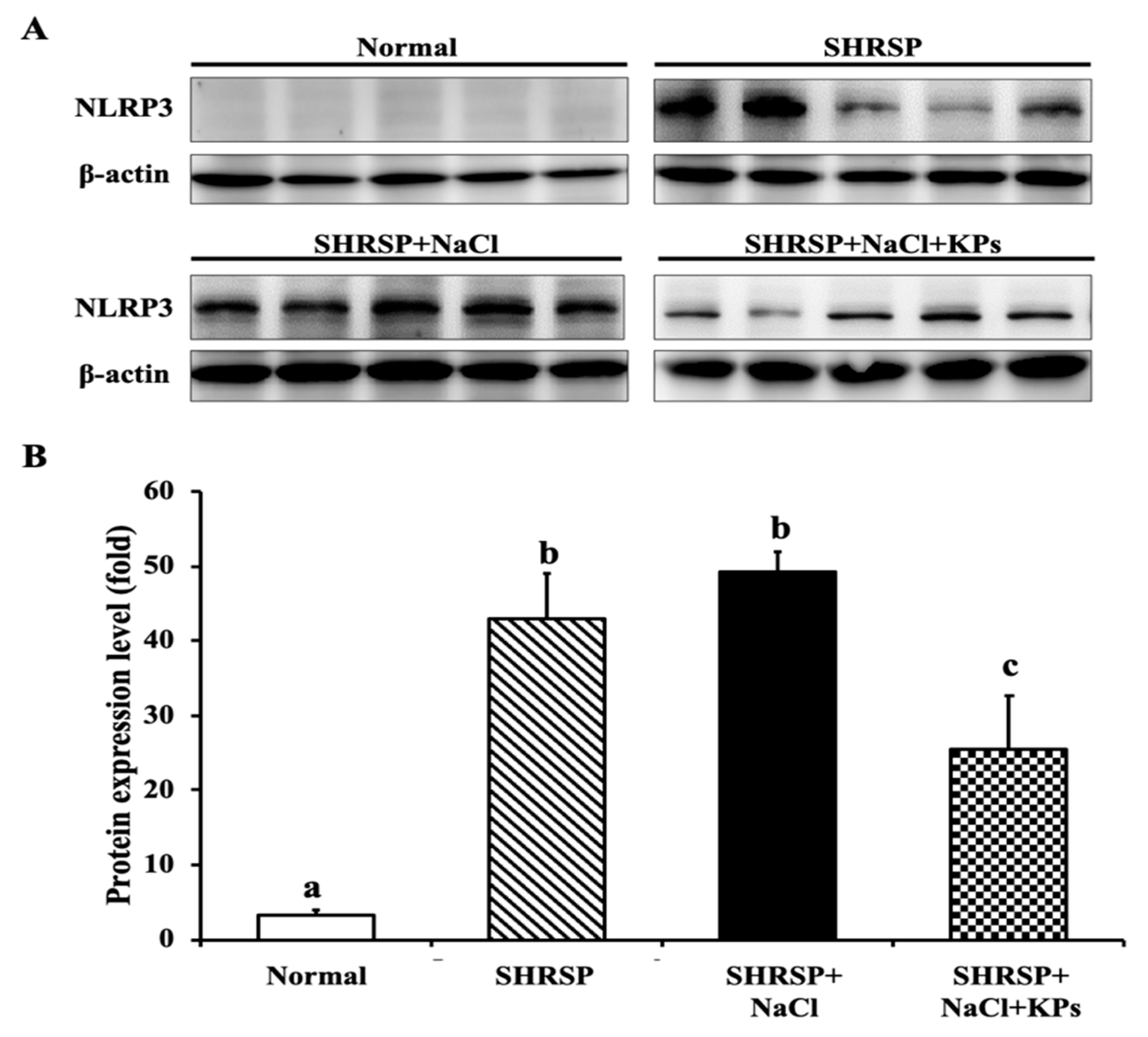
3.7. KPs Reduced Salt-Induced Renal Inflammatory Cytokines, the NLRP3 Inflammasome and Fibrotic Activation in SHRSP Rats
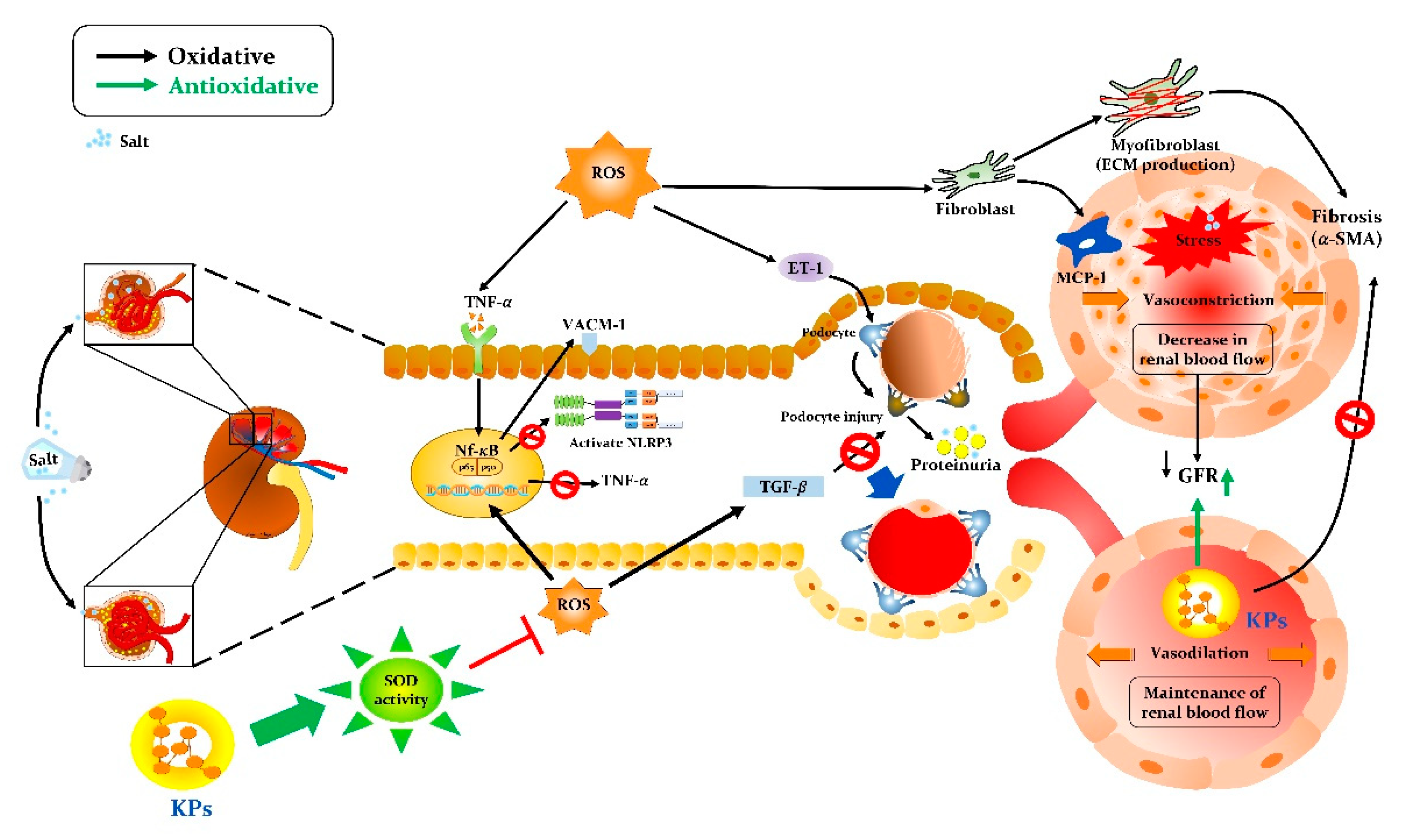
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grisk, O.; DiBona, G.F. Cardiopulmonary baroreflex in NaCl-induced hypertension in borderline hypertensive rats. Hypertension 1997, 1, 464–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotchen, T.A. Contributions of sodium and chloride to NaCl-induced hypertension. Hypertension 2005, 45, 849–850. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, X.C.; Lu, L.; Cao, Y.; Sun, R.R.; Chen, S.; Zhang, P.Y. Cardiovascular disease and its relationship with chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2918–2926. [Google Scholar] [PubMed]
- Gelosa, P.; Banfi, C.; Gianella, A.; Brioschi, M.; Pignieri, A.; Nobili, E.; Castiglioni, L.; Cimino, M.; Tremoli, E.; Sironi, L. Peroxisome proliferator-activated receptor α agonism prevents renal damage and the oxidative stress and inflammatory processes affecting the brains of stroke-prone rats. J. Pharm. Exp. Ther. 2010, 335, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.E.; Tighiouart, H.; Amin, M.G.; Stark, P.C.; MacLeod, B.; Griffith, J.L.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J. Am. Soc. Nephrol. 2004, 15, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- McClellan, W.M.; Flanders, W.D.; Langston, R.D.; Jurkovitz, C.; Presley, R. Anemia and renal insufficiency are independent risk factors for death among patients with congestive heart failure admitted to community hospitals: A population-based study. J. Am. Soc. Nephrol. 2002, 13, 1928–1936. [Google Scholar] [CrossRef] [Green Version]
- Walsh, C.R.; O’Donnell, C.J.; Camargo, C.A., Jr.; Giugliano, R.P.; Lloyd-Jones, D.M. Elevated serum creatinine is associated with 1-year mortality after acute myocardial infarction. Am. Heart. J. 2002, 144, 1003–1011. [Google Scholar] [CrossRef]
- Yin, K.; McGiff, J.C.; Bell-Quilley, C.P. Role of chloride in the variable response of the kidney to cyclooxygenase inhibition. Am. J. Physiol. 1995, 268, F561–F568. [Google Scholar] [CrossRef]
- Schmidlin, O.; Tanaka, M.; Bollen, A.W.; Yi, S.L.; Morris, R.C., Jr. Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension 2005, 5, 867–873. [Google Scholar] [CrossRef] [Green Version]
- Thornton, R.M.; Wyss, J.M.; Oparil, S. Impaired reflex response to volume expansion in NaCl-sensitive spontaneously hypertensive rats. Hypertension 1989, 14, 518–523. [Google Scholar] [CrossRef] [Green Version]
- Huang, B.S.; Leenen, F.H.H. Dietary Na+ and cardiopulmonary baroreflex control of renal sympathetic nerve activity in SHR. Am. J. Physiol. 1995, 268, H61–H67. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, M.S.; Jirakulsomchok, S.; Shao, Z.H.; Wyss, J.M. High-NaCl rats increase natriuretic and diuretic responses in salt-resistant but not salt-sensitive SHR. Am. J. Physiol. 1991, 260, F890–F898. [Google Scholar] [PubMed]
- St-Onge, M.P.; Farnworth, E.R.; Savard, T.; Chabot, D.; Mafu, A.; Jones, P.J. Kefir consumption does not alter plasma lipid levels or cholesterol fractional synthesis rates relative to milk in hyperlipidemic men: A randomized controlled trial. BMC Complement. Altern. Med. 2002, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R.; Mainville, I. Kefir: A fermented milk product. In Farnworth ER; Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003; pp. 77–112. [Google Scholar]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic analysis reveals proteolytic activity of kefir microorganisms on bovine milk proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tu, M.Y.; Chen, H.L.; Tung, Y.T.; Kao, C.C.; Hu, F.C.; Chen, C.M. Short-term effects of kefir-fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS ONE 2015, 10, e0144231. [Google Scholar] [CrossRef] [PubMed]
- Ebner, J.; Aşçı Arslan, A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide profiling of bovine kefir reveals 236 unique peptides released from caseins during its production by starter culture or kefir grains. J. Proteom. 2015, 117, 41–57. [Google Scholar] [CrossRef]
- Baum, F.; Fedorova, M.; Ebner, J.; Hoffmann, R.; Pischetsrieder, M. Analysis of the endogenous peptide profile of milk: Identification of 248 mainly casein-derived peptides. J. Proteome Res. 2013, 12, 5447–5462. [Google Scholar] [CrossRef]
- Chen, H.L.; Tung, Y.T.; Chuang, C.H.; Tu, M.Y.; Tsai, T.C.; Chang, S.Y.; Chen, C.M. Kefir improves bone mass and microarchitecture in an ovariectomized rat model of postmenopausal osteoporosis. Osteoporos. Int. 2015, 26, 589–599. [Google Scholar] [CrossRef]
- Chen, H.L.; Hung, K.F.; Yen, C.C.; Laio, C.H.; Wang, J.L.; Lan, Y.W.; Chong, K.Y.; Fan, H.C.; Chen, C.M. Kefir peptides alleviate particulate matter <4 μm (PM4.0)-induced pulmonary inflammation by inhibiting the NF-κB pathway using luciferase transgenic mice. Sci. Rep. 2019, 9, 11529. [Google Scholar] [CrossRef]
- Churchill, P.C.; Churchill, M.C.; Griffin, K.A.; Picken, M.; Webb, R.C.; Kurtz, T.W.; Bidani, A.K. Increased genetic susceptibility to renal damage in the stroke-prone spontaneously hypertensive rat. Kidney Int. 2002, 61, 1794–1800. [Google Scholar] [CrossRef] [Green Version]
- Tung, Y.T.; Chen, H.L.; Wu, H.S.; Ho, M.H.; Chong, K.Y.; Chen, C.M. Kefir peptides prevent hyperlipidemia and obesity in high-fat-diet-induced obese rats via lipid metabolism modulation. Mol. Nutr. Food. Res. 2018, 62, 1700505. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.W.; Tung, Y.T.; Chen, H.L.; Yang, S.H.; Liu, C.Y.; Lu, M.; Pai, H.J.; Lin, C.C.; Chen, C.M. Myostatin propeptide gene delivery by gene gun ameliorates muscle atrophy in a rat model of botulinum toxin-induced nerve denervation. Life Sci. 2016, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Palygin, O.; Levchenko, V.; Ilatovskaya, D.V.; Pavlov, T.S.; Pochynyuk, O.M.; Jacob, H.J.; Geurts, A.M.; Hodges, M.R.; Staruschenko, A. Essential role of Kir5.1 channels in renal salt handling and blood pressure control. JCI Insight 2017, 2, e92331. [Google Scholar] [CrossRef] [PubMed]
- Hinojosa-Laborde, C.; Jespersen, B.; ShadeRieg, R. Physiology lab demonstration: Glomerular filtration rate in a rat. J. Vis. Exp. 2015, 101, e52425. [Google Scholar] [CrossRef] [Green Version]
- Shackelford, C.; Long, G.; Wolf, J.; Okerberg, C.; Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol. Pathol. 2002, 30, 93–96. [Google Scholar] [CrossRef] [Green Version]
- Heagerty, A.M.; Aalkjaer, C.; Bund, S.J.; Korsgaard, N.; Mulvany, M.J. Small artery structure in hypertension: Dual processes of remodeling and growth. Hypertension 1993, 21, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Reddi, A.S.; Nimmagadda, V.R.; Arora, R. Effect of antihypertensive therapy on renal artery structure in type 2 diabetic rats with hypertension. Hypertension 2001, 37, 1273–1278. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.M.; Ling, Y.H.; Huuskes, B.M.; Ferens, D.M.; Saini, N.; Chan, C.T.; Diep, H.; Kett, M.M.; Samuel, C.S.; Kemp-Harper, B.K.; et al. Pharmacological inhibition of the NLRP3 inflammasome reduces blood pressure, renal damage, and dysfunction in salt-sensitive hypertension. Cardiovasc. Res. 2019, 115, 776–787. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Chao, J.; Li, H.J.; Yao, Y.Y.; Shen, B.; Gao, L.; Bledsoe, G.; Chao, L. Kinin infusion prevents renal inflammation, apoptosis, and fibrosis via inhibition of oxidative stress and mitogen-activated protein kinase activity. Hypertension 2007, 49, 490–497. [Google Scholar] [CrossRef]
- Kuro, T.; Okahara, A.; Nose, M.; Ikuse, T.; Matsumura, Y. Effects of SA7060, a novel dual inhibitor of neutral endopeptidase and angiotensin-converting enzyme, on deoxycorticosterone acetate-salt-induced hypertension in rats. Biol. Pharm. Bull. 2000, 23, 820–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pu, Q.; Amiri, F.; Gannon, P.; Schiffrin, E.L. Dual angiotensin-converting enzyme/neutral endopeptidase inhibition on cardiac and renal fibrosis and inflammation in DOCA-salt hypertensive rats. J. Hypertens. 2005, 23, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Vlachojannis, J.G.; Tsakas, S.; Petropoulou, C.; Goumenos, D.S.; Alexandri, S. Endothelin-1 in the kidney and urine of patients with glomerular disease and proteinuria. Clin. Nephrol. 2002, 58, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Littman, M.P.; Robertson, J.L.; Bovee, K.C. Spontaneous systemic hypertension in dogs: Five cases (1981–1983). J. Am. Vet. Med. Assoc. 1988, 193, 486–494. [Google Scholar] [PubMed]
- Glassock, R.J.; Warnock, D.G.; Delanaye, P. The global burden of chronic kidney disease: Estimates, variability and pitfalls. Nat. Rev. Nephrol. 2017, 13, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Obata, J.; Kuroyanagi, R.; Kimura, H.; Ikeda, Y.; Takano, H.; Naito, A.; Sato, T.; Yoshida, Y. Involvement of angiotensin II in glomerulosclerosis of stroke-prone spontaneously hypertensive rats. Kidney Int. 1996, 55, S109–S112. [Google Scholar]
- Gewin, L.; Zent, R. How does TGF-β mediate tubulointerstital fibrosis? Semin. Nephrol. 2012, 32, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.L.; Topley, N.; Ito, T.; Phillips, A. Interleukin-6 regulation of transforming growth factor (TGF)-beta receptor compartmentalization and turnover enhances TGF-beta1 signaling. J. Biol. Chem. 2005, 280, 12239–12245. [Google Scholar] [CrossRef] [Green Version]
- Lenda, D.M.; Sauls, B.A.; Boegehold, M.A. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am. J. Physiol. 2000, 279, H7–H14. [Google Scholar] [CrossRef]
- Brasier, A.R. The nuclear factor-kappaB-interleukin-6 signaling pathway mediating vascular inflammation. Cardiovasc. Res. 2010, 86, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, C.M.; Minto, A.W.; Dorf, M.E.; Proudfoot, A.; Wells, T.N.; Salant, D.J.; Gutierrez-Ramos, J.C. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J. Exp. Med. 1997, 185, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T.; Hirooka, Y.; Kimura, Y.; Ito, K.; Shimokawa, H.; Takeshita, A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 2004, 109, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Michihara, A.; Shimatani, M.; Anraku, M.; Tomida, H.; Akasaki, K. High levels of oxidative stress exist in the brain than serum or kidneys in stroke-prone spontaneously hypertensive rats at ten weeks of age. Biol. Pharm. Bull. 2010, 33, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, R.D., Jr.; Meng, S.; Tian, N. Renal and vascular oxidative stress and salt-sensitivity of arterial pressure. Acta Physiol. Scand. 2003, 179, 243–250. [Google Scholar] [CrossRef]
- Badid, C.; Vincent, M.; Fouque, D.; Laville, M.; Desmoulière, A. Myofibroblast: A prognostic marker and target cell in progressive renal disease. Ren. Fail. 2001, 23, 543–549. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; Van, G.Y.; Qi, M. Myofibroblast and alpha 1 (III) collagen expression in experimental tubulointerstitial nephritis. Kidney Int. 1997, 51, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Alpers, C.E.; Hudkins, K.L.; Gown, A.M.; Johnson, R.J. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int. 1992, 41, 1134–1142. [Google Scholar] [CrossRef] [Green Version]
- Eardly, K.S.; Cockwell, P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005, 68, 437–455. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Bai, Y.; Wu, L.; Hong, W.; Liang, Y.; Chen, B.; Bai, Y. Inhibition of macrophage migration inhibitory factor protects against inflammation and matrix deposition in kidney tissues after injury. Mediat. Inflamm. 2016, 2016, 2174682. [Google Scholar] [CrossRef] [Green Version]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, S.M.; Dowling, J.K.; Ling, Y.H.; Diep, H.; Chan, C.T.; Ferens, D.; Kett, M.M.; Pinar, A.; Samuel, C.S.; Vinh, A.; et al. Inflammasome activity is essential for one kidney/deoxycorticosterone acetate/salt-induced hypertension in mice. Br. J. Pharmacol. 2016, 173, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, H.; Satoh, M.; Sasaki, T.; Taniguchi, S.; Takahashi, M.; Kashihara, N. Excess aldosterone is a critical danger signal for inflammasome activation in the development of renal fibrosis in mice. FASEB J. 2015, 29, 3899–3910. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Normal | SHRSP | SHRSP+NaCl | SHRSP+NaCl+KPs | |
|---|---|---|---|---|---|
| Body weight (g) | 398 ± 5.82 a | 322 ± 8.29 b | 202 ± 7.19 c | 317 ± 5.86 b | |
| Kidneys index (%) 1 | 0.677 ± 0.019 a | 0.914 ± 0.048 bc | 0.936 ± 0.076 c | 0.835 ± 0.021 bc | |
| Serum | BUN (mg/dl) | 15.8 ± 0.3 a | 26.8 ± 2.0 b | 31.7 ± 3.1 b | 30.4 ± 4.5 b |
| CRE (mg/dl) | 0.4 ± 0.03 a | 0.4 ± 0.04 a | 0.5 ± 0.08 a | 0.4 ± 0.04 a | |
| CRE/BUN ratio | 0.022 ± 0.001 a | 0.015 ± 0.001 a | 0.015 ± 0.002 a | 0.014 ± 0.001 a | |
| GFR/g kidney weight (ml/min/g) | 2.19 ± 0.14 a | 1.69 ± 0.31 b | 0.71 ± 0.49 c | 1.60 ± 0.13 b | |
| Urine | FeNa (%) 2 | 0.05 ± 0.01 a | 0.23 ± 0.05 a | 1.47 ± 0.52 b | 0.23 ± 0.02 a |
| FeK (%) | 7.32 ± 0.66 a | 8.68 ± 1.62 a | 5.91 ± 0.82 a | 8.65 ± 1.31 a | |
| FeCl (%) | 0.16 ± 0.01 a | 0.44 ± 0.08 a | 2.33 ± 0.67 b | 0.39 ± 0.05 a | |
| UCRE (mg/dl) | 24 ± 3.25 a | 63 ± 3.69 b | 20 ± 2.87 a | 55 ± 3.45 b | |
| UPRO (mg/dl) | 42 ± 2.74 a | 764 ± 73.59 b | 901 ± 66.71 c | 762 ± 26.0 b | |
| UPC | 0.57 ± 0.04 a | 12.1 ± 1.16 b | 49.3 ± 8.72 c | 14.0 ± 0.76 b |
| Lesions 1 | Group 2 | |||
|---|---|---|---|---|
| Normal | SHRSP | SHRSP+NaCl | SHRSP+NaCl+KPs | |
| Afferent arteriolopathy, segmental glomerulosclerosis, and tubular atrophy in the juxtamedullary cortex | 0 a | 1.3 ± 0.63 a | 2.5 ± 0.29 b | 0.7 ± 0.33 a |
| Cast, hyaline, tubule, focal | 0 a | 2.0 ± 0.41 b | 3.0 ± 0.41 b | 2.0 ± 0.00 b |
| Chronic progressive nephrosis | 0 a | 2.5 ± 0.29 c | 3.3 ± 0.25 c | 1.3 ± 0.67 b |
| Hypertrophy, media, arterioles, focal | 0 a | 1.8 ± 0.85 b | 3.0 ± 0.00 b | 0.3 ± 0.33 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-H.; Chen, H.-L.; Fan, H.-C.; Tung, Y.-T.; Kuo, C.-W.; Tu, M.-Y.; Chen, C.-M. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats. Antioxidants 2020, 9, 790. https://doi.org/10.3390/antiox9090790
Chen Y-H, Chen H-L, Fan H-C, Tung Y-T, Kuo C-W, Tu M-Y, Chen C-M. Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats. Antioxidants. 2020; 9(9):790. https://doi.org/10.3390/antiox9090790
Chicago/Turabian StyleChen, Yu-Hsuan, Hsiao-Ling Chen, Hueng-Chuen Fan, Yu-Tang Tung, Chia-Wen Kuo, Min-Yu Tu, and Chuan-Mu Chen. 2020. "Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats" Antioxidants 9, no. 9: 790. https://doi.org/10.3390/antiox9090790
APA StyleChen, Y.-H., Chen, H.-L., Fan, H.-C., Tung, Y.-T., Kuo, C.-W., Tu, M.-Y., & Chen, C.-M. (2020). Anti-Inflammatory, Antioxidant, and Antifibrotic Effects of Kefir Peptides on Salt-Induced Renal Vascular Damage and Dysfunction in Aged Stroke-Prone Spontaneously Hypertensive Rats. Antioxidants, 9(9), 790. https://doi.org/10.3390/antiox9090790