Oxidative Stress and Inflammation Induced by Waterpipe Tobacco Smoking Despite Possible Protective Effects of Exercise Training: A Review of the Literature
Abstract
:1. Introduction
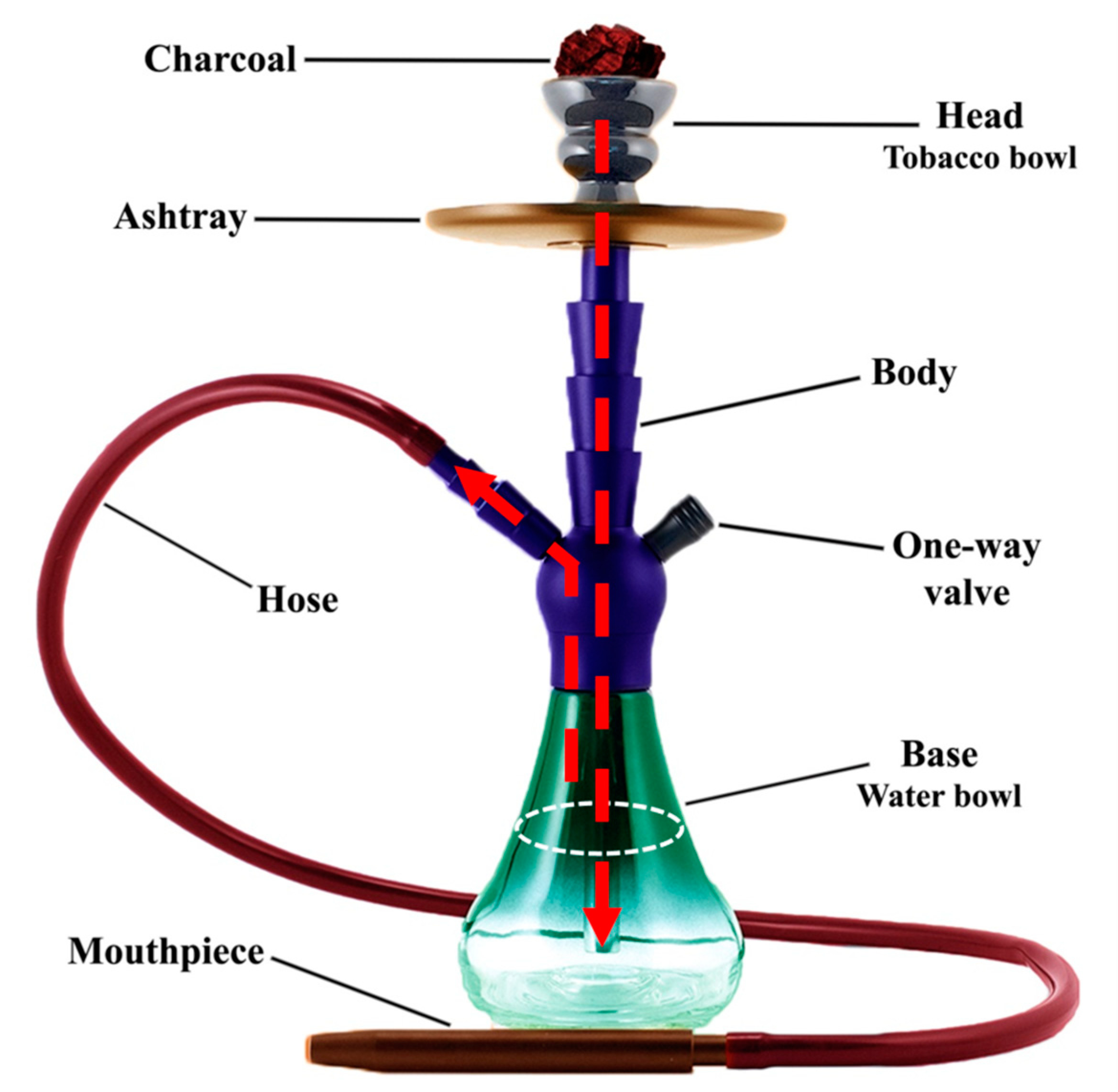
2. An Overview of Waterpipes
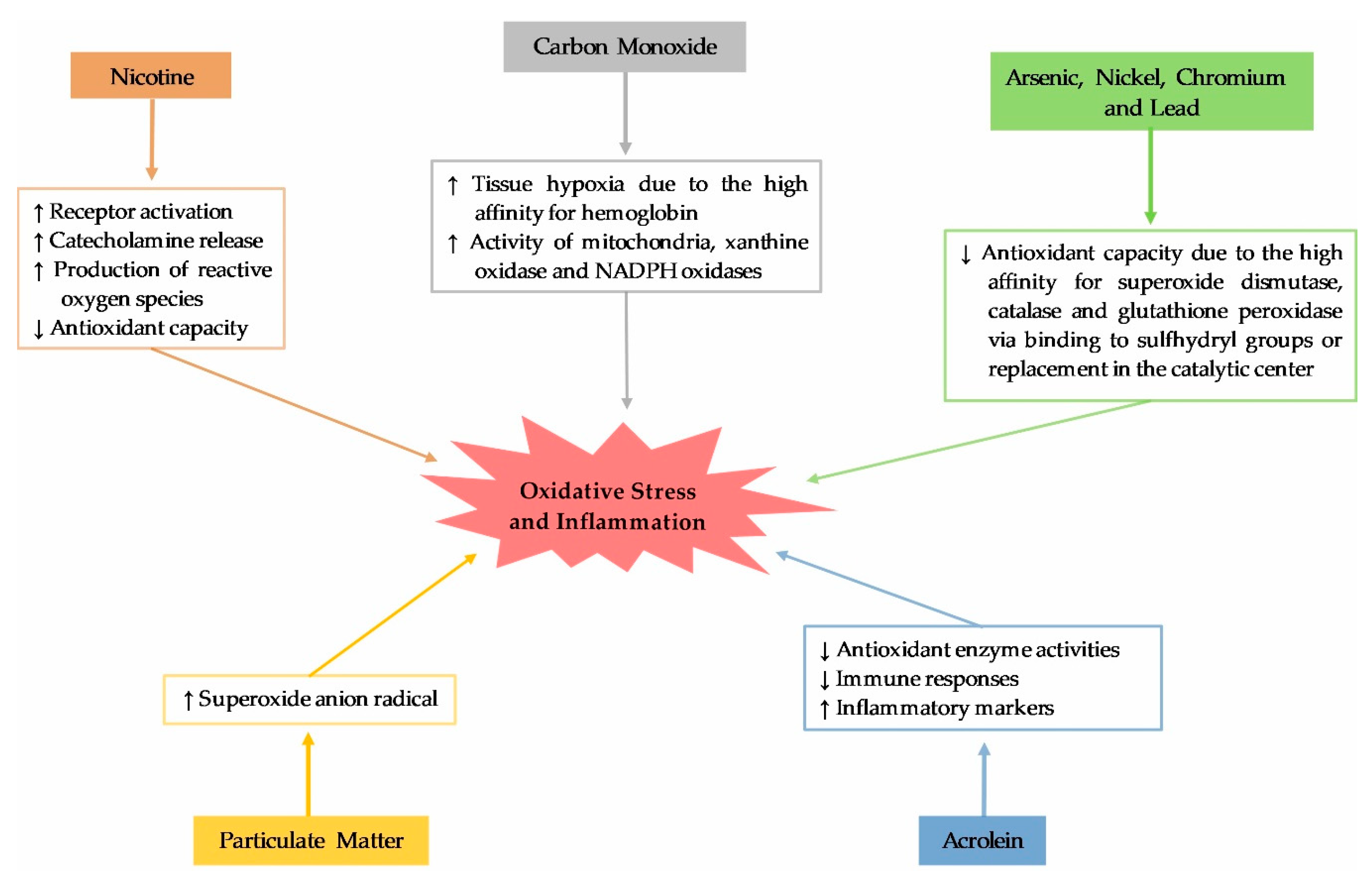
3. Toxicants Released from Waterpipe Smoking
4. Waterpipe Smoking, Oxidative Stress and Inflammation
5. Impact of Exercise Training on Oxidative Stress and Inflammatory Markers in Waterpipe Smokers
5.1. Acute Responses after Exercise
5.2. Effects of Regular Exercise Training
6. Waterpipe Effects on Exercise Capacity and Lung Functions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zafeiridou, M.; Hopkinson, N.S.; Voulvoulis, N. Cigarette Smoking: An Assessment of Tobacco’s Global Environmental Footprint Across Its Entire Supply Chain, and Policy Strategies to Reduce It; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- World Health Organization. Tobacco; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Giovino, G.A.; Mirza, S.A.; Samet, J.M.; Gupta, P.C.; Jarvis, M.J.; Bhala, N.; Peto, R.; Zatonski, W.; Hsia, J.; Morton, J. Tobacco use in 3 billion individuals from 16 countries: An analysis of nationally representative cross-sectional household surveys. Lancet 2012, 380, 668–679. [Google Scholar] [CrossRef]
- World Health Organization Study Group on Tobacco Product Regulation. Advisory Note: Waterpipe Tobacco Smoking: Health Effects, Research Needs and Recommended Actions for Regulators, 2nd ed.; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Martinasek, M.P.; McDermott, R.J.; Martini, L. Waterpipe (hookah) tobacco smoking among youth. Curr. Probl. Pediatr. Adolesc. Health Care 2011, 41, 34–57. [Google Scholar] [CrossRef]
- Waziry, R.; Jawad, M.; Ballout, R.A.; Al Akel, M.; Akl, E.A. The effects of waterpipe tobacco smoking on health outcomes: An updated systematic review and meta-analysis. Int. J. Epidemiol. 2017, 46, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jawad, M.; Charide, R.; Waziry, R.; Darzi, A.; Ballout, R.A.; Akl, E.A. The prevalence and trends of waterpipe tobacco smoking: A systematic review. PLoS ONE 2018, 13, e0192191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhaee, M.R.; Joukar, S.; Zolfaghari, M.R.; Rostamzadeh, F.; Masoumi-Ardakani, Y.; Iranpour, M.; Nazari, M. Effects of Endurance Exercise Training on Cardiac Dysfunction Induced by Waterpipe Tobacco Smoking. Addict. Health 2019, 11, 100. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, D.; Momenan, A.A.; Amouzegar, A.; Azizi, F.; Hadaegh, F. Tobacco smoking: Findings from 20 years of the Tehran Lipid and Glucose Study. Int. J. Endocrinol. Metab. 2018, 16. [Google Scholar] [CrossRef]
- Akl, E.A.; Jawad, M.; Lam, W.Y.; Obeid, R.; Irani, J. Motives, beliefs and attitudes towards waterpipe tobacco smoking: A systematic review. Harm. Reduct. J. 2013, 10, 12. [Google Scholar] [CrossRef] [Green Version]
- Khabour, O.F.; Alzoubi, K.H.; Bani-Ahmad, M.; Dodin, A.; Eissenberg, T.; Shihadeh, A. Acute exposure to waterpipe tobacco smoke induces changes in the oxidative and inflammatory markers in mouse lung. Inhal. Toxicol. 2012, 24, 667–675. [Google Scholar] [CrossRef] [Green Version]
- Arazi, H.; Taati, B.; Rafati Sajedi, F.; Suzuki, K. Salivary Antioxidants Status Following Progressive Aerobic Exercise: What Are the Differences between Waterpipe Smokers and Non-Smokers? Antioxidants 2019, 8, 418. [Google Scholar] [CrossRef] [Green Version]
- Badran, M.; Laher, I. Waterpipe (shisha, hookah) smoking, oxidative stress and hidden disease potential. Redox Biol. 2020, 101455. [Google Scholar] [CrossRef]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Waterpipe tobacco smoking and its human health impacts. J. Hazard. Mater. 2016, 317, 229–236. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Study Group on Tobacco Product Regulation. Advisory Note: Waterpipe Tobacco Smoking: Health Effects, Research Needs and Recommended Actions for Regulators; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Khabour, O.F.; Alzoubi, K.H.; Al-Sawalha, N.; Ahmad, M.B.; Shihadeh, A.; Eissenberg, T. The effect of chronic exposure to waterpipe tobacco smoke on airway inflammation in mice. Life Sci. 2018, 200, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawalha, N.A.; Migdadi, A.a.M.; Alzoubi, K.H.; Khabour, O.F.; Qinna, N.A. Effect of waterpipe tobacco smoking on airway inflammation in murine model of asthma. Inhal. Toxicol. 2017, 29, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Short-term nose-only water-pipe (shisha) smoking exposure accelerates coagulation and causes cardiac inflammation and oxidative stress in mice. Cell. Physiol. Biochem. 2015, 35, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Raza, H.; Yuvaraju, P.; Beegam, S.; John, A.; Yasin, J.; Hameed, R.S.; Adeghate, E.; Ali, B.H. Nose-only water-pipe smoking effects on airway resistance, inflammation, and oxidative stress in mice. J. Appl. Physiol. 2013, 115, 1316–1323. [Google Scholar] [CrossRef] [Green Version]
- Golbidi, S.; Li, H.; Laher, I. Oxidative stress: A unifying mechanism for cell damage induced by noise,(water-pipe) smoking, and emotional stress—Therapeutic strategies targeting redox imbalance. Antioxid. Redox Signal. 2018, 28, 741–759. [Google Scholar] [CrossRef]
- Nemes, R.; Koltai, E.; Taylor, A.W.; Suzuki, K.; Gyori, F.; Radak, Z. Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 2018, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Goel, R.; Bitzer, Z.; Reilly, S.; Trushin, N.; Reinhart, L.; Elias, R.; Richie, J.P. Tobacco Smoke Free Radicals and Related Biomarkers of Oxidative Stress. Free Radic. Biol. Med. 2017, 112, 130–131. [Google Scholar] [CrossRef]
- Suzuki, K. Chronic inflammation as an immunological abnormality and effectiveness of exercise. Biomolecules 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Arazi, H.; Taati, B.; Suzuki, K. A review of the effects of leucine metabolite (β-hydroxy-β-methylbutyrate) supplementation and resistance training on inflammatory markers: A new approach to oxidative stress and cardiovascular risk factors. Antioxidants 2018, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Nieman, D.C.; Wentz, L.M. The compelling link between physical activity and the body’s defense system. J. Sport Health Sci. 2019, 8, 201–217. [Google Scholar] [CrossRef]
- Simpson, R.J.; Campbell, J.P.; Gleeson, M.; Krüger, K.; Nieman, D.C.; Pyne, D.B.; Turner, J.E.; Walsh, N.P. Can exercise affect immune function to increase susceptibility to infection? Exerc. Immunol. Rev. 2020, 26, 8–22. [Google Scholar] [PubMed]
- Eissenberg, T.; Shihadeh, A. Waterpipe tobacco and cigarette smoking: Direct comparison of toxicant exposure. Am. J. Prev. Med. 2009, 37, 518–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattab, A.; Javaid, A.; Iraqi, G.; Alzaabi, A.; Kheder, A.B.; Koniski, M.-L.; Shahrour, N.; Taright, S.; Idrees, M.; Polatli, M. Smoking habits in the Middle East and North Africa: Results of the BREATHE study. Respir. Med. 2012, 106, S16–S24. [Google Scholar] [CrossRef] [Green Version]
- Shihadeh, A.; Azar, S.; Antonios, C.; Haddad, A. Towards a topographical model of narghile water-pipe café smoking: A pilot study in a high socioeconomic status neighborhood of Beirut, Lebanon. Pharmacol. Biochem. Behav. 2004, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Raddaha, A.H.A.; Dempsey, D.; Havel, C.; Peng, M.; Yu, L.; Benowitz, N.L. Nicotine, carbon monoxide, and carcinogen exposure after a single use of a water pipe. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2345–2353. [Google Scholar] [CrossRef] [Green Version]
- Shihadeh, A.; Saleh, R. Polycyclic aromatic hydrocarbons, carbon monoxide,“tar”, and nicotine in the mainstream smoke aerosol of the narghile water pipe. Food Chem. Toxicol. 2005, 43, 655–661. [Google Scholar] [CrossRef]
- Monzer, B.; Sepetdjian, E.; Saliba, N.; Shihadeh, A. Charcoal emissions as a source of CO and carcinogenic PAH in mainstream narghile waterpipe smoke. Food Chem. Toxicol. 2008, 46, 2991–2995. [Google Scholar] [CrossRef]
- Elsayed, Y.; Dalibalta, S.; Abu-Farha, N. Chemical analysis and potential health risks of hookah charcoal. Sci. Total Environ. 2016, 569, 262–268. [Google Scholar] [CrossRef]
- Saleh, R.; Shihadeh, A. Elevated toxicant yields with narghile waterpipes smoked using a plastic hose. Food Chem. Toxicol. 2008, 46, 1461–1466. [Google Scholar] [CrossRef]
- El-Nachef, W.N.; Hammond, S.K. Exhaled carbon monoxide with waterpipe use in US students. J. Am. Med. Assoc. 2008, 299, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Shihadeh, A.; Salman, R.; Jaroudi, E.; Saliba, N.; Sepetdjian, E.; Blank, M.D.; Cobb, C.O.; Eissenberg, T. Does switching to a tobacco-free waterpipe product reduce toxicant intake? A crossover study comparing CO, NO, PAH, volatile aldehydes,“tar” and nicotine yields. Food Chem. Toxicol. 2012, 50, 1494–1498. [Google Scholar] [CrossRef] [Green Version]
- Intorp, M.; Purkis, S.; Whittaker, M.; Wright, W. Determination of “Hoffmann analytes” in cigarette mainstream smoke. The Coresta 2006 joint experiment. Contrib. Tob. Res. 2009, 23, 161–202. [Google Scholar] [CrossRef] [Green Version]
- Al Rashidi, M.; Shihadeh, A.; Saliba, N. Volatile aldehydes in the mainstream smoke of the narghile waterpipe. Food Chem. Toxicol. 2008, 46, 3546–3549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, D.; Hoffmann, I.; El-Bayoumy, K. The less harmful cigarette: A controversial issue. A tribute to Ernst L. Wynder. Chem. Res. Toxicol. 2001, 14, 767–790. [Google Scholar] [CrossRef] [PubMed]
- Schubert, J.; Müller, F.D.; Schmidt, R.; Luch, A.; Schulz, T.G. Waterpipe smoke: Source of toxic and carcinogenic VOCs, phenols and heavy metals? Arch. Toxicol. 2015, 89, 2129–2139. [Google Scholar] [CrossRef]
- Chen, P.; Moldoveanu, S. Mainstream smoke chemical analyses for 2R4F Kentucky reference cigarette. Contrib. Tob. Res. 2003, 20, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Shihadeh, A. Investigation of mainstream smoke aerosol of the argileh water pipe. Food Chem. Toxicol. 2003, 41, 143–152. [Google Scholar] [CrossRef]
- Hoffmann, D.; Hoffmann, I. Letters to the Editor-Tobacco smoke components. Contrib. Tob. Res. 1998, 18, 49–52. [Google Scholar] [CrossRef] [Green Version]
- Tarrant, J.; Mills, K.; Williard, C. Development of an improved method for the determination of polycyclic aromatic hydrocarbons in mainstream tobacco smoke. J. Chromatogr. A 2009, 1216, 2227–2234. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; McAdam, K. A feasibility study on oxidation state of arsenic in cut tobacco, mainstream cigarette smoke and cigarette ash by X-ray absorption spectroscopy. Spectrochim. Acta Part B Atom. Spectr. 2009, 64, 1294–1301. [Google Scholar] [CrossRef]
- Katurji, M.; Daher, N.; Sheheitli, H.; Saleh, R.; Shihadeh, A. Direct measurement of toxicants inhaled by water pipe users in the natural environment using a real-time in situ sampling technique. Inhal. Toxicol. 2010, 22, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, M.V.; Stellman, S.D.; Zang, E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J. Natl. Cancer Inst. 2000, 92, 106–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Zhang, H.; Qi, W.; Zhang, Y.; Li, J.; Li, Z.; Lin, Y.; Bai, X.; Liu, X.; Chen, X. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death. Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Hall, S.; Reed, D.K.; Reed, C.T.; Dixit, M. Nicotine enhances high-fat diet-induced oxidative stress in the kidney. Nicotine Tob. Res. 2016, 18, 1628–1634. [Google Scholar] [CrossRef]
- Akyol, S.; Erdogan, S.; Idiz, N.; Celik, S.; Kaya, M.; Ucar, F.; Dane, S.; Akyol, O. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: An in-depth analysis. Redox Rep. 2014, 19, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Piantadosi, C.A. Carbon monoxide, reactive oxygen signaling, and oxidative stress. Free Radic. Biol. Med. 2008, 45, 562–569. [Google Scholar] [CrossRef] [Green Version]
- Milnerowicz, H.; Ściskalska, M.; Dul, M. Pro-inflammatory effects of metals in persons and animals exposed to tobacco smoke. J. Trace Elem. Med. Biol. 2015, 29, 1–10. [Google Scholar] [CrossRef]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of particulate matter air pollution on cardiovascular oxidative stress pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef]
- Sun, Q.; Yue, P.; Ying, Z.; Cardounel, A.J.; Brook, R.D.; Devlin, R.; Hwang, J.-S.; Zweier, J.L.; Chen, L.C.; Rajagopalan, S. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1760–1766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rababa’h, A.M.; Sultan, B.B.; Alzoubi, K.H.; Khabour, O.F.; Ababneh, M.A. Exposure to waterpipe smoke induces renal functional and oxidative biomarkers variations in mice. Inhal. Toxicol. 2016, 28, 508–513. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H.; Adeghate, E. Nose-Only Water-Pipe Smoke Exposure in Mice Elicits Renal Histopathological Alterations, Inflammation, Oxidative Stress, DNA Damage, and Apoptosis. Front. Physiol. 2020, 11, 46. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Ghorbani, S.; Beiki, Y.; Brandes, M.; Saeidi, A.; Leicht, A. Influence of waterpipe smoking on hematological parameters and cognitive function before and after supramaximal exercise. Sci. Sports 2017, 32, e147–e154. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Halboup, A.M.; Alomari, M.A.; Khabour, O.F. Swimming exercise protective effect on waterpipe tobacco smoking-induced impairment of memory and oxidative stress. Life Sci. 2019, 239, 117076. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Yuvaraju, P.; Beegam, S.; Ali, B.H. Exercise training mitigates water pipe smoke exposure-induced pulmonary impairment via inhibiting NF-κB and activating Nrf2 signalling pathways. Oxid. Med. Cell. Longev. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koubaa, A.; Triki, M.; Trabelsi, H.; Baati, H.; Sahnoun, Z.; Hakim, A. The effect of a 12-week moderate intensity interval training program on the antioxidant defense capability and lipid profile in men smoking cigarettes or hookah: A cohort study. Sci. World J. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- Koubaa, A.; Triki, M.; Trabelsi, H.; Masmoudi, L.; Sahnoun, Z.; Hakim, A. Changes in antioxidant defense capability and lipid profile after 12-week low-intensity continuous training in both cigarette and hookah smokers: A follow-up study. PLoS ONE 2015, 10, e0130563. [Google Scholar] [CrossRef] [Green Version]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and adaptative response mediated by Nrf2 during physical exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merry, T.L.; Ristow, M. Nuclear factor erythroid-derived 2-like 2 (NFE2L2, Nrf2) mediates exercise-induced mitochondrial biogenesis and the anti-oxidant response in mice. J. Physiol. 2016, 594, 5195–5207. [Google Scholar] [CrossRef] [PubMed]
- Margaritelis, N.; Paschalis, V.; Theodorou, A.; Kyparos, A.; Nikolaidis, M. Redox basis of exercise physiology. Redox Biol. 2020, 101499. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-W.; Chang, S.-J. Moderate exercise suppresses NF-κB signaling and activates the SIRT1-AMPK-PGC1α axis to attenuate muscle loss in diabetic db/db mice. Front. Physiol. 2018, 9, 636. [Google Scholar] [CrossRef]
- Louzada, R.A.; Bouviere, J.; Matta, L.P.; Werneck-de-Castro, J.P.; Dupuy, C.; Carvalho, D.P.; Fortunato, R.S. Redox Signaling in Widespread Health Benefits of Exercise. Antioxid. Redox Signal. 2020. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative biology of exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Hawari, F.I.; Obeidat, N.A.; Ayub, H.; Ghonimat, I.; Eissenberg, T.; Dawahrah, S.; Beano, H. The acute effects of waterpipe smoking on lung function and exercise capacity in a pilot study of healthy participants. Inhal. Toxicol. 2013, 25, 492–497. [Google Scholar] [CrossRef]
- Koubaa, A.; Trabelsi, H.; Masmoudi, L.; Triki, M.; Sahnoun, Z.; Zeghal, K.; Hakim, A. Water pipe tobacco smoking and cigarette smoking: Comparative analysis of the smoking effects on antioxidant status, lipid profile and cardiopulmonary quality in sedentary smokers Tunisian. Int. J. Invent. Pharm. Sci. 2013, 2, 51–57. [Google Scholar]
- Koubaa, A.; Triki, M.; Trabelsi, H.; Masmoudi, L.; Zeghal, K.N.; Sahnoun, Z.; Hakim, A. Effect of low-intensity continuous training on lung function and cardiorespiratory fitness in both cigarette and hookah smokers. Afr. Health Sci. 2015, 15, 1170–1181. [Google Scholar] [CrossRef] [Green Version]
- Hawari, F.; Obeidat, N.; Ghonimat, I.; Ayub, H.; Dawahreh, S. The effect of habitual waterpipe tobacco smoking on pulmonary function and exercise capacity in young healthy males: A pilot study. Respir. Med. 2017, 122, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Koubaa, A.; Triki, M.; Trabelsi, H.; Masmoudi, L.; Zeghal, L.; Sahnoun, Z.; Hakim, A. Lung function profiles and aerobic capacity of adult cigarette and hookah smokers after 12 weeks intermittent training. Libyan J. Med. 2015, 10. [Google Scholar] [CrossRef]
| Toxicant | WTS | Cigarette | Approximate Fold Difference (X) |
|---|---|---|---|
| Volatile Aldehydes, µg [36,37,38,39] | |||
| Formaldehyde | 58.7 to 630 | 20.6 to 100 | 3 to 6 |
| Acetaldehyde | 383 to 2520 | 587.4 | 0.7 to 4 |
| Propionaldehyde | 51.7 to 403 | 49 | 1 to 8 |
| Acrolein | 892 | 60 to 240 | 4 to 15 |
| Acetone | 118 | 270.4 | 0.5 |
| Volatile Organic Compounds, µg [40,41] | |||
| Toluene | 9.92 | 64.9 | 0.15 |
| Benzene | 271 | 43.4 | 6 |
| Isoprene | 4 | 298 | <0.1 |
| Heavy Metals, ng [42,43] | |||
| Lead | 6870 | 34 to 85 | 81 to 202 |
| Chromium | 1340 | 4 to 70 | 19 to 335 |
| Nickel | 990 | 600 | 1.5 |
| Arsenic | 165 | 40 to 120 | 1.5 to 4 |
| Cobalt | 70 | 0.13 to 0.2 | 350 to 538 |
| Beryllium | 65 | 300 | 0.2 |
| Carcinogenic polycyclic aromatic hydrocarbon, ng [36,44] | |||
| Chrysene | 106 | 16.2 | 6.5 |
| Benz(a)anthracene | 86.4 | 14.1 | 6 |
| Benzo(b + k)fluoranthenes | 64.7 | 7.6 | 8.5 |
| Benzo(a)pyrene | 51.8 | 6.6 | 8 |
| Indeno(1,2,3-cd)pyrene | 47.3 | 3.8 | 12.5 |
| Others [36,37,45,46,47] | |||
| Nicotine, mg | 1.04 to 4.82 | 0.73 to 2.39 | 1.5 to 2 |
| Carbon monoxide, mg | 150 to 155 | 12 to 22.5 | 7 to 12.5 |
| Tar, mg | 464 to 640 | 9.4 to 29 | 22 to 49 |
| Particulate matter, mg | 770 to 1193 | 11 | 70 to 108 |
| Nitric oxide, µg | 437 | 218.1 | 2 |
| The Authors | Subjects | Purpose | Exercise Protocol | Key Findings |
|---|---|---|---|---|
| Arazi et al. [12] | Sedentary women (11 waterpipe smokers, 12 non-smokers) | Comparing the salivary antioxidative responses following a bout of exhaustive aerobic exercise | Start at 1.7 mph and a gradient of 10% for the first 3 min, the gradient increased by 2% every 3 min, and the speed was 2.5, 3.4, 4.2, 5, 5.5, and 6 mph in the subsequent stages (Bruce treadmill test) | Smaller increase in POX activity, larger decline in DPPH activity, and lower salivary flow rate for smokers ↔ UA |
| Ahmadian et al. [58] | Sedentary men (10 waterpipe smokers, 10 non-smokers) | The influence of WTS on cognitive function and hematological parameters following an acute supramaximal exercise | 30 s Wingate supramaximal exercise test using a cycle ergometer | Greater increases in white blood cell, neutrophil, hematocrit and lymphocyte values for smokers ↔ PLT, PDW, MPV |
| Nakhaee et al. [8] | Wistar male rats | The effects of waterpipe exposure with/without swimming exercise on heart histology and inflammation status | 5 days/week for 4 weeks, 1 h/day, moderate intensity | ↔ MDA, GPX, SOD, IL-10, IL-1β, and IL-6 ↓ TNF-α ↑ Catalase |
| Alzoubi et al. [59] | Wistar male rats | The neuroprotective effects of swimming exercise on hippocampus oxidative markers induced by exposure to waterpipe | 5 days/week for 4 weeks, 1 h/day, moderate intensity | ↑ GPX, Catalase, and GSH/GSSG ↓ GSSG ↔ TBARs, GSH |
| Nemmar et al. [60] | C57BL/6 mice | The impact of regular exercise training on lung inflammation and impairment of pulmonary function induced by exposure to waterpipe | Treadmill running, 5 days/week for 8 weeks, 40 min/day, moderate intensity | ↓ TNF-α, IL-6, NF-κB, 8-isoprostane, intra-alveolar macrophages, airway resistance, lung DNA damage, and focal damage to alveolar septae ↑ Nrf2 |
| Koubaa et al. [61] | Sedentary men (12 waterpipe smokers, 11 cigarette smokers, and 12 non-smokers) | The impact of interval training program on the antioxidant defense capability and lipid profile | Race track running, 3 days/week for 12 weeks, 30 min/day, 2-min intervals interspersed with recovery periods of 1 min, moderate-intensity (70% of VO2max) | ↑ TAS, SOD, GPx, GR, α-tocopherol, and HDL-C ↓ MDA, and TC/HDL-C ↔ LDL-C, TC, TG, HDL-C/TG |
| Koubaa et al. [62] | Sedentary men (14 waterpipe smokers, 15 cigarette smokers, and 14 non-smokers) | The effect of continuous training program on antioxidant defense capability and lipid profile | Race track running, 3 days/week for 12 weeks, 20–30 min/day, low-intensity (40% of VO2max) | ↑ TAS, SOD, GPx, GR, α-tocopherol, and HDL-C ↓ MDA, LDL-C, TC, and TC/HDL-C ↔ MDA, GR, α-tocopherol, TG, HDL-C/TG |
| The Authors | Subjects | Purpose | Exercise Protocol | Key Findings |
|---|---|---|---|---|
| Hawari et al. [70] | 24 healthy men | The acute effects of WTS on exercise capacity and lung function | Cardiopulmonary exercise test using a cycle ergometer: 2-min 20-Watt warm up and 25-Watt increase every 2-min for a maximum time of 10 min | ↓ VO2, O2 pulse, FEF25–75% ↑ HR/VO2, baseline respiratory rate, RPE at mid and peak exercise ↔ FEV1, FVC, DLco, breathing reserve |
| Koubaa et al. [71] | 68 sedentary men (22 waterpipe smokers, 23 cigarette smokers, 23 non-smokers) | Evaluate and compare the effect of smoking on antioxidant status, aerobic capacity, pulmonary function and lipid profile in waterpipe and cigarette smokers | Cardiopulmonary exercise test using a cycle ergometer: 5-min warm up with 6 km/h, 1 km/h increase every 2 min | ↓ VO2max, MAS, FVC, FEV1, PEF, FEF25–75%, FEF50% ↔ FEV1/FVC |
| Koubaa et al. [72] | 43 sedentary men (14 waterpipe smokers, 15 cigarette smokers, 14 non-smokers) | The effects of continuous training on lungs function and cardiorespiratory fitness in smokers | Race track running, 3 days/week for 12 weeks, 20–30 min/day, low-intensity (40% of VO2max) | ↑ FVC, FEV1, FEF50%, VO2max, vVO2max ↔ PEF, FEV1/FVC, FEF25–75% |
| Koubaa et al. [74] | 35 sedentary men (10 waterpipe smokers, 12 cigarette smokers, 11 non-smokers) | The effects of aerobic interval training program on aerobic capacity and pulmonary function in smokers | Race track running, 3 days/week for 12 weeks, 30 min/day, 2-min intervals interspersed with recovery periods of 1 min, moderate-intensity (70% of VO2max) | ↑ VO2max, vVO2max, PEF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taati, B.; Arazi, H.; Suzuki, K. Oxidative Stress and Inflammation Induced by Waterpipe Tobacco Smoking Despite Possible Protective Effects of Exercise Training: A Review of the Literature. Antioxidants 2020, 9, 777. https://doi.org/10.3390/antiox9090777
Taati B, Arazi H, Suzuki K. Oxidative Stress and Inflammation Induced by Waterpipe Tobacco Smoking Despite Possible Protective Effects of Exercise Training: A Review of the Literature. Antioxidants. 2020; 9(9):777. https://doi.org/10.3390/antiox9090777
Chicago/Turabian StyleTaati, Behzad, Hamid Arazi, and Katsuhiko Suzuki. 2020. "Oxidative Stress and Inflammation Induced by Waterpipe Tobacco Smoking Despite Possible Protective Effects of Exercise Training: A Review of the Literature" Antioxidants 9, no. 9: 777. https://doi.org/10.3390/antiox9090777








