Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. I/R Model
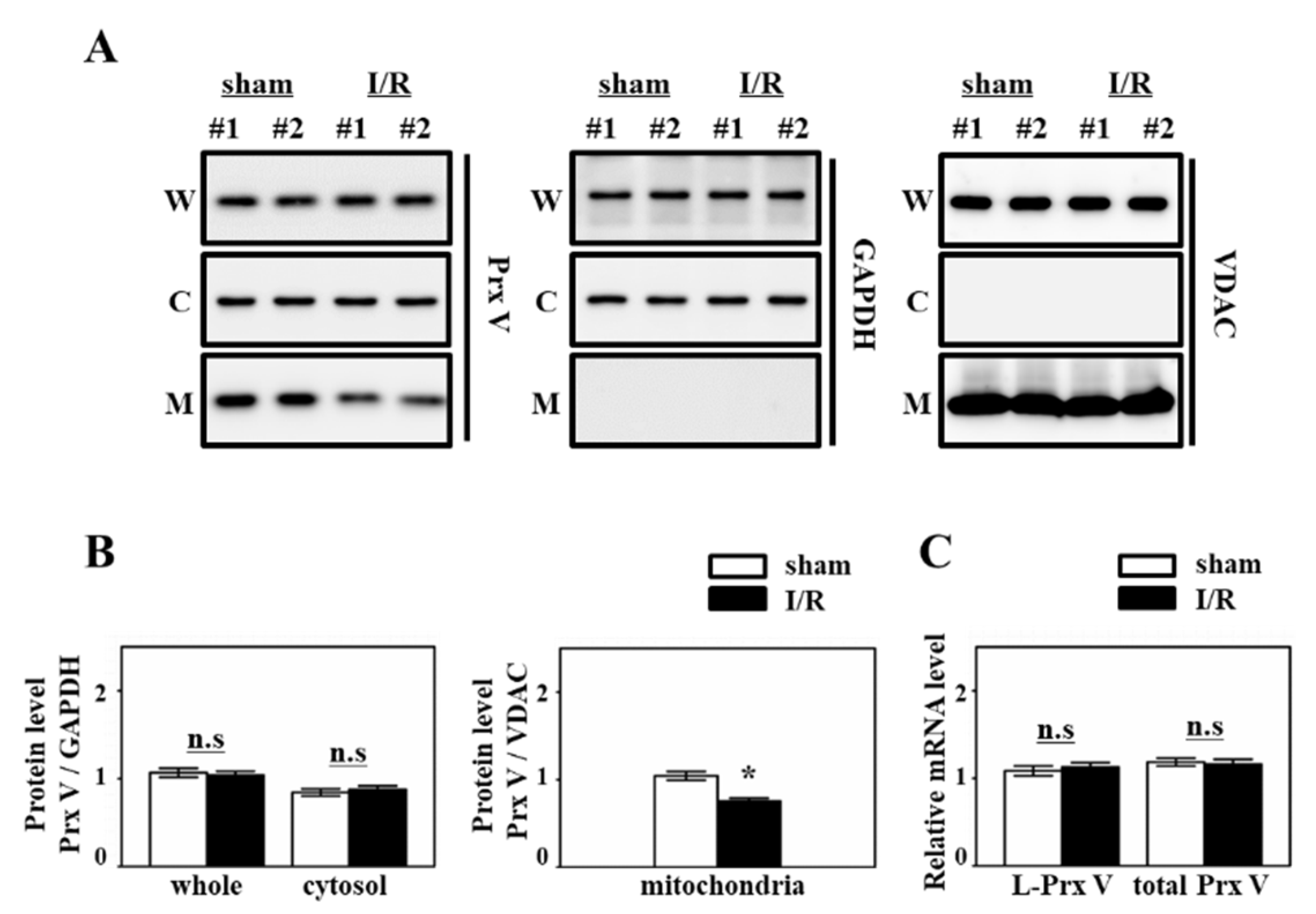
2.3. Subcellular Fractionation
2.4. Western Blotting
2.5. Histology
2.6. Measurements of Blood Parameters
2.7. RNA Isolation
2.8. Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Quantitative Real-Time PCR (qPCR)
2.9. Statistical Analysis
3. Results
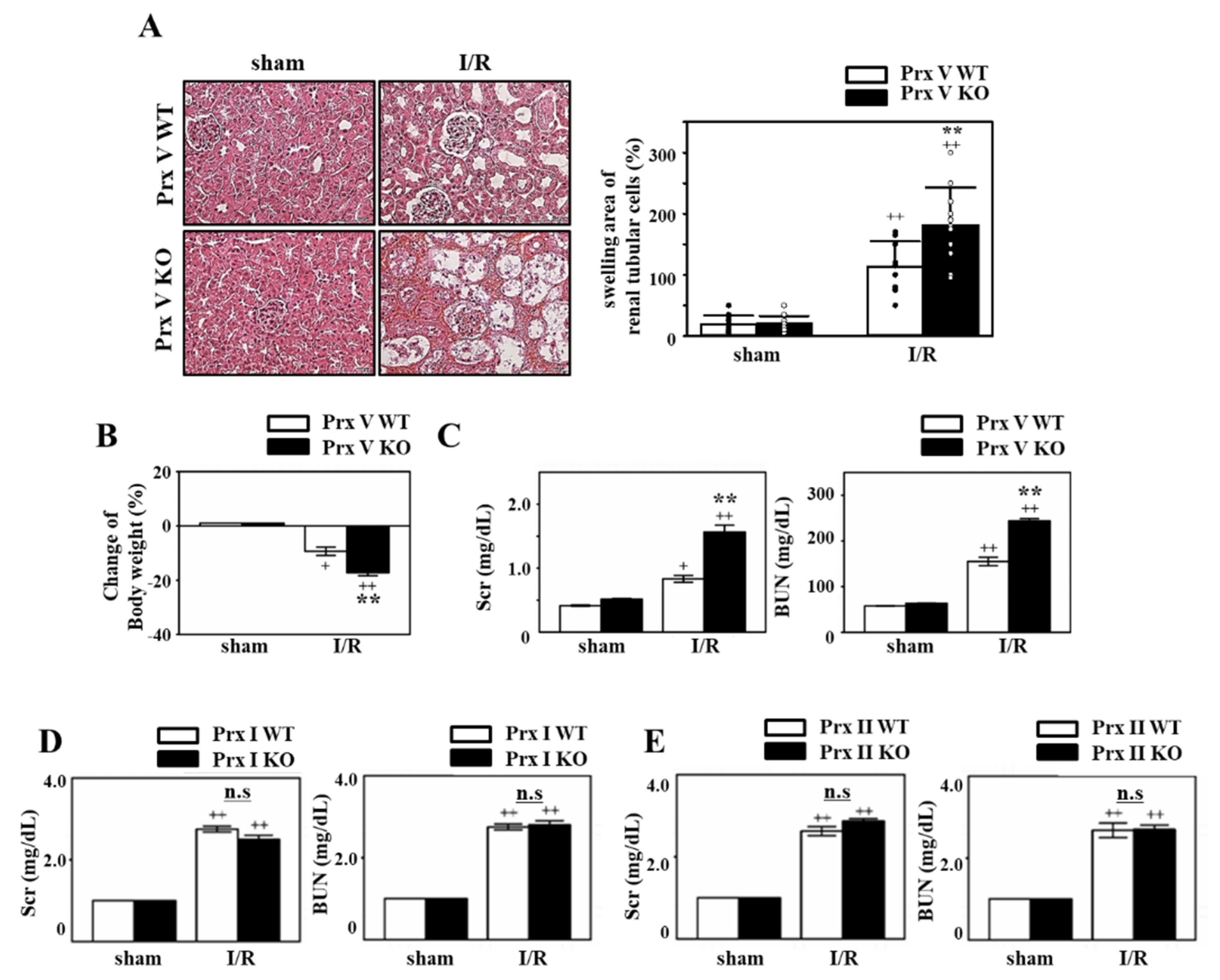
3.1. IR-Induced AKI is Exacerbated by Ablation of Prx V
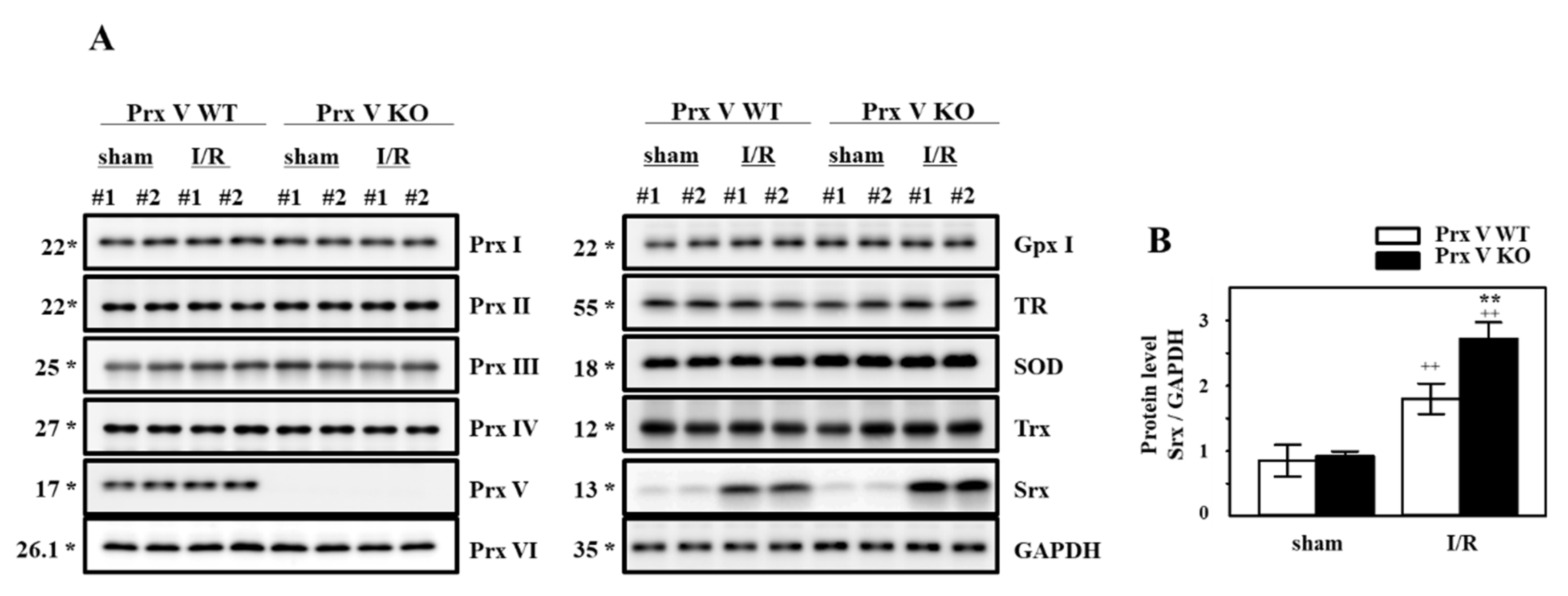
3.2. Ablation of Prx V Induces More Inflammatory Responses by Renal I/R Injury
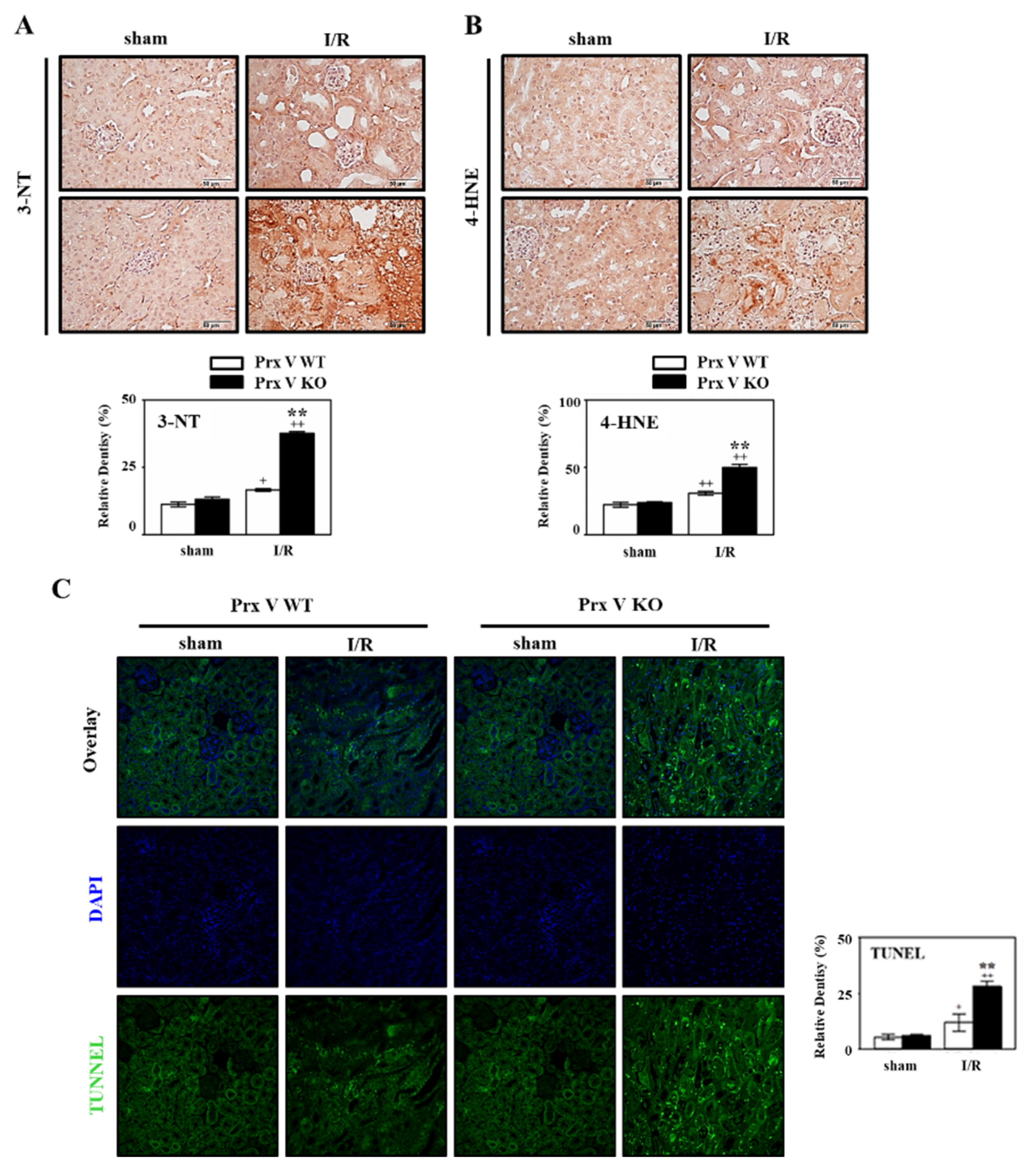
3.3. Ablation of Prx V Induces Highly Enhanced Oxidative Stress, ER Stress, and Apoptosis by Renal I/R Injury
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AKI | acute kidney injury |
| ATF 4 | AMP-dependent transcription factor |
| BUN | Blood urea nitrogen |
| Edem | ER degradation-enhancing a-mannosidase-like protein 1 |
| Gpx | glutathione peroxidase |
| I/R | Ischemia/reperfusion |
| KO | knockout |
| L-Prx V | long form Prx V |
| MTS | mitochondrial targeting sequence |
| NF-kB | nuclear factor-kB |
| Prxs | peroxiredoxins |
| ROS | Reactive oxygen species |
| Scr | Serum creatinine |
| S-Prx V | short form Prx V |
| Srx | Sulfiredoxin |
| SOD | superoxide dismutase |
| Trx | thioredixin |
| TR | thioredoxin reductase |
| TRB 3 | Tribbles homolog 3 |
| WT | wild type |
| XBP1s | x box binding protein 1 |
References
- Scott, R.P.; Quaggin, S.E. Review series: The cell biology of renal filtration. J. Cell Biol. 2015, 209, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Goleg, F.A.; Kong, N.C.; Sahathevan, R. Dialysis-treated end-stage kidney disease in Libya: Epidemiology and risk factors. Int. Urol. Nephrol. 2014, 46, 1581–1587. [Google Scholar] [CrossRef] [PubMed]
- Burrows, N.R.; Li, Y.; Williams, D.E. Racial and ethnic differences in trends of end-stage renal disease: United States, 1995 to 2005. Adv. Chronic Kidney Dis. 2008, 15, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Cowley, A.W., Jr.; Abe, M.; Mori, T.; O’Connor, P.M.; Ohsaki, Y.; Zheleznova, N.N. Reactive oxygen species as important determinants of medullary flow, sodium excretion; hypertension. Am. J. Physiol. Renal. Physiol. 2015, 308, F179–F197. [Google Scholar] [CrossRef]
- Dendooven, A.; Ishola, D.A., Jr.; Nguyen, T.Q.; Van der Giezen, D.M.; Kok, R.J.; Goldschmeding, R.; Joles, J.A. Oxidative stress in obstructive nephropathy. Int. J. Exp. Pathol. 2011, 92, 202–210. [Google Scholar] [CrossRef]
- Okamura, D.M.; Pennathur, S. The balance of powers: Redox regulation of fibrogenic pathways in kidney injury. Redox Biol. 2015, 6, 495–504. [Google Scholar] [CrossRef]
- Siegel, N.J.; Feldman, R.A.; Lytton, B.; Hayslett, J.P.; Kashgarian, M. Renal cortical blood flow distribution in obstructive nephropathy in rats. Circ. Res. 1977, 40, 379–384. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 2009, 130, 41–50. [Google Scholar] [CrossRef]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Renal. Inj. Prev. 2015, 4, 20–27. [Google Scholar]
- Wood, Z.A.; Schroder, E.; Robin Harris, J.; Poole, L.B. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2003, 28, 32–40. [Google Scholar] [CrossRef]
- Rhee, S.G.; Chae, H.Z.; Kim, K. Peroxiredoxins: A historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 2005, 38, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.H.; Sung, S.H.; Cho, E.J.; Lee, S.K.; Lee, H.E.; Woo, H.A.; Yu, D.Y.; Kil, I.S.; Rhee, S.G. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology 2011, 53, 945–953. [Google Scholar] [CrossRef]
- Bae, S.H.; Sung, S.H.; Lee, H.E.; Kang, H.T.; Lee, S.K.; Oh, S.Y.; Woo, H.A.; Kil, I.S.; Rhee, S.G. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxid. Redox Signal. 2012, 17, 1351–1361. [Google Scholar] [CrossRef]
- Knoops, B.; Goemaere, J.; Van der Eecken, V.; Declercq, J.P. Peroxiredoxin 5: Structure, mechanism, and function of the mammalian atypical 2-Cys peroxiredoxin. Antioxid. Redox Signal. 2011, 15, 817–829. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, S.J.; Kim, J.H.; Seong, J.B.; Kim, K.M.; Woo, H.A.; Lee, D.S. Peroxiredoxin 5 regulates adipogenesis-attenuating oxidative stress in obese mouse models induced by a high-fat diet. Free Radic. Biol. Med. 2018, 123, 27–38. [Google Scholar] [CrossRef]
- Van der Eecken, V.; Clippe, A.; Dekoninck, S.; Goemaere, J.; Walbrecq, G.; Van Veldhoven, P.P.; Knoops, B. Abolition of peroxiredoxin-5 mitochondrial targeting during canid evolution. PLoS ONE 2013, 8, e72844. [Google Scholar] [CrossRef][Green Version]
- Garg, S.G.; Gould, S.B. The Role of Charge in Protein Targeting Evolution. Trends Cell. Biol. 2016, 26, 894–905. [Google Scholar] [CrossRef]
- Daskalakis, V.; Farantos, S.C.; Guallar, V.; Varotsis, C. Regulation of electron and proton transfer by the protein matrix of cytochrome c oxidase. J. Phys. Chem. B 2011, 115, 3648–3655. [Google Scholar] [CrossRef]
- Rousseau, D.L.; Sassaroli, M.; Ching, Y.C.; Dasgupta, S. The role of water near cytochrome a in cytochrome c oxidase. Ann. N. Y. Acad. Sci. 1988, 550, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Ma, S.K.; Bae, E.H.; Lee, J.; Kim, S.W. Peroxiredoxin 5 Protects TGF-beta Induced Fibrosis by Inhibiting Stat3 Activation in Rat Kidney Interstitial Fibroblast Cells. PLoS ONE 2016, 11, e0149266. [Google Scholar]
- Wei, Q.; Dong, Z. Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am. J. Physiol. Renal. Physiol. 2012, 303, F1487–F1494. [Google Scholar] [CrossRef] [PubMed]
- Noh, Y.H.; Baek, J.Y.; Jeong, W.; Rhee, S.G.; Chang, T.S. Sulfiredoxin Translocation into Mitochondria Plays a Crucial Role in Reducing Hyperoxidized Peroxiredoxin III. J. Biol. Chem. 2009, 284, 8470–8477. [Google Scholar] [CrossRef]
- Kil, I.S.; Ryu, K.W.; Lee, S.K.; Kim, J.Y.; Chu, S.Y.; Kim, J.H.; Park, S.; Rhee, S.G. Circadian Oscillation of Sulfiredoxin in the Mitochondria. Mol. Cell 2015, 59, 651–663. [Google Scholar] [CrossRef]
- Chang, T.S.; Cho, C.S.; Park, S.; Yu, S.; Kang, S.W.; Rhee, S.G. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J. Biol. Chem. 2004, 279, 41975–41984. [Google Scholar] [CrossRef]
- Woo, H.A.; Chae, H.Z.; Hwang, S.C.; Yang, K.S.; Kang, S.W.; Kim, K.; Rhee, S.G. Reversing the inactivation of peroxiredoxins caused by cysteine sulfinic acid formation. Science 2003, 300, 653–656. [Google Scholar] [CrossRef]
- Lee, S.R.; Kim, J.R.; Kwon, K.S.; Yoon, H.W.; Levine, R.L.; Ginsburg, A.; Rhee, S.G. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999, 274, 4722–4734. [Google Scholar] [CrossRef]
- Baek, J.Y.; Park, S.; Park, J.; Jang, J.Y.; Wang, S.B.; Kim, S.R.; Woo, H.A.; Lim, K.M.; Chang, T.S. Protective Role of Mitochondrial Peroxiredoxin III against UVB-Induced Apoptosis of Epidermal Keratinocytes. J. Investig. Dermatol. 2017, 137, 1333–1342. [Google Scholar] [CrossRef]
- Cho, C.S.; Yoon, H.J.; Kim, J.Y.; Woo, H.A.; Rhee, S.G. Circadian rhythm of hyperoxidized peroxiredoxin II is determined by hemoglobin autoxidation and the 20S proteasome in red blood cells. Proc. Natl. Acad. Sci. USA 2014, 111, 12043–12048. [Google Scholar] [CrossRef]
- Bae, S.H.; Woo, H.A.; Sung, S.H.; Lee, H.E.; Lee, S.K.; Kil, I.S.; Rhee, S.G. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxid. Redox Signal. 2009, 11, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Kil, I.S.; Lee, S.K.; Ryu, K.W.; Woo, H.A.; Hu, M.C.; Bae, S.H.; Rhee, S.G. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Mol. Cell 2012, 46, 584–594. [Google Scholar] [CrossRef]
- Knoops, B.; Argyropoulou, V.; Becker, S.; Ferte, L.; Kuznetsova, O. Multiple Roles of Peroxiredoxins in Inflammation. Mol. Cells 2016, 39, 60–64. [Google Scholar] [PubMed]
- Choi, H.I.; Chung, K.J.; Yang, H.Y.; Ren, L.; Sohn, S.; Kim, P.R.; Kook, M.S.; Choy, H.E.; Lee, T.H. Peroxiredoxin V selectively regulates IL-6 production by modulating the Jak2-Stat5 pathway. Free Radic. Biol. Med. 2013, 65, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.Y.; Yang, X.C.; Su, J.; Kang, J.S.; Wu, Y.; Xue, Y.N.; Dong, Y.T.; Sun, L.K. Inhibition of autophagic flux by ROS promotes apoptosis during DTT-induced ER/oxidative stress in HeLa cells. Oncol. Rep. 2016, 35, 3471–3479. [Google Scholar] [CrossRef]
- Li, G.; Scull, C.; Ozcan, L.; Tabas, I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress; PKR activation to induce apoptosis. J. Cell Biol. 2010, 191, 1113–1125. [Google Scholar] [CrossRef]
- Qiao, X.; Li, R.S.; Li, H.; Zhu, G.Z.; Huang, X.G.; Shao, S.; Bai, B. Intermedin protects against renal ischemia-reperfusion injury by inhibition of oxidative stress. Am. J. Physiol. Renal. Physiol. 2013, 304, F112–F119. [Google Scholar] [CrossRef]
- Ponticelli, C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 2014, 29, 1134–1140. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion—From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Garvin, J.L. Effects of Reactive Oxygen Species on Tubular Transport along the Nephron. Antioxidants 2017, 6, 23. [Google Scholar] [CrossRef]
- Knoops, B.; Clippe, A.; Bogard, C.; Arsalane, K.; Wattiez, R.; Hermans, C.; Duconseille, E.; Falmagne, P.; Bernard, A. Cloning and characterization of AOEB166, a novel mammalian antioxidant enzyme of the peroxiredoxin family. J. Biol. Chem. 1999, 274, 30451–30458. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.S.; Kang, S.W.; Kim, K.; Baines, I.C.; Lee, T.H.; Rhee, S.G. Identification of a new type of mammalian peroxiredoxin that forms an intramolecular disulfide as a reaction intermediate. J. Biol. Chem. 2000, 275, 20346–20354. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Avraham, S.; Jiang, S.; London, R.; Van Veldhoven, P.P.; Subramani, S.; Rogers, R.A.; Avraham, H. Characterization of human and murine PMP20 peroxisomal proteins that exhibit antioxidant activity in vitro. J. Biol. Chem. 1999, 274, 29897–29904. [Google Scholar] [CrossRef] [PubMed]
- Woo, H.A.; Jeong, W.; Chang, T.S.; Park, K.J.; Park, S.J.; Yang, J.S.; Rhee, S.G. Reduction of cysteine sulfinic acid by sulfiredoxin is specific to 2-cys peroxiredoxins. J. Biol. Chem. 2005, 280, 3125–3128. [Google Scholar] [CrossRef]
- Woo, H.A.; Rhee, S.G. Immunoblot Detection of Proteins That Contain Cysteine Sulfinic or Sulfonic Acids with Antibodies Specific for the Hyperoxidized Cysteine-Containing Sequence. In Methods in Redox Signaling; Mary Ann Liebert Inc.: New Rochelle, NY, USA, 2010; pp. 19–23. [Google Scholar]

| Target Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Size |
|---|---|---|---|
| GADPH | AGAACATCATCCCTGCATCC | GGTCCTCAGTGTAGCCCAAG | 228 |
| LPrx V | AGAAGCAGGTTGGGAGTGTG | CTTTCTTGCCCTTGAACAGC | 158 |
| SPrx V | GGCATTTACACCTGGCTGTT | CGACGATTCCCAAAGAGAGA | 242 |
| Nrf-2 | CTCTCTGAACTCCTGGACGG | GGGTCTCCGTAAATGGAAG | 182 |
| Srx | GGAAGGAAGAAAGGAGATGG | AGAGTTCAGGCTATGGGGAT | 155 |
| ATF4 | ATGGCCGGCTATGGATGAT | CGAAGTCAAACTCTTTCAGATCCATT | 113 |
| TRB3 | CTCTGAGGCTCCAGGACAAG | GGCTCAGGCTCATCTCTCAC | 142 |
| XBP1s | GAGTCCGCAGCAGGTG | GTGTCAGAGTCCATGGGA | 149 |
| Edem 1 | GCAATGAAGGAGAAGGAGACCC | TAGAAGGCGTGTAGGCAGATGG | 157 |
| IL-1β | TCGTGCTGTCGGACCCATAT | GTCGTTGCTTGGTTCTCCTTGT | 110 |
| TNF-α | GCCACCACGCTCTTCTG | GGTGTGGGTGAGGAGCA | 294 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Lee, E.G.; Yi, H.J.; Kim, N.H.; Rhee, S.G.; Woo, H.A. Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice. Antioxidants 2020, 9, 769. https://doi.org/10.3390/antiox9080769
Park J, Lee EG, Yi HJ, Kim NH, Rhee SG, Woo HA. Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice. Antioxidants. 2020; 9(8):769. https://doi.org/10.3390/antiox9080769
Chicago/Turabian StylePark, Jiyoung, Eun Gyeong Lee, Ho Jin Yi, Nam Hee Kim, Sue Goo Rhee, and Hyun Ae Woo. 2020. "Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice" Antioxidants 9, no. 8: 769. https://doi.org/10.3390/antiox9080769
APA StylePark, J., Lee, E. G., Yi, H. J., Kim, N. H., Rhee, S. G., & Woo, H. A. (2020). Ablation of Peroxiredoxin V Exacerbates Ischemia/Reperfusion-Induced Kidney Injury in Mice. Antioxidants, 9(8), 769. https://doi.org/10.3390/antiox9080769





