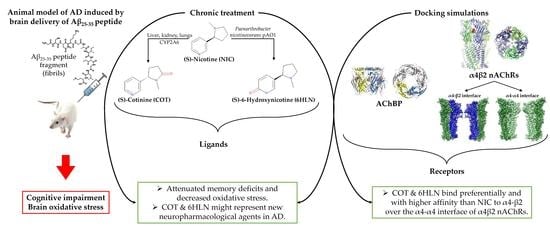
Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Molecular Docking Simulations
2.1.1. Tridimensional (3D) Structures of Ligands and Receptors
2.1.2. Docking Software and Parameters
2.2. Animals
2.3. Neurosurgery
2.4. Drug Treatment and Group Division
2.5. Behavior Assessment
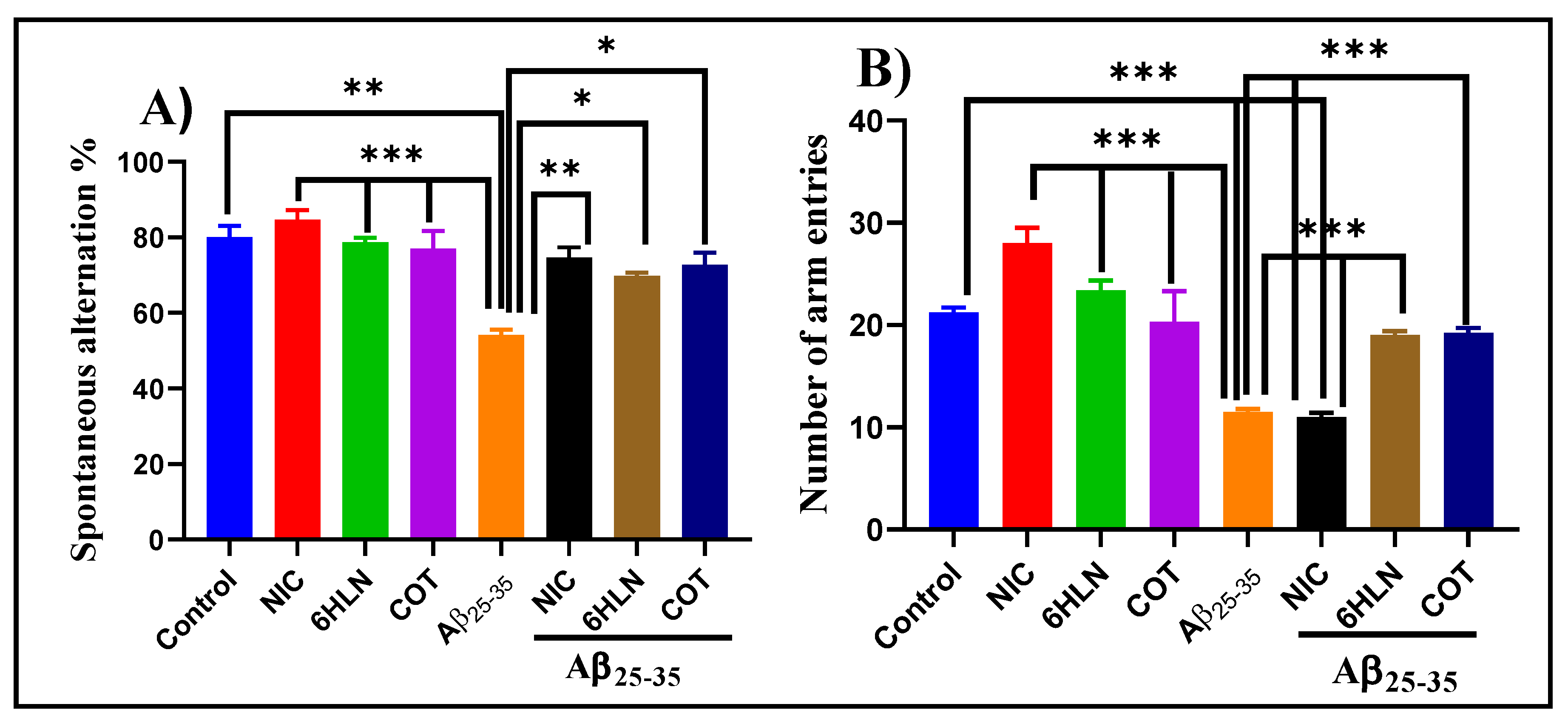
2.5.1. Y-Maze Task
2.5.2. Novel Object Recognition Task
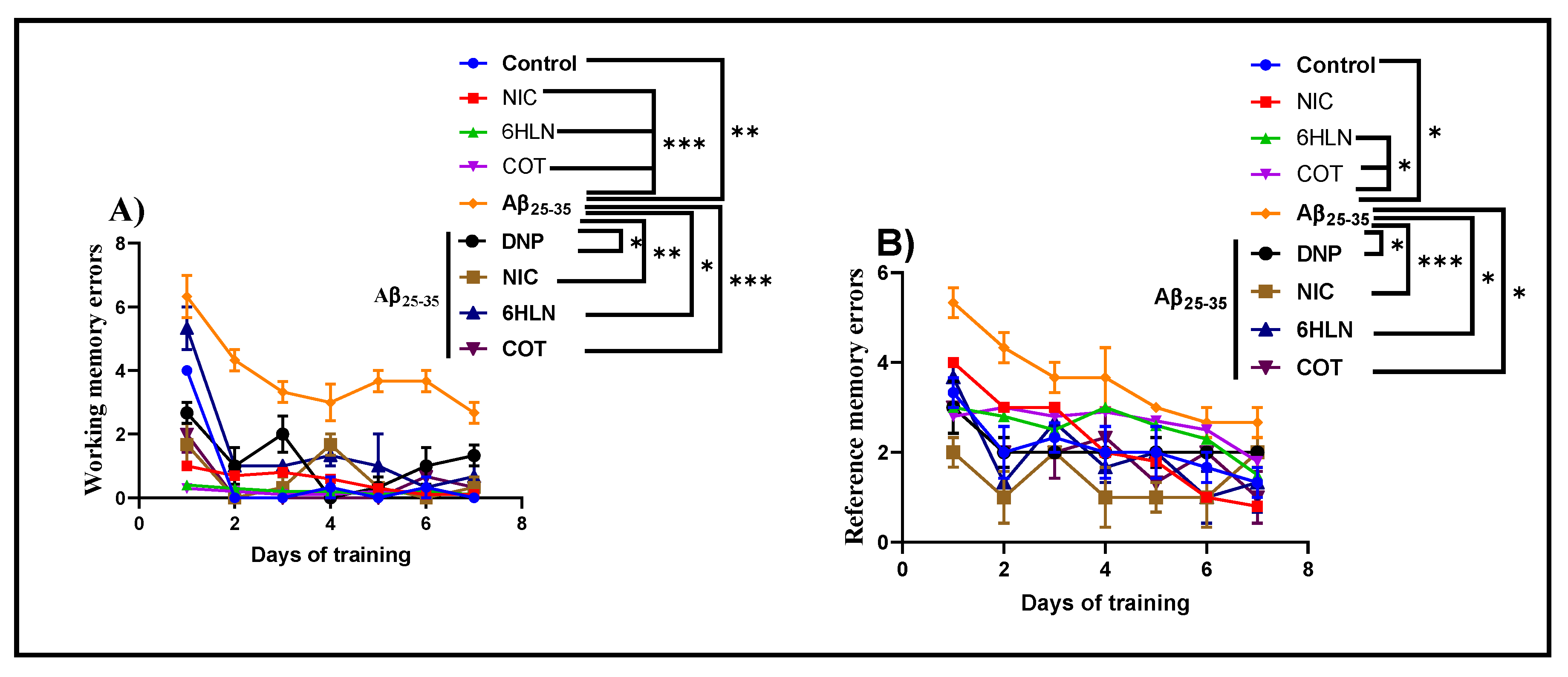
2.5.3. Radial Arm Maze Task
2.6. Biochemical Parameters Assessment
2.6.1. Protein Concentration Estimation
2.6.2. AChE Activity Determination
2.6.3. SOD Activity Determination
2.6.4. CAT Activity Determination
2.6.5. GPX Activity Determination
2.6.6. Determination of the Total GSH Content
2.6.7. Determination of Carbonylated Proteins Level
2.6.8. Determination of MDA Level
2.7. RNA Isolation and Real Time Quantitative PCR (qRT-PCR)
2.8. Statistic Analysis
3. Results and Discussion
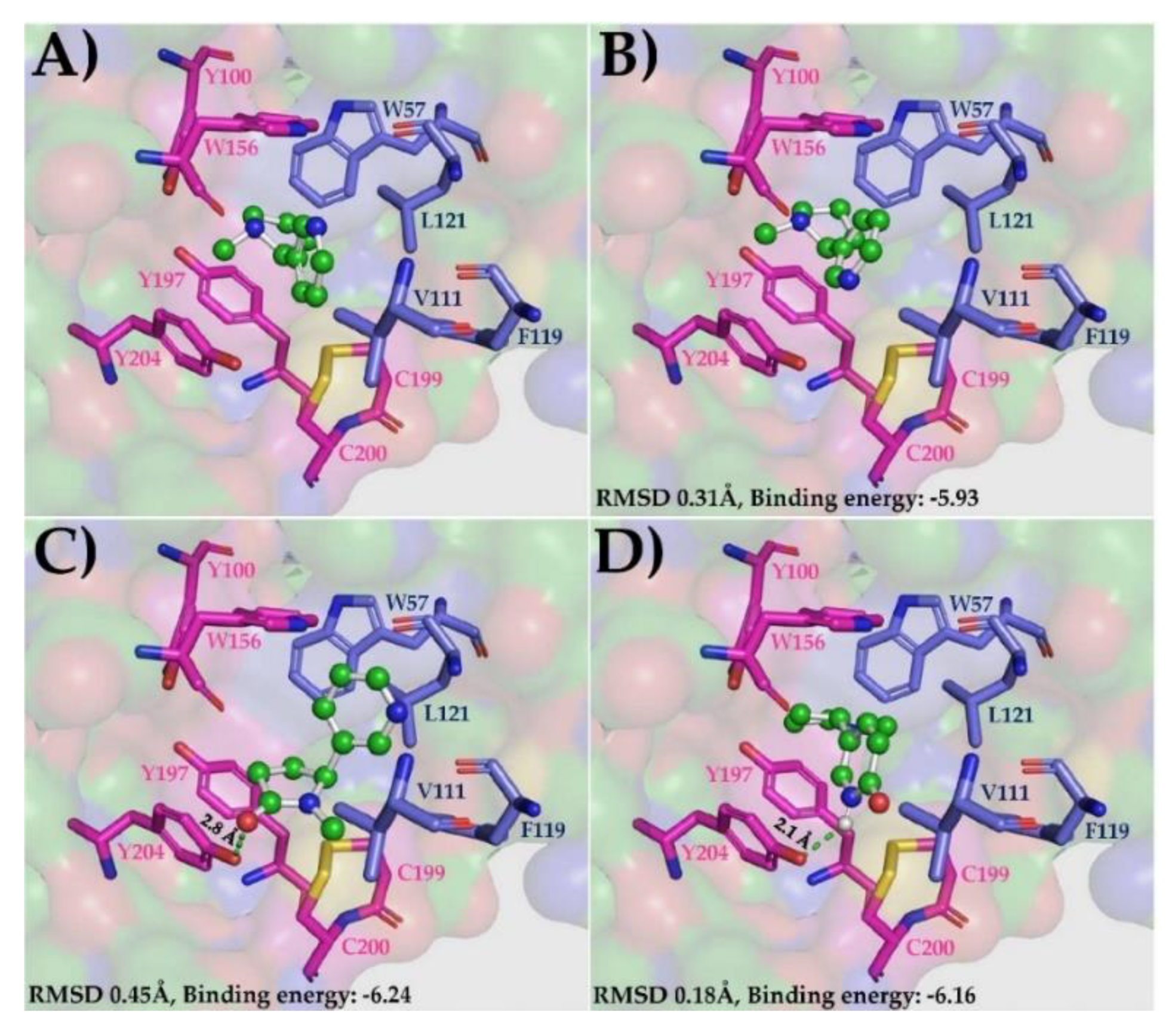
3.1. Molecular Docking Simulations
3.2. Effects of Nicotinic Derivatives on Cognitive Functions
3.3. Effects of Nicotinic Derivatives on AChE Specific Activity
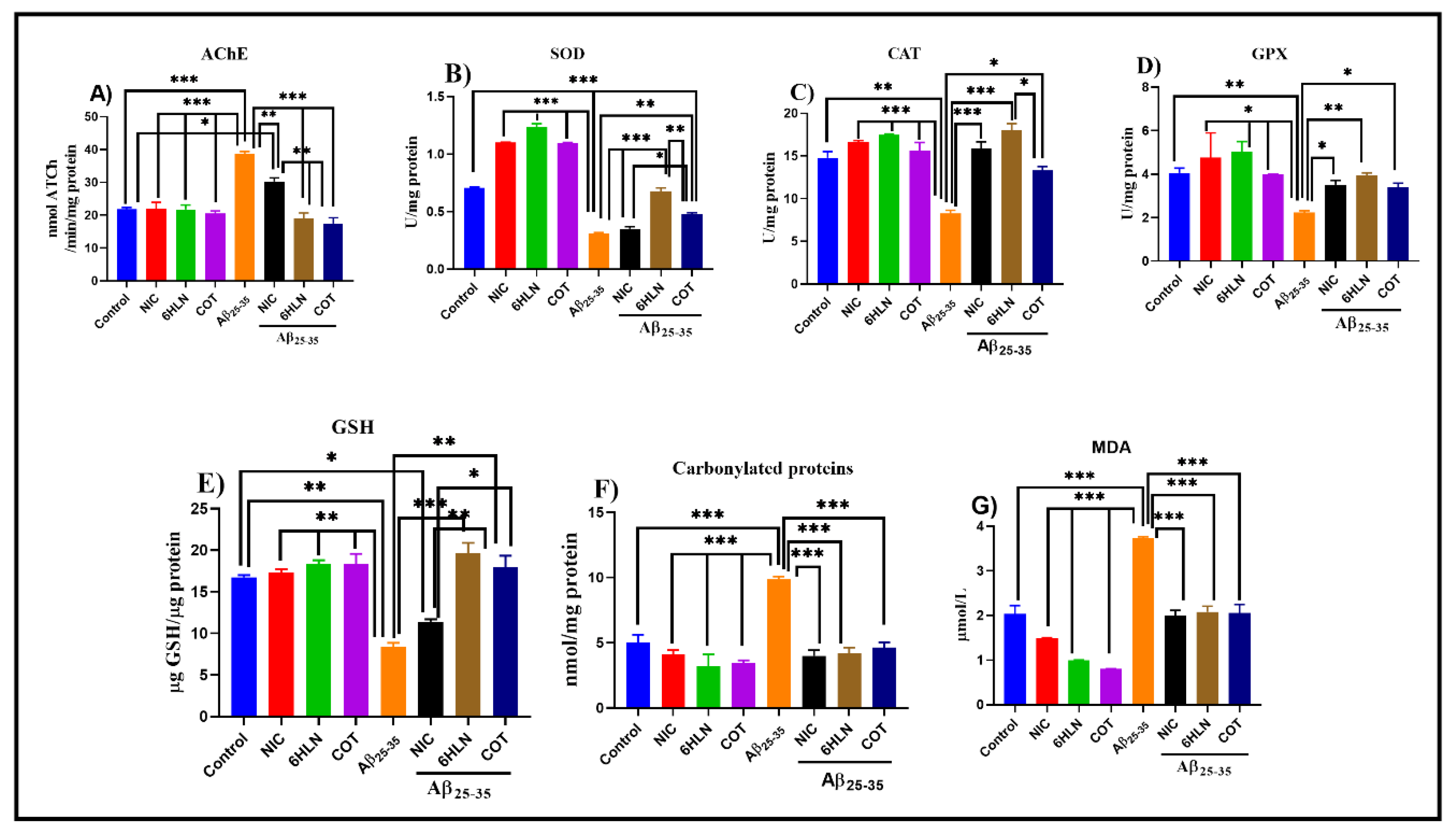
3.4. Effects of Nicotinic Derivatives on Oxidative Status
3.5. Effects of Nicotinic Derivatives on Gene Expression
3.5.1. Effects of Nicotinic Derivatives on Bdnf Expression
3.5.2. Effects of Nicotinic Derivatives on Arc Expression
3.5.3. Effects of Nicotinic Derivatives on il-1β Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Dementia: Number of People Affected to Triple in Next 30 Years; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Auld, D.S.; Kornecook, T.J.; Bastianetto, S.; Quirion, R. Alzheimer’s disease and the basal forebrain cholinergic system: Relations to β-amyloid peptides, cognition, and treatment strategies. Prog. Neurobiol. 2002, 68, 209–245. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Hardy, J.; Selkoe, D.J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science 2002, 297, 353–356. [Google Scholar] [CrossRef]
- Barage, S.H.; Sonawane, K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 2015, 52, 1–18. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Bartus, R.; Dean, R.; Beer, B.; Lippa, A. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Posadas, I.; López-Hernández, B.; Ceña, V. Nicotinic receptors in neurodegeneration. Curr. Neuropharmacol. 2013, 11, 298–314. [Google Scholar] [CrossRef]
- Lombardo, S.; Maskos, U. Role of the nicotinic acetylcholine receptor in Alzheimer’s disease pathology and treatment. Neuropharmacology 2015, 96, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Parri, H.R.; Hernandez, C.M.; Dineley, K.T. Research update: Alpha7 nicotinic acetylcholine receptor mechanisms in Alzheimer’s disease. Biochem. Pharm. 2011, 82, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Castello, N.A.; Cheng, D.; Kitazawa, M.; Baglietto-Vargas, D.; Green, K.N.; Esbenshade, T.A.; Bitner, R.S.; Decker, M.W.; LaFerla, F.M. α7 nicotinic receptor agonist enhances cognition in aged 3xTg-AD mice with robust plaques and tangles. Am. J. Pathol. 2014, 184, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Kamari, F.; Vafaee, M.S.; Sadigh-Eteghad, S. Revisiting nicotine’s role in the ageing brain and cognitive impairment. Rev. Neurosci. 2017, 28, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Sofuoglu, M.; Herman, A.I.; Robinson, C.; Waters, A.J. Cognitive Effects of Nicotine. In The Effects of Drug Abuse on the Human Nervous System; Elsevier: Amsterdam, The Netherlands, 2014; pp. 367–385. ISBN 9780124186798. [Google Scholar]
- Guan, Z.-Z.; Yu, W.-F.; Nordberg, A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem. Int. 2003, 43, 243–249. [Google Scholar] [CrossRef]
- Nizri, E.; Irony-Tur-Sinai, M.; Lory, O.; Orr-Urtreger, A.; Lavi, E.; Brenner, T. Activation of the cholinergic anti-inflammatory system by nicotine attenuates neuroinflammation via suppression of Th1 and Th17 responses. J. Immunol. 2009, 183, 6681–6688. [Google Scholar] [CrossRef]
- Nordberg, A.; Hellström-Lindahl, E.; Lee, M.; Johnson, M.; Mousavi, M.; Hall, R.; Perry, E.; Bednar, I.; Court, J. Chronic nicotine treatment reduces beta-amyloidosis in the brain of a mouse model of Alzheimer’s disease (APPsw). J. Neurochem. 2002, 81, 655–658. [Google Scholar] [CrossRef]
- Benowitz, N.L.; Burbank, A.D. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc. Med. 2016, 26, 515–523. [Google Scholar] [CrossRef]
- Benowitz, N.L. Nicotine addiction. N. Engl. J. Med. 2010, 362, 2295–2303. [Google Scholar] [CrossRef]
- Buccafusco, J.J. Neuronal nicotinic receptor subtypes: Defining therapeutic targets. Mol. Interv. 2004, 4, 285–295. [Google Scholar] [CrossRef]
- Echeverria, V.; Zeitlin, R. Cotinine: A Potential New Therapeutic Agent against Alzheimer’s disease. CNS Neurosci. Ther. 2012, 18, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Grizzell, J.A.; Echeverria, V. New Insights into the Mechanisms of Action of Cotinine and its Distinctive Effects from Nicotine. Neurochem. Res. 2015, 40, 2032–2046. [Google Scholar] [CrossRef] [PubMed]
- Igloi, G.L.; Brandsch, R. Sequence of the 165-Kilobase Catabolic Plasmid pAO1 from Arthrobacter nicotinovorans and Identification of a pAO1-Dependent Nicotine Uptake System. J. Bacteriol. 2003, 185, 1976–1986. [Google Scholar] [CrossRef] [PubMed]
- Brandsch, R. Microbiology and biochemistry of nicotine degradation. Appl. Microbiol. Biotechnol. 2006, 69, 493–498. [Google Scholar] [CrossRef]
- Boiangiu, R.; Guzun, D.; Mihăşan, M. Time dependent accumulation of nicotine derivatives in the culture medium of Arthrobacter nicotinovorans pAO1. Analele Ştiinţifice Ale Universităţii Alexandru Ioan Cuza din Iași, Sectiunea II A Genetica si Biologie Moleculara 2014, 15, 19–23. [Google Scholar]
- Hritcu, L.; Ionita, R.; Motei, D.E.; Babii, C.; Stefan, M.; Mihasan, M. Nicotine versus 6-hydroxy-l-nicotine against chlorisondamine induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2017, 86, 102–108. [Google Scholar] [CrossRef]
- Patel, S.; Grizzell, J.A.; Holmes, R.; Zeitlin, R.; Solomon, R.; Sutton, T.L.; Rohani, A.; Charry, L.C.; Iarkov, A.; Mori, T.; et al. Cotinine halts the advance of Alzheimer’s disease-like pathology and associated depressive-like behavior in Tg6799 mice. Front. Aging Neurosci. 2014, 6, 162. [Google Scholar] [CrossRef]
- Echeverria, V.; Zeitlin, R.; Burgess, S.; Patel, S.; Barman, A.; Thakur, G.; Mamcarz, M.; Wang, L.; Sattelle, D.B.; Kirschner, D.A.; et al. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer’s disease mice. J. Alzheimer’s Dis. 2011, 24, 817–835. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Celie, P.H.N.; van Rossum-Fikkert, S.E.; van Dijk, W.J.; Brejc, K.; Smit, A.B.; Sixma, T.K. Nicotine and carbamylcholine binding to nicotinic acetylcholine receptors as studied in AChBP crystal structures. Neuron 2004, 41, 907–914. [Google Scholar] [CrossRef]
- Walsh, R.M.; Roh, S.-H.; Gharpure, A.; Morales-Perez, C.L.; Teng, J.; Hibbs, R.E. Structural principles of distinct assemblies of the human α4β2 nicotinic receptor. Nature 2018, 557, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Leite, T.B.; Gomes, D.; Miteva, M.A.; Chomilier, J.; Villoutreix, B.O.; Tufféry, P. Frog: A FRee Online druG 3D conformation generator. Nucleic Acids Res. 2007, 35, W568–W572. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger, Inc. The PyMOL Molecular Graphics System, Version 2.2.3; Schrödinger, Inc.: New York, NY, USA, 2015. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An environment for comparative protein modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef]
- Postu, P.A.; Noumedem, J.A.K.; Cioanca, O.; Hancianu, M.; Mihasan, M.; Ciorpac, M.; Gorgan, D.L.; Petre, B.A.; Hritcu, L. Lactuca capensis reverses memory deficits in Aβ1-42-induced an animal model of Alzheimer’s disease. J. Cell. Mol. Med. 2017, 22, 111–122. [Google Scholar] [CrossRef]
- Fedotova, J.; Soultanov, V.; Nikitina, T.; Roschin, V.; Ordyan, N.; Hritcu, L. Cognitive-enhancing activities of the polyprenol preparation Ropren® in gonadectomized β-amyloid (25–35) rat model of Alzheimer’s disease. Physiol. Behav. 2016, 157, 55–62. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 9780080475134. [Google Scholar]
- Hamisha, K.N.; Tfilin, M.; Yanai, J.; Turgeman, G. Mesenchymal Stem Cells Can Prevent Alterations in Behavior and Neurogenesis Induced by Aß25–35 Administration. J. Mol. Neurosci. 2015, 55, 1006–1013. [Google Scholar] [CrossRef]
- Bate, S.T.; Clark, R.A. The Design and Statistical Analysis of Animal Experiments; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781139344319. [Google Scholar]
- Jackson, L.L. VTE on an elevated T-maze. J. Comp. Psychol. 1943, 36, 99–107. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules 2020, 25, 1519. [Google Scholar] [CrossRef] [PubMed]
- Pardo Andreu, G.L.; Maurmann, N.; Reolon, G.K.; de Farias, C.B.; Schwartsmann, G.; Delgado, R.; Roesler, R. Mangiferin, a naturally occurring glucoxilxanthone improves long-term object recognition memory in rats. Eur. J. Pharm. 2010, 635, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Foyet, H.S.; Asongalem, A.E.; Oben, E.K.; Cioanca, O.; Hancianu, M.; Hritcu, L. Effects of the Methanolic Extract of Vitellaria paradoxa Stem Bark Against Scopolamine-Induced Cognitive Dysfunction and Oxidative Stress in the Rat Hippocampus. Cell. Mol. Neurobiol. 2016, 36, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. B 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Hritcu, L.; Boiangiu, R.S.; de Morais, M.C.; de Sousa, D.P.-C. A Natural Compound, Improves β -Amyloid-Peptide 1-42-Induced Memory Impairment and Oxidative Stress in the Rat Hippocampus. Biomed. Res. Int. 2020. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.J.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Winterbourn, C.; Hawkins, R.; Brian, M.; Carrell, R. The estimation of red cell superoxide dismutase activity. J. Lab. Clin. Med. 1975, 85, 337. [Google Scholar]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Tokumura, A. Glutathione peroxidase activity in tissues of vitamin E-deficient mice. J. Nutr. Sci. Vitam. 1976, 22, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
- Salbitani, G.; Vona, V.; Bottone, C.; Petriccione, M.; Carfagna, S. Sulfur deprivation results in oxidative perturbation in chlorella sorokiniana (211/8k). Plant. Cell Physiol. 2015, 56, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Oliver, C.N.; Ahn, B.W.; Moerman, E.J.; Goldstein, S.; Stadtman, E.R. Age-related changes in oxidized proteins. J. Biol. Chem. 1987, 262, 5488–5491. [Google Scholar] [PubMed]
- Luo, S.; Wehr, N.B. Protein carbonylation: Avoiding pitfalls in the 2,4-dinitrophenylhydrazine assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef]
- Domijan, A.-M.; Ralić, J.; Radić Brkanac, S.; Rumora, L.; Žanić-Grubišić, T. Quantification of malondialdehyde by HPLC-FL—Application to various biological samples. Biomed. Chromatogr. 2015, 29, 41–46. [Google Scholar] [CrossRef]
- Vaideș-Negustor, R.N.; Mihăşan, M. Side comparation of two methods for quantifying malondialdehyde levels in animal tissue extracts. J. Exp. Molec. Biol. 2020, 20, 61–66. [Google Scholar]
- Ionita, R.; Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Hancianu, M.; Cioanca, O.; Hritcu, L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Taly, A.; Corringer, P.-J.; Guedin, D.; Lestage, P.; Changeux, J.-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009, 8, 733–750. [Google Scholar] [CrossRef]
- Pohanka, M. Alpha7 Nicotinic Acetylcholine Receptor Is a Target in Pharmacology and Toxicology. Int. J. Mol. Sci. 2012, 13, 2219–2238. [Google Scholar] [CrossRef]
- Mihășan, M.; Căpățînă, L.; Neagu, E.; Ștefan, M.; Hrițcu, L. In-silico identification of 6-hydroxy-L-nicotine as a novel neuroprotective drug. Rom. Biotechnol. Lett. 2013, 18, 8333–8340. [Google Scholar]
- Sabri, O.; Meyer, P.M.; Gräf, S.; Hesse, S.; Wilke, S.; Becker, G.-A.; Rullmann, M.; Patt, M.; Luthardt, J.; Wagenknecht, G.; et al. Cognitive correlates of α4β2 nicotinic acetylcholine receptors in mild Alzheimer’s dementia. Brain 2018, 141, 1840–1854. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Callahan, P.M.; Bertrand, D. R-(+) and S-(-) isomers of cotinine augment cholinergic responses in vitro and in vivo. J. Pharmacol. Exp. Ther. 2015, 352, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Dineley, K.T.; Westerman, M.; Bui, D.; Bell, K.; Ashe, K.H.; Sweatt, J.D. β-Amyloid activates the mitogen-activated protein kinase cascade via hippocampal α7 nicotinic acetylcholine receptors: In Vitro and in Vivo mechanisms related to Alzheimer’s disease. J. Neurosci. 2001, 21, 4125–4133. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Takeda, K.; Ichijo, H. The ASK1-MAP Kinase Signaling in ER Stress and Neurodegenerative Diseases. Curr. Mol. Med. 2006, 6, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Toral-Rios, D.; Pichardo-Rojas, P.S.; Alonso-Vanegas, M.; Campos-Peña, V. GSK3β and Tau Protein in Alzheimer’s Disease and Epilepsy. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Grizzell, J.A.; Patel, S.; Barreto, G.E.; Echeverria, V. Cotinine improves visual recognition memory and decreases cortical Tau phosphorylation in the Tg6799 mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 78, 75–81. [Google Scholar] [CrossRef]
- Grizzell, J.A.; Iarkov, A.; Holmes, R.; Mori, T.; Echeverria, V. Cotinine reduces depressive-like behavior, working memory deficits, and synaptic loss associated with chronic stress in mice. Behav. Brain Res. 2014, 268, 55–65. [Google Scholar] [CrossRef]
- Alijevic, O.; McHugh, D.; Rufener, L.; Mazurov, A.; Hoeng, J.; Peitsch, M. An electrophysiological characterization of naturally occurring tobacco alkaloids and their action on human α4β2 and α7 nicotinic acetylcholine receptors. Phytochemistry 2020, 170, 1121187. [Google Scholar] [CrossRef]
- O’Leary, K.; Parameswaran, N.; McIntosh, J.M.; Quik, M. Cotinine selectively activates a subpopulation of α3/α6β2 * nicotinic receptors in monkey striatum. J. Pharmacol. Exp. Ther. 2008, 325, 646–654. [Google Scholar] [CrossRef]
- Wildeboer-Andrud, K.M.; Zheng, L.; Choo, K.S.; Stevens, K.E. No Title. Pharmacol. Biochem. Behav. 2014, 117. [Google Scholar]
- Fox, A.M.; Moonschi, F.H.; Richards, C.I. The Nicotine Metabolite, Cotinine, Alters the Assembly and Trafficking of a Subset of Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2015, 290, 24403–24412. [Google Scholar] [CrossRef] [PubMed]
- Harpsøe, K.; Ahring, P.K.; Christensen, J.K.; Jensen, M.L.; Peters, D.; Balle, T. Unraveling the high- and low-sensitivity agonist responses of nicotinic acetylcholine receptors. J. Neurosci. 2011, 31, 10759–10766. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, B.; Sheeja Malar, D.; Sathya, S.; Pandima Devi, K. Antiaggregation Potential of Padina gymnospora against the Toxic Alzheimer’s Beta-Amyloid Peptide 25-35 and Cholinesterase Inhibitory Property of Its Bioactive Compounds. PLoS ONE 2015, 10, e0141708. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Pryor, N.; Moss, M.A.; Hestekin, C.N. Unraveling the early events of amyloid-ß protein (Aß) aggregation: Techniques for the determination of Aβ aggregate size. Int. J. Mol. Sci. 2012, 13, 3038–3072. [Google Scholar] [CrossRef]
- Aliaga, E.; Silhol, M.; Bonneau, N.; Maurice, T.; Arancibia, S.; Tapia-Arancibia, L. Dual response of BDNF to sublethal concentrations of β-amyloid peptides in cultured cortical neurons. Neurobiol. Dis. 2010, 37, 208–217. [Google Scholar] [CrossRef]
- Soleimani Asl, S.; Bergen, H.; Ashtari, N.; Amiri, S.; Łos, M.J.; Mehdizadeh, M. Pelargonidin exhibits restoring effects against amyloid β-induced deficits in the hippocampus of male rats. Med. J. Islam. Repub. Iran 2019, 33, 135. [Google Scholar]
- Ye, T.; Li, X.; Zhou, P.; Ye, S.; Gao, H.; Hua, R.; Ma, J.; Wang, Y.; Cai, B. Chrysophanol improves memory ability of d-galactose and Aβ25–35 treated rat correlating with inhibiting tau hyperphosphorylation and the CaM–CaMKIV signal pathway in hippocampus. 3 Biotech 2020, 10, 111. [Google Scholar] [CrossRef]
- Hritcu, L.; Stefan, M.; Brandsch, R.; Mihasan, M.; Hrițcu, L.; Ștefan, M.; Brandsch, R.; Mihășan, M. 6-hydroxy-l-nicotine from Arthrobacter nicotinovorans sustain spatial memory formation by decreasing brain oxidative stress in rats. J. Physiol. Biochem. 2013, 69, 25–34. [Google Scholar] [CrossRef]
- Hritcu, L.; Stefan, M.; Brandsch, R.; Mihasan, M. Enhanced behavioral response by decreasing brain oxidative stress to 6-hydroxy-l-nicotine in Alzheimer’s disease rat model. Neurosci. Lett. 2015, 591, 41–47. [Google Scholar] [CrossRef]
- Barreto, G.E.; Iarkov, A.; Moran, V.E. Beneficial effects of nicotine, cotinine and its metabolites as potential agents for Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 340. [Google Scholar] [PubMed]
- Terry, A.V.; Hernandez, C.M.; Hohnadel, E.J.; Bouchard, K.P.; Buccafusco, J.J. Cotinine, a neuroactive metabolite of nicotine: Potential for treating disorders of impaired cognition. CNS Drug Rev. 2005, 11, 229–252. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, C.; Barreto, G.E.; Iarkov, A.; Tarasov, V.V.; Aliev, G.; Echeverria, V. Cotinine: A Therapy for Memory Extinction in Post-traumatic Stress Disorder. Mol. Neurobiol. 2018, 55, 6700–6711. [Google Scholar] [CrossRef] [PubMed]
- Iarkov, A.; Appunn, D.; Echeverria, V. Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemother. Pharmacol. 2016, 78, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Beurel, E.; Jope, R.S. Cotinine administration improves impaired cognition in the mouse model of Fragile X syndrome. Eur. J. Neurosci. 2017, 45, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, V.; Cuello, A.C. Intracellular A-Beta Amyloid, A Sign for Worse Things to Come? Mol. Neurobiol. 2002, 26, 299–316. [Google Scholar] [CrossRef]
- Salomon, A.R.; Marcinowski, K.J.; Friedland, R.P.; Zagorski, M.G. Nicotine Inhibits Amyloid Formation by the β-Peptide †. Biochemistry 1996, 35, 13568–13578. [Google Scholar] [CrossRef]
- Szymańska, I.; Radecka, H.; Radecki, J.; Kaliszan, R. Electrochemical impedance spectroscopy for study of amyloid beta-peptide interactions with (-) nicotine ditartrate and (-) cotinine. Biosens. Bioelectron. 2007, 22, 1955–1960. [Google Scholar] [CrossRef]
- Burgess, S.; Zeitlin, R.; Echeverria, V. Cotinine Inhibits Amyloid-β Peptide Neurotoxicity and Oligomerization. J. Clin. Toxicol. 2012, 01, 2011–2013. [Google Scholar] [CrossRef]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and Clinical Significance of Biomarkers of Oxidative Stress in Humans. Oxid. Med. Cell. Longev. 2017, 2017, 32. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Boyd-Kimball, D. Oxidative Stress, Amyloid-β Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J. Alzheimers. Dis. 2018, 62, 1345–1367. [Google Scholar] [CrossRef] [PubMed]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, L.; Weng, Q.; Xiao, L.; Li, Q. Curcumin analogues attenuate Aβ25-35-induced oxidative stress in PC12 cells via Keap1/Nrf2/HO-1 signaling pathways. Chem. Biol. Interact. 2019, 305, 171–179. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Wang, H.; Zhao, L.; Ma, Z.; Li, T.; Liu, J.; Sun, M.; Jian, Y.; Yao, L.; et al. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life Sci. 2019, 221, 259–266. [Google Scholar] [CrossRef]
- Mocanu, E.M.; Mazarachi, A.L.; Mihasan, M. In vitro stability and antioxidant potential of the neuprotective metabolite 6-hydroxy-nicotine. J. Exp. Mol. Biol. 2018, 19, 53–58. [Google Scholar]
- Srivastava, E.D.; Hallett, M.B.; Rhodes, J. Effect of Nicotine and Cotinine on the Production of Oxygen Free Radicals by Neutrophils in Smokers and Non-smokers. Hum. Toxicol. 1989, 8, 461–463. [Google Scholar] [CrossRef]
- Soto-Otero, R.; Méndez-Alvarez, E.; Hermida-Ameijeiras, A.; López-Real, A.M.; Labandeira-García, J.L. Effects of (-)-nicotine and (-)-cotinine on 6-hydroxydopamine-induced oxidative stress and neurotoxicity: Relevance for Parkinson’s disease. Biochem. Pharmacol. 2002, 64, 125–135. [Google Scholar] [CrossRef]
- De Aguiar, R.B.; Parfitt, G.M.; Jaboinski, J.; Barros, D.M. Neuroactive effects of cotinine on the hippocampus: Behavioral and biochemical parameters. Neuropharmacology 2013, 71, 292–298. [Google Scholar] [CrossRef]
- Zeitlin, R.; Patel, S.; Solomon, R.; Tran, J.; Weeber, E.J.; Echeverria, V. Cotinine enhances the extinction of contextual fear memory and reduces anxiety after fear conditioning. Behav. Brain Res. 2012, 228, 284–293. [Google Scholar] [CrossRef]
- Tanila, H. The role of BDNF in Alzheimer’s disease. Neurobiol. Dis. 2017, 97, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Machaalani, R.; Chen, H. Brain derived neurotrophic factor (BDNF), its tyrosine kinase receptor B (TrkB) and nicotine. Neurotoxicology 2018, 65, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Minichiello, L. TrkB signalling pathways in LTP and learning. Nat. Rev. Neurosci. 2009, 10, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Sabirzhanov, B.; Keifer, J. Oligomeric Amyloid-β Inhibits the Proteolytic Conversion of Brain-derived Neurotrophic Factor (BDNF), AMPA Receptor Trafficking, and Classical Conditioning. J. Biol. Chem. 2010, 285, 34708–34717. [Google Scholar] [CrossRef]
- Ramser, E.M.; Gan, K.J.; Decker, H.; Fan, E.Y.; Suzuki, M.M.; Ferreira, S.T.; Silverman, M.A. Amyloid-β oligomers induce tau-independent disruption of BDNF axonal transport via calcineurin activation in cultured hippocampal neurons. Mol. Biol. Cell 2013, 24, 2494–2505. [Google Scholar] [CrossRef]
- Arancibia, S.; Silhol, M.; Moulière, F.; Meffre, J.; Höllinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef]
- Kitiyanant, N.; Kitiyanant, Y.; Svendsen, C.N.; Thangnipon, W. BDNF-, IGF-1- and GDNF-secreting human neural progenitor cells rescue amyloid β-induced toxicity in cultured rat septal neurons. Neurochem. Res. 2012, 37, 143–152. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Y.; Lian, Y.; Chen, Y.; Wu, T.; Zheng, Y.; Zong, H.; Sun, L.; Zhang, R.; Wang, Z.; et al. Brain-Derived Neurotrophic Factor Ameliorates Learning Deficits in a Rat Model of Alzheimer’s Disease Induced by Aβ1-42. PLoS ONE 2015, 10, e0122415. [Google Scholar] [CrossRef]
- Gao, J.; Adam, B.L.; Terry, A.V. Evaluation of nicotine and cotinine analogs as potential neuroprotective agents for Alzheimer’s disease. Bioorganic Med. Chem. Lett. 2014, 24, 1472–1478. [Google Scholar] [CrossRef][Green Version]
- Riveles, K.; Huang, L.Z.; Quik, M. Cigarette smoke, nicotine and cotinine protect against 6-hydroxydopamine-induced toxicity in SH-SY5Y cells. Neurotoxicology 2008, 29, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Alme, M.N.; Bittins, M.; Kuipers, S.D.; Nair, R.R.; Pai, B.; Panja, D.; Schubert, M.; Soule, J.; Tiron, A.; et al. The Arc of synaptic memory. Exp. Brain Res. 2010, 200, 125–140. [Google Scholar] [CrossRef] [PubMed]
- Kerrigan, T.L.; Randall, A.D. A new player in the “synaptopathy” of Alzheimer’s disease—Arc/Arg 3.1. Front. Neurol. 2013, 4, 9. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Wegenast-Braun, B.M.; Fulgencio Maisch, A.; Eicke, D.; Radde, R.; Herzig, M.C.; Staufenbiel, M.; Jucker, M.; Calhoun, M.E. Independent Effects of Intra- and Extracellular Aβ on Learning-Related Gene Expression. Am. J. Pathol. 2009, 175, 271–282. [Google Scholar] [CrossRef]
- Palop, J.J. Vulnerability of Dentate Granule Cells to Disruption of Arc Expression in Human Amyloid Precursor Protein Transgenic Mice. J. Neurosci. 2005, 25, 9686–9693. [Google Scholar] [CrossRef]
- Dickey, C.A.; Gordon, M.N.; Mason, J.E.; Wilson, N.J.; Diamond, D.M.; Guzowski, J.F.; Morgan, D. Amyloid suppresses induction of genes critical for memory consolidation in APP + PS1 transgenic mice. J. Neurochem. 2004, 88, 434–442. [Google Scholar] [CrossRef]
- Kristensen, S.E.; Thomsen, M.S.; Hansen, H.H.; Timmermann, D.B.; Hay-Schmidt, A.; Mikkelsen, J.D. The α7 nicotinic receptor agonist SSR180711 increases activity regulated cytoskeleton protein (Arc) gene expression in the prefrontal cortex of the rat. Neurosci. Lett. 2007, 418, 154–158. [Google Scholar] [CrossRef]
- Waltereit, R.; Dammermann, B.; Wulff, P.; Scafidi, J.; Staubli, U.; Kauselmann, G.; Bundman, M.; Kuhl, D. Arg3.1/Arc mRNA Induction by Ca 2+ and cAMP Requires Protein Kinase A and Mitogen-Activated Protein Kinase/Extracellular Regulated Kinase Activation. J. Neurosci. 2001, 21, 5484–5493. [Google Scholar] [CrossRef]
- Bramham, C.R.; Worley, P.F.; Moore, M.J.; Guzowski, J.F. The Immediate Early Gene Arc/Arg3.1: Regulation, Mechanisms, and Function. J. Neurosci. 2008, 28, 11760–11767. [Google Scholar] [CrossRef]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2018, 125, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Alvarez, X.A.; Fernández-Novoa, L.; Franco, A.; Mangues, R.; Pellicer, A.; Nishimura, T. Brain interleukin-1 beta in Alzheimer’s disease and vascular dementia. Methods Find. Exp. Clin. Pharmacol. 1994, 16, 141–151. [Google Scholar]
- Simard, A.R.; Soulet, D.; Gowing, G.; Julien, J.P.; Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron 2006, 49, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Craft, J.M.; Watterson, D.M.; Hirsch, E.; Van Eldik, L.J. Interleukin 1 receptor antagonist knockout mice show enhanced microglial activation and neuronal damage induced by intracerebroventricular infusion of human beta-amyloid. J. Neuroinflamm. 2005, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Blasko, I.; Veerhuis, R.; Stampfer-Kountchev, M.; Saurwein-Teissl, M.; Eikelenboom, P.; Grubeck-Loebenstein, B. Costimulatory effects of interferon-β and interleukin-1β or tumor necrosis factor α on the synthesis of Aβ1-40 and Aβ1-42 by human astrocytes. Neurobiol. Dis. 2000, 7, 682–689. [Google Scholar] [CrossRef]
- Griffin, W.S.T.; Liu, L.; Li, Y.; Mrak, R.E.; Barger, S.W. Interleukin-1 mediates Alzheimer and Lewy body pathologies. J. Neuroinflamm. 2006, 3, 5. [Google Scholar] [CrossRef]
- Rehani, K.; Scott, D.A.; Renaud, D.; Hamza, H.; Williams, L.R.; Wang, H.; Martin, M. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim. Biophys. Acta 2008, 1783, 375–382. [Google Scholar] [CrossRef]
- Bagaitkar, J.; Zeller, I.; Renaud, D.E.; Scott, D.A. Cotinine inhibits the pro-inflammatory response initiated by multiple cell surface Toll-like receptors in monocytic THP cells. Tob. Induc. Dis. 2012, 10, 18. [Google Scholar] [CrossRef]





| Receptor | Site Name | Site Description | Resolution (Å) | PDB Entry | Reference |
|---|---|---|---|---|---|
| AChBP | AChBP | The interface between two identical subunits | 2.2 | 1UW6 | [32] |
| α4β2 nAChR (3α:2β) | α4-α4 | The interface between two α4 subunits | 3.9 | 6CNK | [33] |
| α4-β2 | The interface between an α4 and β2 subunit |
| Gene | Product Size (bp) | Primer | Sequence |
|---|---|---|---|
| bdnf (exon 5) | 101 | Forward | 5′-ATT ACC TGG ATG CCG CAA AC-3′ |
| Reverse | 5′-TGA CCC ACT CGC TAA TAC TGT-3′ | ||
| arc | 115 | Forward | 5′-CCCTGCAGCCCAAGTTCAAG-3 |
| Reverse | 5′-GAAGGCTCAGCTGCCTGCTC-3′ | ||
| il-1β | 144 | Forward | 5′-AGC ACC TTC TTT TCC TTC ATC TT-3′ |
| Reverse | 5′-CAG ACA GCA GGC ATT TT-3′ |
| Ligands | Binding Poses * | Receptor (Binding Site) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AChBP | α4-α4 | α4-β2 | ||||||||
| RMSD | Binding Energy | Ligand Efficiency | RMSD | Binding Energy | Ligand Efficiency | RMSD | Binding Energy | Ligand Efficiency | ||
| (S)-Nicotine | 1 | 0.46 | −7.05 | −0.59 | 0.55 | −5.57 | −0.46 | 0.47 | −6.06 | −0.51 |
| 2 | 0.45 | −6.95 | −0.58 | 0.56 | −5.51 | −0.46 | 0.31 | −5.93 | −0.49 | |
| 3 | 0.29 | −6.59 | −0.55 | 0.23 | −5.5 | −0.46 | 0.32 | −5.93 | −0.49 | |
| (R)-Nicotine | 1 | 0.36 | −6.88 | −0.57 | 0.5 | −5.52 | −0.46 | 0.48 | −5.87 | −0.49 |
| 2 | 0.37 | −6.79 | −0.57 | 0.51 | −5.51 | −0.46 | 0.62 | −5.86 | −0.49 | |
| 3 | 0.57 | −6.77 | −0.56 | 0.51 | −5.49 | −0.46 | 0.47 | −5.85 | −0.49 | |
| (S)-Cotinine | 1 | 0.27 | −6.6 | −0.51 | 0.4 | −5.65 | −0.43 | 0.45 | −6.24 | −0.48 |
| 2 | 0.5 | −6.56 | −0.5 | 0.45 | −5.62 | −0.43 | 0.61 | −6.17 | −0.47 | |
| 3 | 0.56 | −6.46 | −0.5 | 0.47 | −5.61 | −0.43 | 0.45 | −6 | −0.46 | |
| (R)-Cotinine | 1 | 0.6 | −6.55 | −0.5 | 0.45 | −5.65 | −0.43 | 0.45 | −6.17 | −0.47 |
| 2 | 0.44 | −6.47 | −0.5 | 0.54 | −5.62 | −0.43 | 0.44 | −6.17 | −0.47 | |
| 3 | 0.42 | −6.42 | −0.49 | 0.43 | −5.62 | −0.43 | 0.45 | −6.13 | −0.47 | |
| (S)-6-Hydroxynicotine | 1 | 0.33 | −7.2 | −0.55 | 0.28 | −5.71 | −0.44 | 0.18 | −6.16 | −0.47 |
| 2 | 0.27 | −7.18 | −0.55 | 0.37 | −5.69 | −0.44 | 0.22 | −6.16 | −0.47 | |
| 3 | 0.25 | −7.17 | −0.55 | 0.39 | −5.67 | −0.44 | 0.18 | −6.16 | −0.47 | |
| (R)-6-Hydroxynicotine | 1 | 0.64 | −7.34 | −0.56 | 0.42 | −5.73 | −0.44 | 0.51 | −6.48 | −0.5 |
| 2 | 0.5 | −7.34 | −0.56 | 0.65 | −5.69 | −0.44 | 0.6 | −6.37 | −0.49 | |
| 3 | 0.65 | −7.32 | −0.56 | 0.51 | −5.66 | −0.44 | 0.5 | −6.36 | −0.49 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Petre, B.A.; Hritcu, L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants 2020, 9, 768. https://doi.org/10.3390/antiox9080768
Boiangiu RS, Mihasan M, Gorgan DL, Stache BA, Petre BA, Hritcu L. Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants. 2020; 9(8):768. https://doi.org/10.3390/antiox9080768
Chicago/Turabian StyleBoiangiu, Razvan Stefan, Marius Mihasan, Dragos Lucian Gorgan, Bogdan Alexandru Stache, Brindusa Alina Petre, and Lucian Hritcu. 2020. "Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease" Antioxidants 9, no. 8: 768. https://doi.org/10.3390/antiox9080768
APA StyleBoiangiu, R. S., Mihasan, M., Gorgan, D. L., Stache, B. A., Petre, B. A., & Hritcu, L. (2020). Cotinine and 6-Hydroxy-L-Nicotine Reverses Memory Deficits and Reduces Oxidative Stress in Aβ25-35-Induced Rat Model of Alzheimer’s Disease. Antioxidants, 9(8), 768. https://doi.org/10.3390/antiox9080768