Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders
Abstract
1. Introduction
2. Natural Sources of Lycopene and Its Use Amongst Different Societies
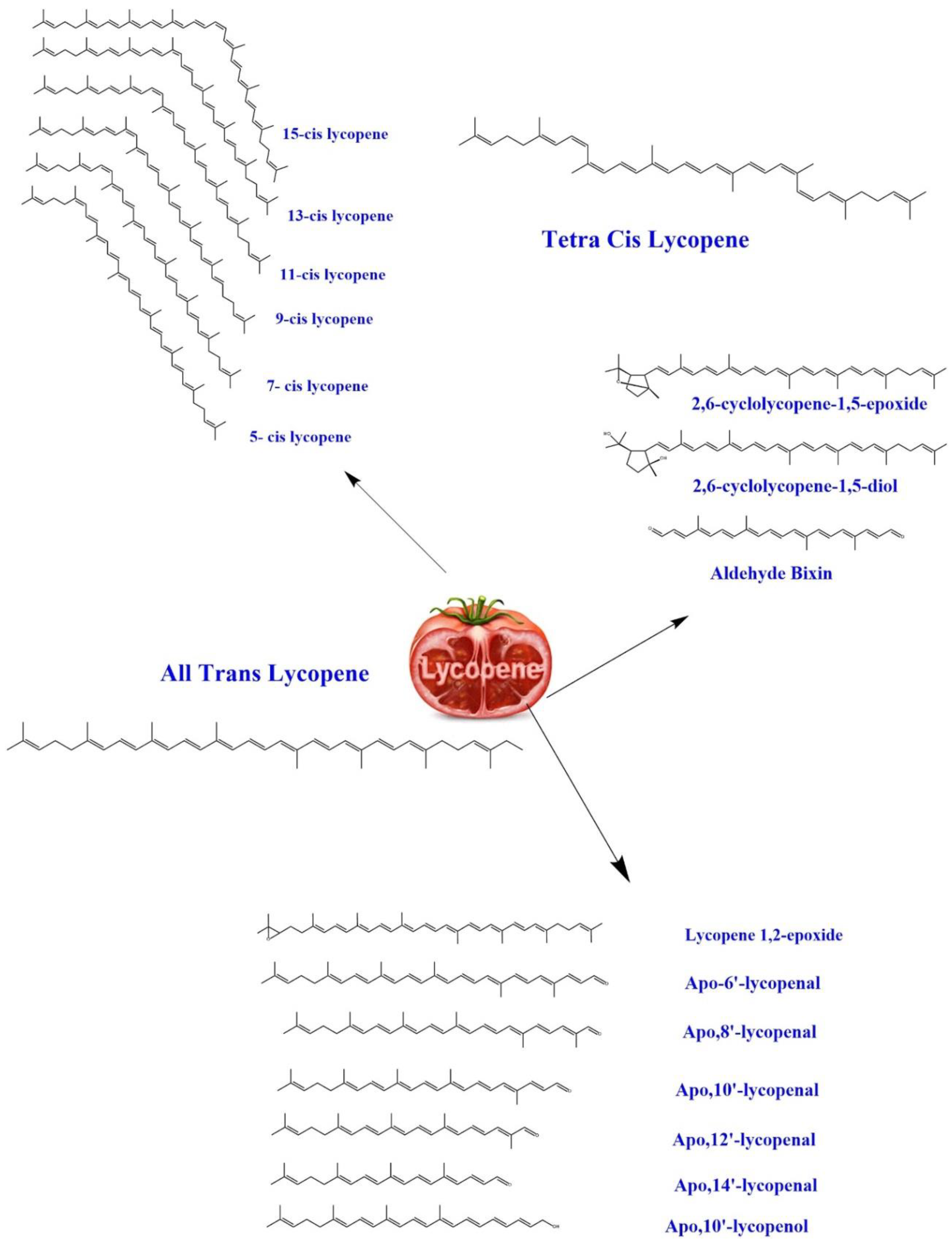
3. Biochemistry of Lycopene
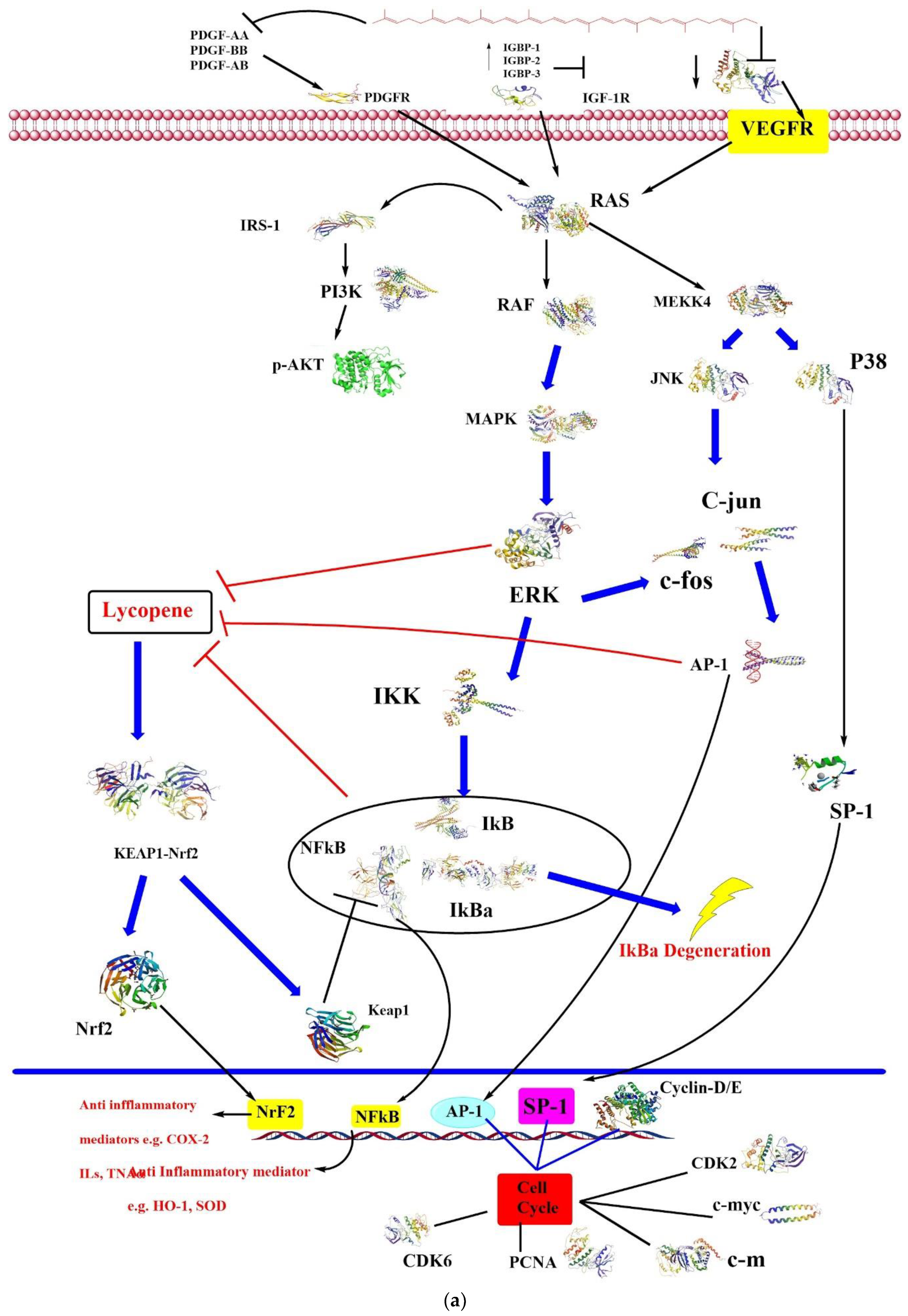
4. Biological Effects
4.1. Anticancer
4.2. Antidiabetic
4.3. Cardioprotective
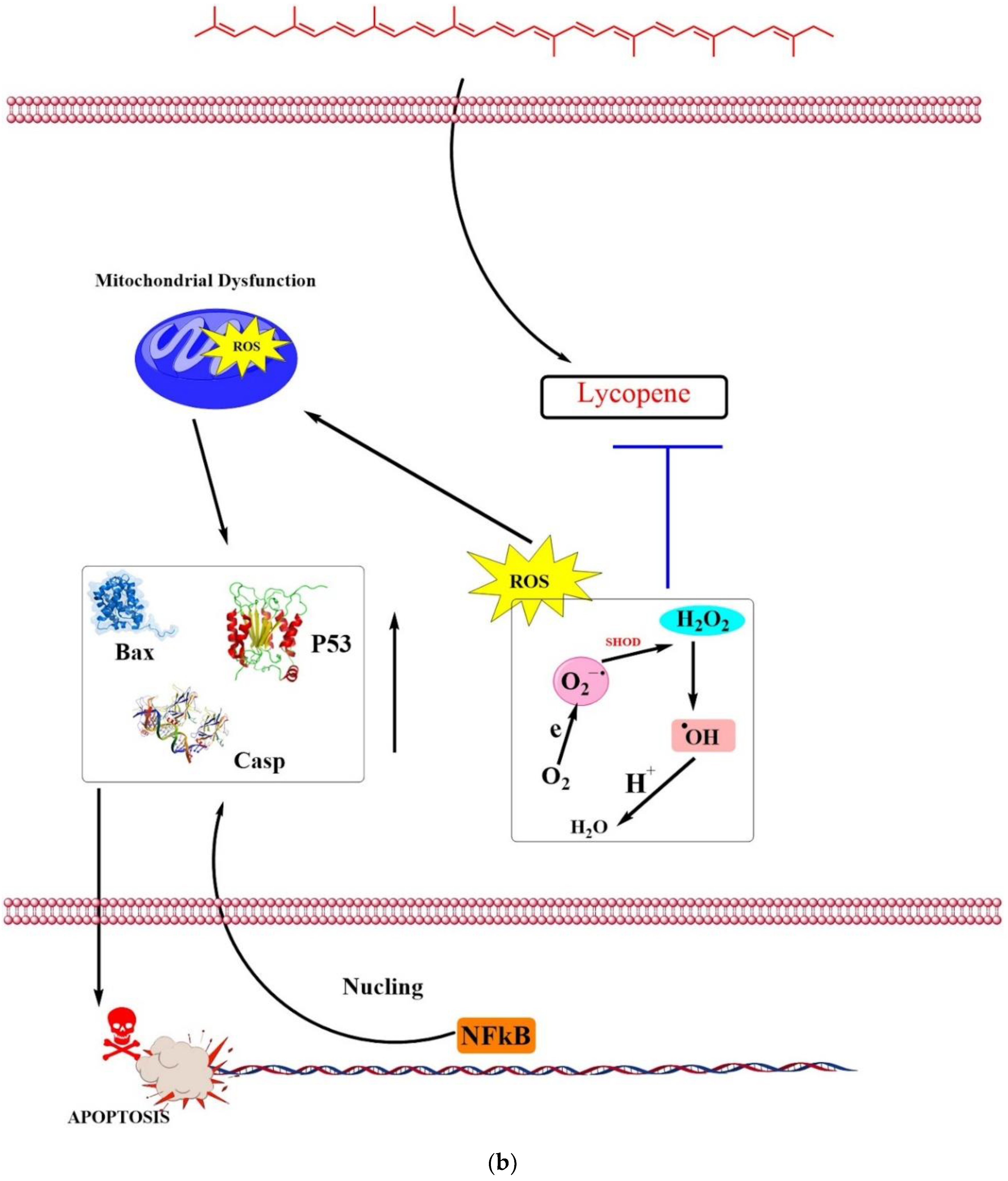
4.4. Antioxidative
4.5. Anti-Inflammatory Activity
4.6. Hepatoprotective
4.7. Against Dermatologic Diseases
4.8. Neuroprotective
4.9. Bone Protective
4.10. Targeting Reproductive Disorders
5. Protective Effects of Lycopene Against Different Toxins
6. Recommended Dose
7. Safety and Toxicity
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pennathur, S.; Maitra, D.; Byun, J.; Sliskovic, I.; Abdulhamid, I.; Saed, G.M.; Diamond, M.P.; Abu-Soud, H.M. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic. Biol. Med. 2010, 49, 205–213. [Google Scholar] [CrossRef]
- Yin, Y.; Zheng, Z.; Jiang, Z. Effects of lycopene on metabolism of glycolipid in type 2 diabetic rats. Biomed. Pharmacother. 2019, 109, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and vascular health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: A review. BioFactors 2019, 45, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Erdman, J.W., Jr.; Clinton, S.K. Complex interactions between dietary and genetic factors impact lycopene metabolism and distribution. Arch. Biochem. Biophys. 2013, 539, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxidative Med. Cell. Longev. 2016, 2016, 3218605. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.C.; Dudenbostel, T.; Crowe-White, K. Watermelon juice: A novel functional food to increase circulating lycopene in older adult women. Plant Foods Hum. Nutr. 2019, 74, 200–203. [Google Scholar] [CrossRef]
- Naviglio, D.; Sapio, L.; Langilla, C.; Ragone, A.; Illiano, M.; Naviglio, S.; Gallo, M. Beneficial Effects and Perspective Strategies for Lycopene Food Enrichment: A Systematic Review. Syst. Rev. Pharm. 2019, 10, 383–392. [Google Scholar]
- Saini, R.K.; Rengasamy, K.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective effects of lycopene in cancer, cardiovascular, and neurodegenerative diseases: An update on epidemiological and mechanistic perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef]
- Przybylska, S. Lycopene–a bioactive carotenoid offering multiple health benefits: A review. Int. J. Food Sci. Technol. 2020, 55, 11–32. [Google Scholar] [CrossRef]
- Joshi, B.; Kar, S.K.; Yadav, P.K.; Yadav, S.; Shrestha, L.; Bera, T.K. Therapeutic and medicinal uses of lycopene: A systematic review. Int. J. Res. Med Sci. 2020, 8, 1195. [Google Scholar] [CrossRef]
- Rowles, J.; Ranard, K.; Smith, J.; An, R.; Erdman, J. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Rowles, J.L.; Ranard, K.M.; Applegate, C.C.; Jeon, S.; An, R.; Erdman, J.W. Processed and raw tomato consumption and risk of prostate cancer: A systematic review and dose–response meta-analysis. Prostate Cancer Prostatic Dis. 2018, 21, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Carini, F.; David, S.; Tomasello, G.; Mazzola, M.; Damiani, P.; Rappa, F.; Battaglia, L.; Cappello, F.; Jurjus, A.; Geagea, A.G. Colorectal cancer: An update on the effects of lycopene on tumor progression and cell proliferation. J. Biol. Regul. Homeost. Agents 2017, 31, 769–774. [Google Scholar]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and metabolic syndrome: A systematic review of the literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [CrossRef]
- Kwatra, B. A review on potential properties and therapeutic applications of lycopene. Int. J. Med Biomed. Stud. 2020, 4. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8. [Google Scholar] [CrossRef]
- Chen, D.; Huang, C.; Chen, Z. A review for the pharmacological effect of lycopene in central nervous system disorders. Biomed. Pharmacother. 2019, 111, 791–801. [Google Scholar] [CrossRef]
- Chernyshova, M.P.; Pristenskiy, D.V.; Lozbiakova, M.V.; Chalyk, N.E.; Bandaletova, T.Y.; Petyaev, I.M. Systemic and skin-targeting beneficial effects of lycopene-enriched ice cream: A pilot study. J. Dairy Sci. 2019, 102, 14–25. [Google Scholar] [CrossRef]
- Salehi, B.; Lopez-Jornet, P.; Pons-Fuster López, E.; Calina, D.; Sharifi-Rad, M.; Ramírez-Alarcón, K.; Forman, K.; Fernández, M.; Martorell, M.; Setzer, W.N. Plant-derived bioactives in oral mucosal lesions: A key emphasis to curcumin, lycopene, chamomile, aloe vera, green tea and coffee properties. Biomolecules 2019, 9, 106. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Bramley, P.M. Is lycopene beneficial to human health? Phytochemistry 2000, 54, 233–236. [Google Scholar] [CrossRef]
- Barber, N.; Barber, J. Lycopene and prostate cancer. Prostate Cancer Prostatic Dis. 2002, 5, 6–12. [Google Scholar] [CrossRef]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- O’Neill, M.; Carroll, Y.; Corridan, B.; Olmedilla, B.; Granado, F.; Blanco, I.; Van den Berg, H.; Hininger, I.; Rousell, A.-M.; Chopra, M. A European carotenoid database to assess carotenoid intakes and its use in a five-country comparative study. Br. J. Nutr. 2001, 85, 499–507. [Google Scholar] [CrossRef]
- Porrini, M.; Riso, P. What are typical lycopene intakes? J. Nutr. 2005, 135, 2042S–2045S. [Google Scholar] [CrossRef]
- Jacques, P.F.; Lyass, A.; Massaro, J.M.; Vasan, R.S.; D’Agostino Sr, R.B. Relationship of lycopene intake and consumption of tomato products to incident CVD. Br. J. Nutr. 2013, 110, 545–551. [Google Scholar] [CrossRef]
- Authority, E.F.S. Revised exposure assessment for lycopene as a food colour. EFSA J. 2010, 8, 1444. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Holzapfel, B.M.; Champ, S.; Feldthusen, J.; Clements, J.; Hutmacher, D.W. The potential role of lycopene for the prevention and therapy of prostate cancer: From molecular mechanisms to clinical evidence. Int. J. Mol. Sci. 2013, 14, 14620–14646. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Rao, A. Carotenoids and chronic diseases. Drug Metab. Drug Interact. 2000, 17, 189–210. [Google Scholar] [CrossRef] [PubMed]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W., Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [Google Scholar] [CrossRef] [PubMed]
- Walfisch, Y.; Walfisch, S.; Agbaria, R.; Levy, J.; Sharoni, Y. Lycopene in serum, skin and adipose tissues after tomato-oleoresin supplementation in patients undergoing haemorrhoidectomy or peri-anal fistulotomy. Br. J. Nutr. 2003, 90, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Burton-Freeman, B.M.; Sesso, H.D. Whole food versus supplement: Comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014, 5, 457–485. [Google Scholar] [CrossRef]
- Richelle, M.; Sanchez, B.; Tavazzi, I.; Lambelet, P.; Bortlik, K.; Williamson, G. Lycopene isomerisation takes place within enterocytes during absorption in human subjects. Br. J. Nutr. 2010, 103, 1800–1807. [Google Scholar] [CrossRef]
- Teodoro, A.J.; Perrone, D.; Martucci, R.B.; Borojevic, R. Lycopene isomerisation and storage in an in vitro model of murine hepatic stellate cells. Eur. J. Nutr. 2009, 48, 261–268. [Google Scholar] [CrossRef]
- Moussa, M.; Landrier, J.-F.; Reboul, E.; Ghiringhelli, O.; Coméra, C.; Collet, X.; Fröhlich, K.; Böhm, V.; Borel, P. Lycopene absorption in human intestinal cells and in mice involves scavenger receptor class B type I but not Niemann-Pick C1-like 1. J. Nutr. 2008, 138, 1432–1436. [Google Scholar] [CrossRef]
- Moussa, M.; Gouranton, E.; Gleize, B.; Yazidi, C.E.; Niot, I.; Besnard, P.; Borel, P.; Landrier, J.F. CD36 is involved in lycopene and lutein uptake by adipocytes and adipose tissue cultures. Mol. Nutr. Food Res. 2011, 55, 578–584. [Google Scholar] [CrossRef]
- Ferrucci, L.; Perry, J.R.; Matteini, A.; Perola, M.; Tanaka, T.; Silander, K.; Rice, N.; Melzer, D.; Murray, A.; Cluett, C. Common variation in the β-carotene 15, 15′-monooxygenase 1 gene affects circulating levels of carotenoids: A genome-wide association study. Am. J. Hum. Genet. 2009, 84, 123–133. [Google Scholar] [CrossRef]
- Lindshield, B.L.; Canene-Adams, K.; Erdman, J.W., Jr. Lycopenoids: Are lycopene metabolites bioactive? Arch. Biochem. Biophys. 2007, 458, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Vuong, L.T.; Ruckle, J.; Synal, H.A.; Schulze-König, T.; Wertz, K.; Rümbeli, R.; Liberman, R.G.; Skipper, P.L.; Tannenbaum, S.R. Lycopene bioavailability and metabolism in humans: An accelerator mass spectrometry study. Am. J. Clin. Nutr. 2011, 93, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Moran, N.E.; Cichon, M.J.; Riedl, K.M.; Grainger, E.M.; Schwartz, S.J.; Novotny, J.A.; Erdman, J.W., Jr.; Clinton, S.K. Compartmental and noncompartmental modeling of 13C-lycopene absorption, isomerization, and distribution kinetics in healthy adults. Am. J. Clin. Nutr. 2015, 102, 1436–1449. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Yenice, E.; Tuzcu, M.; Orhan, C.; Mizrak, C.; Ozercan, I.H.; Sahin, N.; Yilmaz, B.; Bilir, B.; Ozpolat, B. Lycopene protects against spontaneous ovarian cancer formation in laying hens. J. Cancer Prev. 2018, 23, 25. [Google Scholar] [CrossRef] [PubMed]
- Cataño, J.G.; Trujillo, C.G.; Caicedo, J.I.; Bravo-Balado, A.; Robledo, D.; Mariño-Alvarez, A.M.; Pedraza, A.; Arcila, M.J.; Plata, M. Efficacy of lycopene intake in primary prevention of prostate cancer: A systematic review of the literature and meta-analysis. Arch. Esp. Urol. 2018, 71, 187–197. [Google Scholar]
- Dos Santos, R.C.; Ombredane, A.S.; Souza, J.M.T.; Vasconcelos, A.G.; Plácido, A.; das GN Amorim, A.; Barbosa, E.A.; Lima, F.C.; Ropke, C.D.; Alves, M.M. Lycopene-rich extract from red guava (Psidium guajava L.) displays cytotoxic effect against human breast adenocarcinoma cell line MCF-7 via an apoptotic-like pathway. Food Res. Int. 2018, 105, 184–196. [Google Scholar] [CrossRef]
- Morgia, G.; Voce, S.; Palmieri, F.; Gentile, M.; Iapicca, G.; Giannantoni, A.; Blefari, F.; Carini, M.; Vespasiani, G.; Santelli, G. Association between selenium and lycopene supplementation and incidence of prostate cancer: Results from the post-hoc analysis of the procomb trial. Phytomedicine 2017, 34, 1–5. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, R.; Bi, T.; Lu, Y.; Jiang, L. Inhibitory effect of lycopene against the growth of human gastric cancer cells. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 184–190. [Google Scholar] [CrossRef]
- Jhou, B.-Y.; Song, T.-Y.; Lee, I.; Hu, M.-L.; Yang, N.-C. Lycopene inhibits metastasis of human liver adenocarcinoma SK-Hep-1 cells by downregulation of NADPH oxidase 4 protein expression. J. Agric. Food Chem. 2017, 65, 6893–6903. [Google Scholar] [CrossRef]
- Holzapfel, N.P.; Shokoohmand, A.; Wagner, F.; Landgraf, M.; Champ, S.; Holzapfel, B.M.; Clements, J.A.; Hutmacher, D.W.; Loessner, D. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am. J. Cancer Res. 2017, 7, 1322. [Google Scholar]
- Baş, H.; Pandır, D. Protective effects of lycopene on furantreated diabetic and non-diabetic rat lung. Biomed. Environ. Sci. 2016, 29, 143–147. [Google Scholar]
- Reddy, P.V.N.; Ambati, M.; Koduganti, R. Systemic lycopene as an adjunct to scaling and root planing in chronic periodontitis patients with type 2 diabetes mellitus. J. Int. Soc. Prev. Community Dent. 2015, 5, S25. [Google Scholar] [PubMed]
- Sandikci, M.; Karagenc, L.; Yildiz, M. Changes in the Pancreas in Experimental Diabetes and the Effect of Lycopene on These Changes: Proliferating, Apoptotic, and Estrogen Receptor α Positive Cells. Anat. Rec. 2017, 300, 2000–2007. [Google Scholar] [CrossRef] [PubMed]
- Uçar, S.; Pandir, D. Furan induced ovarian damage in non-diabetic and diabetic rats and cellular protective role of lycopene. Arch. Gynaecol. Obstet. 2017, 296, 1027–1037. [Google Scholar] [CrossRef]
- Soleymaninejad, M.; Joursaraei, S.G.; Feizi, F.; Jafari Anarkooli, I. The effects of lycopene and insulin on histological changes and the expression level of Bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 2017, 4650939. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Al-Shali, K.Z.; Ahmad, S. Lycopene powers the inhibition of glycation-induced diabetic nephropathy: A novel approach to halt the AGE-RAGE axis menace. Biofactors 2015, 41, 372–381. [Google Scholar] [CrossRef]
- Yegın, S.Ç.; Yur, F.; Çetın, S.; Güder, A. Effect of lycopene on serum nitrite-nitrate levels in diabetic rats. Indian J. Pharm. Sci. 2015, 77, 357. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Pinho, O.; Monteiro, P. Can lycopene be considered an effective protection against cardiovascular disease? Food Chem. 2018, 245, 1148–1153. [Google Scholar] [CrossRef]
- Kumar, R.; Salwe, K.J.; Kumarappan, M. Evaluation of antioxidant, hypolipidemic, and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn. Res. 2017, 9, 161. [Google Scholar]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef]
- He, Y.; Xia, P.; Jin, H.; Zhang, Y.; Chen, B.; Xu, Z. Lycopene Ameliorates Transplant Arteriosclerosis in Vascular Allograft Transplantation by Regulating the NO/cGMP Pathways and Rho-Associated Kinases Expression. Oxidative Med. Cell. Longev. 2016, 2016, 3128280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Liu, H.; Wang, J.; Liu, P.; Tan, X.; Ren, B.; Liu, Z.; Liu, X. Lycopene supplementation attenuates oxidative stress, neuroinflammation, and cognitive impairment in aged CD-1 mice. J. Agric. Food Chem. 2018, 66, 3127–3136. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, L.; Wang, Z.; Cui, Y.; Tan, X.; Yuan, T.; Liu, Q.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates lipopolysaccharide-induced amyloidogenesis and cognitive impairments via mediating neuroinflammation and oxidative stress. J. Nutr. Biochem. 2018, 56, 16–25. [Google Scholar] [CrossRef] [PubMed]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Hamada, O.B. Suppression of inducible nitric oxide synthase and tumor necrosis factor-alpha level by lycopene is comparable to methylprednisolone in acute pancreatitis. Dig. Liver Dis. 2018, 50, 601–607. [Google Scholar] [CrossRef]
- Zhao, B.; Ren, B.; Guo, R.; Zhang, W.; Ma, S.; Yao, Y.; Yuan, T.; Liu, Z.; Liu, X. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem. Toxicol. 2017, 109, 505–516. [Google Scholar] [CrossRef]
- Kawata, A.; Murakami, Y.; Suzuki, S.; Fujisawa, S. Anti-inflammatory activity of β-Carotene, lycopene and tri-n-butylborane, a scavenger of reactive oxygen species. Vivo 2018, 32, 255–264. [Google Scholar]
- Liu, C.-B.; Wang, R.; Yi, Y.-F.; Gao, Z.; Chen, Y.-Z. Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 2018, 53, 66–71. [Google Scholar] [CrossRef]
- Vasconcelos, A.G.; das GN Amorim, A.; dos Santos, R.C.; Souza, J.M.T.; de Souza, L.K.M.; de SL Araújo, T.; Nicolau, L.A.D.; de Lima Carvalho, L.; de Aquino, P.E.A.; da Silva Martins, C. Lycopene rich extract from red guava (Psidium guajava L.) displays anti-inflammatory and antioxidant profile by reducing suggestive hallmarks of acute inflammatory response in mice. Food Res. Int. 2017, 99, 959–968. [Google Scholar] [CrossRef]
- Jiang, W.; Guo, M.-H.; Hai, X. Hepatoprotective and antioxidant effects of lycopene on non-alcoholic fatty liver disease in rat. World J. Gastroenterol. 2016, 22, 10180. [Google Scholar] [CrossRef]
- Yefsah-Idres, A.; Benazzoug, Y.; Otman, A.; Latour, A.; Middendorp, S.; Janel, N. Hepatoprotective effects of lycopene on liver enzymes involved in methionine and xenobiotic metabolism in hyperhomocysteinemic rats. Food Funct. 2016, 7, 2862–2869. [Google Scholar] [CrossRef]
- Tokac, M.; Aydin, S.; Taner, G.; Özkardeş, A.B.; Taşlipinar, M.Y.; DoĞan, M.; Dündar, H.Z.; Kilic, M.; Başaran, A.A.; Başaran, A.N. Hepatoprotective and antioxidant effects of lycopene in acute cholestasis. Turk. J. Med Sci. 2015, 45, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Grether-Beck, S.; Marini, A.; Jaenicke, T.; Stahl, W.; Krutmann, J. Molecular evidence that oral supplementation with lycopene or lutein protects human skin against ultraviolet radiation: Results from a double-blinded, placebo-controlled, crossover study. Br. J. Dermatol. 2017, 176, 1231–1240. [Google Scholar] [CrossRef]
- Lu, C.-W.; Hung, C.-F.; Jean, W.-H.; Lin, T.-Y.; Huang, S.-K.; Wang, S.-J. Lycopene depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase C in rat cerebrocortical nerve terminals. Can. J. Physiol. Pharmacol. 2017, 96, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Kumar, A. Neuroprotective effect of lycopene against PTZ-induced kindling seizures in mice: Possible behavioural, biochemical and mitochondrial dysfunction. Phytother. Res. 2016, 30, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Jamwal, S.; Deshmukh, R.; Kumar, P. Beneficial effects of lycopene against haloperidol induced orofacial dyskinesia in rats: Possible neurotransmitters and neuroinflammation modulation. Eur. J. Pharmacol. 2016, 771, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Costa-Rodrigues, J.; Fernandes, M.H.; Pinho, O.; Monteiro, P.R.R. Modulation of human osteoclastogenesis and osteoblastogenesis by lycopene. J. Nutr. Biochem. 2018, 57, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ardawi, M.-S.M.; Badawoud, M.H.; Hassan, S.M.; Rouzi, A.A.; Ardawi, J.M.; AlNosani, N.M.; Qari, M.H.; Mousa, S.A. Lycopene treatment against loss of bone mass, microarchitecture and strength in relation to regulatory mechanisms in a postmenopausal osteoporosis model. Bone 2016, 83, 127–140. [Google Scholar] [CrossRef]
- Ghyasvand, T.; Goodarzi, M.T.; Amiri, I.; Karimi, J.; Ghorbani, M. Serum levels of lycopene, beta-carotene, and retinol and their correlation with sperm DNA damage in normospermic and infertile men. Int. J. Reprod. Biomed. 2015, 13, 787. [Google Scholar] [CrossRef]
- Aly, H.A.; El-Beshbishy, H.A.; Banjar, Z.M. Mitochondrial dysfunction induced impairment of spermatogenesis in LPS-treated rats: Modulatory role of lycopene. Eur. J. Pharmacol. 2012, 677, 31–38. [Google Scholar] [CrossRef]
- Nedamani, A.R.; Nedamani, E.R.; Salimi, A. The role of lycopene in human health as a natural colorant. Nutr. Food Sci. 2019, 49, 284–298. [Google Scholar]
- Ghadage, S.; Mane, K.; Agrawal, R.; Pawar, V. Tomato lycopene: Potential health benefits. Pharma Innov. J. 2019, 8, 1245–1248. [Google Scholar]
- Chen, P.; Xu, S.; Qu, J. Lycopene protects keratinocytes against UVB radiation-induced carcinogenesis via negative regulation of FOXO3a through the mTORC2/AKT signaling pathway. J. Cell. Biochem. 2018, 119, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, J.; Zhou, Y.; Tuo, M.; Qin, X.; Yu, Q.; Cheng, H.; Li, Y. In vitro effects and mechanisms of lycopene in MCF-7 human breast cancer cells. Genet. Mol. Res. 2017, 16, 13. [Google Scholar] [CrossRef]
- Cha, J.H.; Kim, W.K.; Ha, A.W.; Kim, M.H.; Chang, M.J. Anti-inflammatory effect of lycopene in SW480 human colorectal cancer cells. Nutr. Res. Pract. 2017, 11, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.D.C.P.; Machado, C.L.; Trindade, B.B.; do Canto Lima, I.C.; Gimba, E.R.P.; Teodoro, A.J.; Takiya, C.; Borojevic, R. Lycopene extracts from different tomato-based food products induce apoptosis in cultured human primary prostate cancer cells and regulate TP53, Bax and Bcl-2 transcript expression. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 339. [Google Scholar] [PubMed]
- Jain, A.; Sharma, G.; Kushwah, V.; Thakur, K.; Ghoshal, G.; Singh, B.; Jain, S.; Shivhare, U.; Katare, O. Fabrication and functional attributes of lipidic nanoconstructs of lycopene: An innovative endeavour for enhanced cytotoxicity in MCF-7 breast cancer cells. Colloids Surf. B Biointerfaces 2017, 152, 482–491. [Google Scholar] [CrossRef]
- Ye, M.; Wu, Q.; Zhang, M.; Huang, J. Lycopene inhibits the cell proliferation and invasion of human head and neck squamous cell carcinoma. Mol. Med. Rep. 2016, 14, 2953–2958. [Google Scholar] [CrossRef]
- Wang, X.; Yang, H.-H.; Liu, Y.; Zhou, Q.; Chen, Z.-H. Lycopene consumption and risk of colorectal cancer: A meta-analysis of observational studies. Nutr. Cancer 2016, 68, 1083–1096. [Google Scholar] [CrossRef]
- Gong, X.; Marisiddaiah, R.; Zaripheh, S.; Wiener, D.; Rubin, L.P. Mitochondrial β-carotene 9′, 10′ oxygenase modulates prostate cancer growth via NF-κB inhibition: A lycopene-independent function. Mol. Cancer Res. 2016, 14, 966–975. [Google Scholar] [CrossRef]
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.Q.; Smith, D.E.; Wang, X.D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181. [Google Scholar] [CrossRef]
- Graff, R.E.; Pettersson, A.; Lis, R.T.; Ahearn, T.U.; Markt, S.C.; Wilson, K.M.; Rider, J.R.; Fiorentino, M.; Finn, S.; Kenfield, S.A. Dietary lycopene intake and risk of prostate cancer defined by ERG protein expression. Am. J. Clin. Nutr. 2016, 103, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Chen, B.; Bai, Y.; Miao, T.; Rui, L.; Zhang, H.; Xia, B.; Li, Y.; Gao, S.; Wang, X.-D. Lycopene in protection against obesity and diabetes: A mechanistic review. Pharmacol. Res. 2020, 159, 104966. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, O.; Topsakal, S.; Haligur, M.; Aydogan, A.; Dincoglu, D. Effects of caffeine and lycopene in experimentally induced diabetes mellitus. Pancreas 2016, 45, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.-C.; Peng, L.-S.; Zou, L.; Huang, S.-F.; Xie, Y.; Mu, G.-P.; Zeng, X.-H.; Zhou, X.-L.; Zeng, Y.-C. Protective effect and mechanism of lycopene on endothelial progenitor cells (EPCs) from type 2 diabetes mellitus rats. Biomed. Pharmacother. 2017, 92, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Sultana, M. Lycopene and Cardiovascular Diseases: A Review of the Literature. Int. J. Res. Rev. 2017, 4, 73–86. [Google Scholar]
- Sen, S. The chemistry and biology of lycopene: Antioxidant for human health. Int. J. Adv. Life Sci. Res. 2019, 2, 8–14. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61, 1601009. [Google Scholar] [CrossRef]
- Lin, J.; Li, H.-X.; Xia, J.; Li, X.-N.; Jiang, X.-Q.; Zhu, S.-Y.; Ge, J.; Li, J.-L. The chemopreventive potential of lycopene against atrazine-induced cardiotoxicity: Modulation of ionic homeostasis. Sci. Rep. 2016, 6, 24855. [Google Scholar] [CrossRef]
- Tong, C.; Peng, C.; Wang, L.; Zhang, L.; Yang, X.; Xu, P.; Li, J.; Delplancke, T.; Zhang, H.; Qi, H. Intravenous administration of lycopene, a tomato extract, protects against myocardial ischemia-reperfusion injury. Nutrients 2016, 8, 138. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, P.; Shu, W.; Jia, D. The protective effect of lycopene on hypoxia/reoxygenation-induced endoplasmic reticulum stress in H9C2 cardiomyocytes. Eur. J. Pharmacol. 2016, 774, 71–79. [Google Scholar] [CrossRef]
- Müller, L.; Caris-Veyrat, C.; Lowe, G.; Böhm, V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—A critical review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Hu, H.; Chen, B.; Yue, R.; Zhou, Z.; Liu, Y.; Zhang, S.; Xu, L.; Wang, H.; Yu, Z. Lycopene protects against hypoxia/reoxygenation injury by alleviating ER stress induced apoptosis in neonatal mouse cardiomyocytes. PLoS ONE 2015, 10, e0136443. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhou, W.; Xiong, C.; Tan, G.; Chen, M. Lycopene attenuates inflammation and apoptosis in post-myocardial infarction remodeling by inhibiting the nuclear factor-κB signaling pathway. Mol. Med. Rep. 2015, 11, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Gajendragadkar, P.R.; Hubsch, A.; Mäki-Petäjä, K.M.; Serg, M.; Wilkinson, I.B.; Cheriyan, J. Effects of oral lycopene supplementation on vascular function in patients with cardiovascular disease and healthy volunteers: A randomised controlled trial. PLoS ONE 2014, 9, e99070. [Google Scholar] [CrossRef] [PubMed]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in human health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Tian, Y.; Xiao, Y.; Wang, B.; Sun, C.; Tang, K.; Sun, F. Vitamin E and lycopene reduce coal burning fluorosis-induced spermatogenic cell apoptosis via oxidative stress-mediated JNK and ERK signaling pathways. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef]
- Alvi, S.S.; Ansari, I.A.; Ahmad, M.K.; Iqbal, J.; Khan, M.S. Lycopene amends LPS induced oxidative stress and hypertriglyceridemia via modulating PCSK-9 expression and Apo-CIII mediated lipoprotein lipase activity. Biomed. Pharmacother. 2017, 96, 1082–1093. [Google Scholar] [CrossRef]
- Baykalir, B.G.; Aksit, D.; Dogru, M.S.; Yay, A.H.; Aksit, H.; Seyrek, K.; Atessahin, A. Lycopene ameliorates experimental colitis in rats via reducing apoptosis and oxidative stress. Int. J. Vitam Nutr. Res 2016, 86, 27–35. [Google Scholar] [CrossRef]
- Yu, L.; Wang, W.; Pang, W.; Xiao, Z.; Jiang, Y.; Hong, Y. Dietary lycopene supplementation improves cognitive performances in tau transgenic mice expressing P301L mutation via inhibiting oxidative stress and tau hyperphosphorylation. J. Alzheimer’s Dis. 2017, 57, 475–482. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; da Silva, T.P.; de Araujo, G.R.; Araujo, C.M.; da Silva, R.C.; Lima, W.G.; Bezerra, F.S.; Costa, D.C. Lycopene inhibits reactive oxygen species production in SK-Hep-1 cells and attenuates acetaminophen-induced liver injury in C57BL/6 mice. Chem. -Biol. Interact. 2017, 263, 7–17. [Google Scholar] [CrossRef]
- Lim, S.; Hwang, S.; Yu, J.H.; Lim, J.W.; Kim, H. Lycopene inhibits regulator of calcineurin 1-mediated apoptosis by reducing oxidative stress and down-regulating Nucling in neuronal cells. Mol. Nutr. Food Res. 2017, 61, 1600530. [Google Scholar] [CrossRef]
- Bayomy, N.A.; Elbakary, R.H.; Ibrahim, M.A.; Abdelaziz, E.Z. Effect of lycopene and rosmarinic acid on gentamicin induced renal cortical oxidative stress, apoptosis, and autophagy in adult male albino rat. Anat. Rec. 2017, 300, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Tvrdá, E.; Kováčik, A.; Tušimová, E.; Paál, D.; Mackovich, A.; Alimov, J.; Lukáč, N. Antioxidant efficiency of lycopene on oxidative stress-induced damage in bovine spermatozoa. J. Anim. Sci. Biotechnol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Jiang, Z.; Liao, Y.; Song, Z.; Nan, X. Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem. Res. 2016, 41, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Campos, K.K.D.; Araújo, G.R.; Martins, T.L.; Bandeira, A.C.B.; de Paula Costa, G.; Talvani, A.; Garcia, C.C.M.; Oliveira, L.A.M.; Costa, D.C.; Bezerra, F.S. The antioxidant and anti-inflammatory properties of lycopene in mice lungs exposed to cigarette smoke. J. Nutr. Biochem. 2017, 48, 9–20. [Google Scholar] [CrossRef]
- Göncü, T.; Oğuz, E.; Sezen, H.; Koçarslan, S.; Oğuz, H.; Akal, A.; Adıbelli, F.M.; Çakmak, S.; Aksoy, N. Anti-inflammatory effect of lycopene on endotoxin-induced uveitis in rats. Arq. Bras. Oftalmol. 2016, 79, 357–362. [Google Scholar] [CrossRef]
- Sachdeva, A.K.; Chopra, K. Lycopene abrogates Aβ(1-42)-mediated neuroinflammatory cascade in an experimental model of Alzheimer’s disease. J. Nutr. Biochem. 2015, 26, 736–744. [Google Scholar] [CrossRef]
- Colmán-Martínez, M.; Martínez-Huélamo, M.; Valderas-Martínez, P.; Arranz-Martínez, S.; Almanza-Aguilera, E.; Corella, D.; Estruch, R.; Lamuela-Raventós, R.M. trans-Lycopene from tomato juice attenuates inflammatory biomarkers in human plasma samples: An intervention trial. Mol. Nutr. Food Res. 2017, 61, 1600993. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, S.B. Sarcandra glabra combined with lycopene protect rats from lipopolysaccharide induced acute lung injury via reducing inflammatory response. Biomed. Pharmacother. 2016, 84, 34–41. [Google Scholar] [CrossRef]
- Li, Y.F.; Chang, Y.Y.; Huang, H.C.; Wu, Y.C.; Yang, M.D.; Chao, P.M. Tomato juice supplementation in young women reduces inflammatory adipokine levels independently of body fat reduction. Nutrition 2015, 31, 691–696. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, Y.; Zhou, X.; Pan, W.; Shi, Y.; Wang, M.; Zhang, X.; Qi, D.; Li, L.; Ma, K.; et al. Depression-like behaviors and heme oxygenase-1 are regulated by lycopene in lipopolysaccharide-induced neuroinflammation. J. Neuroimmunol. 2016, 298, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Makon-Sébastien, N.; Francis, F.; Eric, S.; Henri, V.P.; François, L.J.; Laurent, P.; Yves, B.; Serge, C. Lycopene modulates THP1 and Caco2 cells inflammatory state through transcriptional and nontranscriptional processes. Mediat. Inflamm. 2014, 2014, 507272. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, S.A.; Shaik Ibrahim, S.; Devaki, T.; Chakraborty, S.; Agarwal, S.; Pérez-Sánchez, H. Lycopene Prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in albino rats. J. Proteome Res. 2017, 16, 3190–3199. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Xu, Z.; Liu, W.; Yang, H.; Xu, B.; Wei, Y. Effects of lycopene and proanthocyanidins on hepatotoxicity induced by mercuric chloride in rats. Biol. Trace Elem. Res. 2012, 146, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Anusha, M.; Venkateswarlu, M.; Prabhakaran, V.; Taj, S.S.; Kumari, B.P.; Ranganayakulu, D. Hepatoprotective activity of aqueous extract of Portulaca oleracea in combination with lycopene in rats. Indian J. Pharmacol. 2011, 43, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Yucel, Y.; Oguz, E.; Kocarslan, S.; Tatli, F.; Gozeneli, O.; Seker, A.; Sezen, H.; Buyukaslan, H.; Aktumen, A.; Ozgonul, A.; et al. The effects of lycopene on methotrexate-induced liver injury in rats. Bratisl. Lek. Listy 2017, 118, 212–216. [Google Scholar] [CrossRef]
- Bandeira, A.C.B.; da Silva, R.C.; Rossoni, J.V.J.; Figueiredo, V.P.; Talvani, A.; Cangussú, S.D.; Bezerra, F.S.; Costa, D.C. Lycopene pretreatment improves hepatotoxicity induced by acetaminophen in C57BL/6 mice. Bioorg. Med. Chem. 2017, 25, 1057–1065. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Bayramoglu, A.; Altuner, Y.; Uyanoglu, M.; Colak, S. The effects of lycopene on hepatic ischemia/reperfusion injury in rats. Cytotechnology 2015, 67, 487–491. [Google Scholar] [CrossRef]
- Xia, J.; Lin, J.; Zhu, S.Y.; Du, Z.H.; Guo, J.A.; Han, Z.X.; Li, J.L.; Zhang, Y. Lycopene protects against atrazine-induced hepatotoxicity through modifications of cytochrome P450 enzyme system in microsomes. Exp. Toxicol. Pathol. 2016, 68, 223–231. [Google Scholar] [CrossRef]
- Sheik Abdulazeez, S.; Thiruvengadam, D. Effect of lycopene on oxidative stress induced during D-galactosamine/lipopolysaccharide-sensitized liver injury in rats. Pharm. Biol. 2013, 51, 1592–1599. [Google Scholar] [CrossRef]
- Sheriff, S.A.; Devaki, T. Lycopene stabilizes lipoprotein levels during D-galactosamine/lipopolysaccharide induced hepatitis in experimental rats. Asian Pac. J. Trop. Biomed. 2012, 2, 975–980. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, J.; Wang, J.; Li, Y.; Xiao, L.; Duan, D.; Wang, Q. Protective effect of lycopene on high-fat diet-induced cognitive impairment in rats. Neurosci. Lett. 2016, 627, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Lei, L.; Zhang, Z.; Cheng, Y. Neuroprotective effects of lycopene pretreatment on transient global cerebral ischemia-reperfusion in rats: The role of the Nrf2/HO-1 signaling pathway. Mol. Med. Rep. 2016, 13, 412–418. [Google Scholar] [CrossRef]
- Chen, W.; Mao, L.; Xing, H.; Xu, L.; Fu, X.; Huang, L.; Huang, D.; Pu, Z.; Li, Q. Lycopene attenuates Aβ1-42 secretion and its toxicity in human cell and Caenorhabditis elegans models of Alzheimer disease. Neurosci. Lett. 2015, 608, 28–33. [Google Scholar] [CrossRef]
- Prema, A.; Janakiraman, U.; Manivasagam, T.; Thenmozhi, A.J. Neuroprotective effect of lycopene against MPTP induced experimental Parkinson’s disease in mice. Neurosci. Lett. 2015, 599, 12–19. [Google Scholar] [CrossRef]
- Prakash, A.; Kumar, A. Implicating the role of lycopene in restoration of mitochondrial enzymes and BDNF levels in β-amyloid induced Alzheimer’s disease. Eur. J. Pharm. 2014, 741, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.B.; Wang, R.; Pan, H.B.; Ding, Q.F.; Lu, F.B. Effect of lycopene on oxidative stress and behavioral deficits in rotenone induced model of Parkinson’s disease. Zhongguo Ying Yong Sheng Li Xue Za Zhi = Zhongguo Yingyong Shenglixue Zazhi = Chin. J. Appl. Physiol. 2013, 29, 380–384. [Google Scholar]
- Qu, M.; Nan, X.; Gao, Z.; Guo, B.; Liu, B.; Chen, Z. Protective effects of lycopene against methylmercury-induced neurotoxicity in cultured rat cerebellar granule neurons. Brain Res. 2013, 1540, 92–102. [Google Scholar] [CrossRef]
- Kaur, H.; Chauhan, S.; Sandhir, R. Protective effect of lycopene on oxidative stress and cognitive decline in rotenone induced model of Parkinson’s disease. Neurochem. Res. 2011, 36, 1435–1443. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.T.; Welch, A.A. Carotenoid dietary intakes and plasma concentrations are associated with heel bone ultrasound attenuation and osteoporotic fracture risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef]
- Sahni, S.; Hannan, M.T.; Blumberg, J.; Cupples, L.A.; Kiel, D.P.; Tucker, K.L. Protective effect of total carotenoid and lycopene intake on the risk of hip fracture: A 17-year follow-up from the Framingham Osteoporosis Study. J. Bone Miner. Res. 2009, 24, 1086–1096. [Google Scholar] [CrossRef]
- Mackinnon, E.S.; Rao, A.V.; Rao, L.G. Dietary restriction of lycopene for a period of one month resulted in significantly increased biomarkers of oxidative stress and bone resorption in postmenopausal women. J. Nutr. Health Aging 2011, 15, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Ateşşahin, A.; Türk, G.; Karahan, I.; Yilmaz, S.; Ceribaşi, A.O.; Bulmuş, O. Lycopene prevents adriamycin-induced testicular toxicity in rats. Fertil. Steril. 2006, 85 (Suppl. 1), 1216–1222. [Google Scholar] [CrossRef]
- Ateşşahin, A.; Karahan, I.; Türk, G.; Gür, S.; Yilmaz, S.; Ceribaşi, A.O. Protective role of lycopene on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Reprod. Toxicol. 2006, 21, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Ghosh, A.; Dey, A.; Pakhira, B.P.; Ghosh, D. Attenuation of the cyproterone acetate-induced testicular hypofunction by a novel nutraceutical lycopene: A genomic approach. Andrologia 2017, 49, e12709. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Pedraza-Chaverrí, J.; Torres-Ramos, M.; Jiménez-Farfán, D.; Cruz Salgado, A.; Serrano-García, N.; Osorio-Rico, L.; Sotelo, J. Multiple molecular and cellular mechanisms of action of lycopene in cancer inhibition. Evid. -Based Complement. Altern. Med. eCAM 2013, 2013, 705152. [Google Scholar] [CrossRef]
- Giovannucci, E.; Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C. Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 1995, 87, 1767–1776. [Google Scholar] [CrossRef]
- Corridan, B.; O’Donohue, M.; Morrissey, P. Carotenoids and immune response in elderly people. In Proceedings of Proceedings-Nutrition Society of London; Cambridge University Press: Cambridge, UK, 1998; p. 4A. [Google Scholar]
- Khiveh, A.; Hashempur, M.H.; Shakiba, M.; Lotfi, M.H.; Shakeri, A.; Kazemeini, S.; Mousavi, Z.; Jabbari, M.; Kamalinejad, M.; Emtiazy, M. Effects of rhubarb (Rheum ribes L.) syrup on dysenteric diarrhea in children: A randomized, double-blind, placebo-controlled trial. J. Integr. Med. 2017, 15, 365–372. [Google Scholar] [CrossRef]
- Shakeri, A.; Hashempur, M.H.; Mojibian, M.; Aliasl, F.; Bioos, S.; Nejatbakhsh, F. A comparative study of ranitidine and quince (Cydonia oblonga Mill) sauce on gastroesophageal reflux disease (GERD) in pregnancy: A randomised, open-label, active-controlled clinical trial. J. Obstet. Gynaecol. 2018, 38, 899–905. [Google Scholar] [CrossRef]
- Qu, M.; Zhou, Z.; Chen, C.; Li, M.; Pei, L.; Chu, F.; Yang, J.; Wang, Y.; Li, L.; Liu, C.; et al. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem. Int. 2011, 59, 1095–1103. [Google Scholar] [CrossRef]
- Wang, X.-D. Carotenoid Oxidative/Degradative Products and Their Biological Activities; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Jonker, D.; Kuper, C.F.; Fraile, N.; Estrella, A.; Rodríguez Otero, C. Ninety-day oral toxicity study of lycopene from Blakeslea trispora in rats. Regul. Toxicol. Pharmacol. RTP 2003, 37, 396–406. [Google Scholar] [CrossRef]
- Trumbo, P.R. Are there adverse effects of lycopene exposure? J. Nutr. 2005, 135, 2060s–2061s. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Beecher, G.; Burk, R.; Chan, A.; Erdman, j.J.; Jacob, R.; Jialal, I.; Kolonel, L.; Marshall, J.; Taylor Mayne, P.R. Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids. Inst. Med. 2000. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary reference intakes: Vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J. Acad. Nutr. Diet. 2001, 101, 294. [Google Scholar]
- Veeramachaneni, S.; Ausman, L.M.; Choi, S.W.; Russell, R.M.; Wang, X.D. High dose lycopene supplementation increases hepatic cytochrome P4502E1 protein and inflammation in alcohol-fed rats. J. Nutr. 2008, 138, 1329–1335. [Google Scholar] [CrossRef]
- Cassileth, B. Lycopene. Oncology 2010, 24, 296. [Google Scholar]
- Reich, P.; Shwachman, H.; Craig, J.M. Lycopenemia: A variant of carotenemia. N. Engl. J. Med. 1960, 262, 263–269. [Google Scholar] [CrossRef]
- Michael McClain, R.; Bausch, J. Summary of safety studies conducted with synthetic lycopene. Regul. Toxicol. Pharmacol. RTP 2003, 37, 274–285. [Google Scholar] [CrossRef]
| Food Sources | Contents (mg/100 g) |
|---|---|
| Fresh tomatoes | 0.72–4.2 |
| Cooked tomatoes | 3.70 |
| Tomato sauce | 6.20 |
| Tomato paste | 5.40–150 |
| Ketchup | 9.90–13.44 |
| Pumpkin | 0.38–0.46 |
| Sweet potato | 0.02–0.11 |
| Pink grapefruit | 0.35–3.36 |
| Carrot | 0.65–0.78 |
| Pink guava | 5.23–5.5 |
| Watermelon | 2.30–7.20 |
| Apricot | 0.01–0.05 |
| Papaya | 0.11–5.3 |
| Rosehip | 0.68–0.71 |
| Biological Effect | Mechanisms of Action | References |
|---|---|---|
| Anticancer | Reduced nuclear factor-kappa B (NF-κB) expression, serum level of oxidative stress marker malondialdehyde (MDA) Improved nuclear factor erythroid 2 expressions Reduced the expression of signal transducer and activator of transcription 3 (STAT3) Induced the protein inhibitor of activated STAT3 expression | [44,45] |
| Induced cell cycle arrest and modified the potential of mitochondrial membrane, DNA fragmentation | [46,47] | |
| Treatment of HGC-27 cells with lycopene exhibited significant improvement in LC3-I, Phosphorylated Extracellular Signal-Regulated Kinase (p-ERK) proteins expressions | [48] | |
| Reduced NADPH oxidase (NOX) 4 activity Lowered invasion, migration, and adhesion, NOX activity, matrix metalloproteinase (MMP)-9, MMP-2 activities and NOX4 protein expression | [49] | |
| Reduced metastatic load and inhibited cancer antigen 125 (CA125) expression Down-regulated expression of Integrin beta-1 (ITGB1), MMP9, Integrin alpha-5 (ITGA5), Focal Adhesion Kinase (FAK), integrin-linked kinase (ILK) and EMT markers Decreased activity of mitogen-activated protein kinase (MAPK) and inhibited integrin α5 protein expression | [50] | |
| Antidiabetic | Lowered MDA levels, and increased antioxidant enzyme activities | [51] |
| Decreased the glycated haemoglobin (HbA1c) and C-reactive protein (CRP) | [52,53] | |
| Enhanced antioxidant enzymes activities (i.e., superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), glutathione-S-transferase) | [54] | |
| Lowered the expression of BCL2-associated X protein (BAX), but improved B-cell lymphoma-extra large (Bcl-xL) levels and B-cell lymphoma 2 (Bcl-2) | [55] | |
| Downregulated RAGE expression and declined NF-κB and MMP-2 expressions | [56] | |
| Reduced levels of serum nitrate-nitrite level | [57] | |
| Cardioprotective | Decreased low-density lipoprotein-cholesterol (LDL), total cholesterol (TC), and thiobarbituric acid-reacting substances | [58] |
| Lowered very-low-density lipoprotein-cholesterol (VLDL), triglycerides (TG) and increased high-density lipoprotein-cholesterol (HDL) level | [59] | |
| Decreased inflammatory factors (CRP, interleukin (IL)-6), pulse wave velocity, adhesion molecules and endothelial function | [60] | |
| Lowered the expression of Rho-associated protein kinase (ROCK)1, Ki-67, intercellular adhesion molecule-1 (ICAM-1) and ROCK2 Improved the expression of endothelial nitric oxide synthase (eNOS) implanted arteries and cGMP plasma concentration | [61] | |
| Antioxidant | Increased CAT, glutathione (GSH) and SOD activities and mRNAs antioxidant enzyme Reduced age-related neuroinflammatory disorders by decreasing microgliosis (Ionized calcium-binding adaptor protein-1 (IBA-1)) Down regulate the accumulation of amyloid beta (Aβ) 1-42 in the old CD-1 mice’s brain. | [62] |
| Decreased Aβ accumulation, amyloid precursor protein (APP) Reduced the expression of neuronal β-secretase (BACE)1 Improved the expressions of α-secretase A Disintegrin And Metalloproteinase (ADAM)10 Suppressed IBA-1 (a marker of microglial activation) expression, inflammatory mediators Inhibited NF-κB, phosphorylation of MAPKs and Nuclear factor erythroid 2-related factor 2 (Nrf2) activity | [63] | |
| Decreased myeloperoxidase (MPO) activity, tumor necrosis factor-alpha (TNF-α) Down-regulated gene expression of inducible nitric oxide synthase (iNOS) | [64] | |
| Decreased inflammatory cytokines Improved antioxidant enzymes NAD(P)H: quinone oxidoreductase (NQO1) and heme oxygenase-1 (HO-1) mRNA expressions Downregulated inflammatory cytokines TNF-α and IL-1β Improved the glial cells inflammatory markers glial fibrillary acidic protein (GFAP) and IBA-1 expression | [65] | |
| Antiinflammatory | Up-regulated the HO-1 mRNA Gene expression suppression of the TNF-α and lipopolysaccharide (LPS)-stimulated cyclooxygenase-2 (COX-2), nitric oxide synthase 2 (NOS2) | [66] |
| Lowered the IL-1β, TNF-α, IL-6β serum level and increased the NF-κB p65 mRNA, Toll-like receptor 4 (TLR4) and protein expressions | [67] | |
| Inhibited COX-2, iNOS and NF-κB Reduced the leukocytes migration | [68] | |
| Hepatoprotective | Lowered aspartate aminotransferase, alanine aminotransferase, free fatty acid, MDA and LDL Increased the SOD, GSH condensation Down-regulated the expression of Cytochrome P450 2E1 (CYP2E1), TNF-α | [69] |
| Enhanced the S-adenosyl-homocysteine hydrolase, hepatic cystathionine beta-synthase activities | [70] | |
| Reduced nitric oxide (NO) and MDA levels | [71] | |
| Against dermatologic diseases | Decreased Ultraviolet (UV) B-induced cell growth and increased apoptosis Prevented from Forkhead box O3 (FOXO3) phosphorylation when exposing to UVB radiation and cytoplasm sequestering Decreased cyclin-dependent kinase 4 (CDK4) and CDK2 Increased in the expression of Poly (ADP-ribose) polymerase (PARP), BAX and cell apoptosis Inhibited UVA1- and UVA/B-induced upregulation of HO-1 | [72] |
| Neuroprotective | Suppressing 4 aminopyridines (4-AP) evoked glutamate release and elevated intrasynaptosomal Ca2+ level Suppressing release of 4-AP-evoked glutamate | [73] |
| Improved mitochondrial enzymatic activities, kindling score, oxidative stress | [74] | |
| Decreased impairment in biochemical, behavioral, neuroinflammatory and neurochemical markers | [75] | |
| Boneprotective | Osteoblasts differentiation improved | [76] |
| Modified the biomarkers of serum osteocalcin, bone metabolism, crosslinked carboxyterminal telopeptides and N-terminal propeptide of type 1 collagen in serum Downregulated the osteoclast differentiation concurrent Up-regulated the osteoblasts alongside GPx, SOD, and CAT activities | [77] | |
| Targeting reproductive disorders | Reduced lipid peroxidation and sperm DNA fragmentation | [78] |
| Improved the sperm motility and sperm count Improved mitochondrial enzymatic activity, i.e., CAT, SOD, glucocorticoid receptor (GR), GPx and alcohol dehydrogenase (ADH)) and non-enzymatic antioxidant level (GSH and ascorbate) | [79] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.; Ghorat, F.; Ul-Haq, I.; Ur-Rehman, H.; Aslam, F.; Heydari, M.; Shariati, M.A.; Okuskhanova, E.; Yessimbekov, Z.; Thiruvengadam, M.; et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants 2020, 9, 706. https://doi.org/10.3390/antiox9080706
Imran M, Ghorat F, Ul-Haq I, Ur-Rehman H, Aslam F, Heydari M, Shariati MA, Okuskhanova E, Yessimbekov Z, Thiruvengadam M, et al. Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants. 2020; 9(8):706. https://doi.org/10.3390/antiox9080706
Chicago/Turabian StyleImran, Muhammad, Fereshteh Ghorat, Iahtisham Ul-Haq, Habib Ur-Rehman, Farhan Aslam, Mojtaba Heydari, Mohammad Ali Shariati, Eleonora Okuskhanova, Zhanibek Yessimbekov, Muthu Thiruvengadam, and et al. 2020. "Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders" Antioxidants 9, no. 8: 706. https://doi.org/10.3390/antiox9080706
APA StyleImran, M., Ghorat, F., Ul-Haq, I., Ur-Rehman, H., Aslam, F., Heydari, M., Shariati, M. A., Okuskhanova, E., Yessimbekov, Z., Thiruvengadam, M., Hashempur, M. H., & Rebezov, M. (2020). Lycopene as a Natural Antioxidant Used to Prevent Human Health Disorders. Antioxidants, 9(8), 706. https://doi.org/10.3390/antiox9080706







