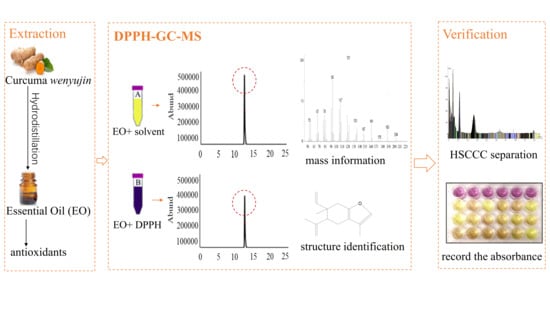
An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
2.3. DPPH Radical Scavenging Assay
2.4. DPPH-GC Offline Experiment and Structure Identification by GC-MS
2.5. HSCCC Separation
2.5.1. Selection of Biphasic Solvent System
2.5.2. Preparation of Solvent System and Sample Solution
2.5.3. HSCCC Separation Procedure
2.6. Statistical Analysis
3. Results and Discussion
3.1. DPPH Radical Scavenging Activity
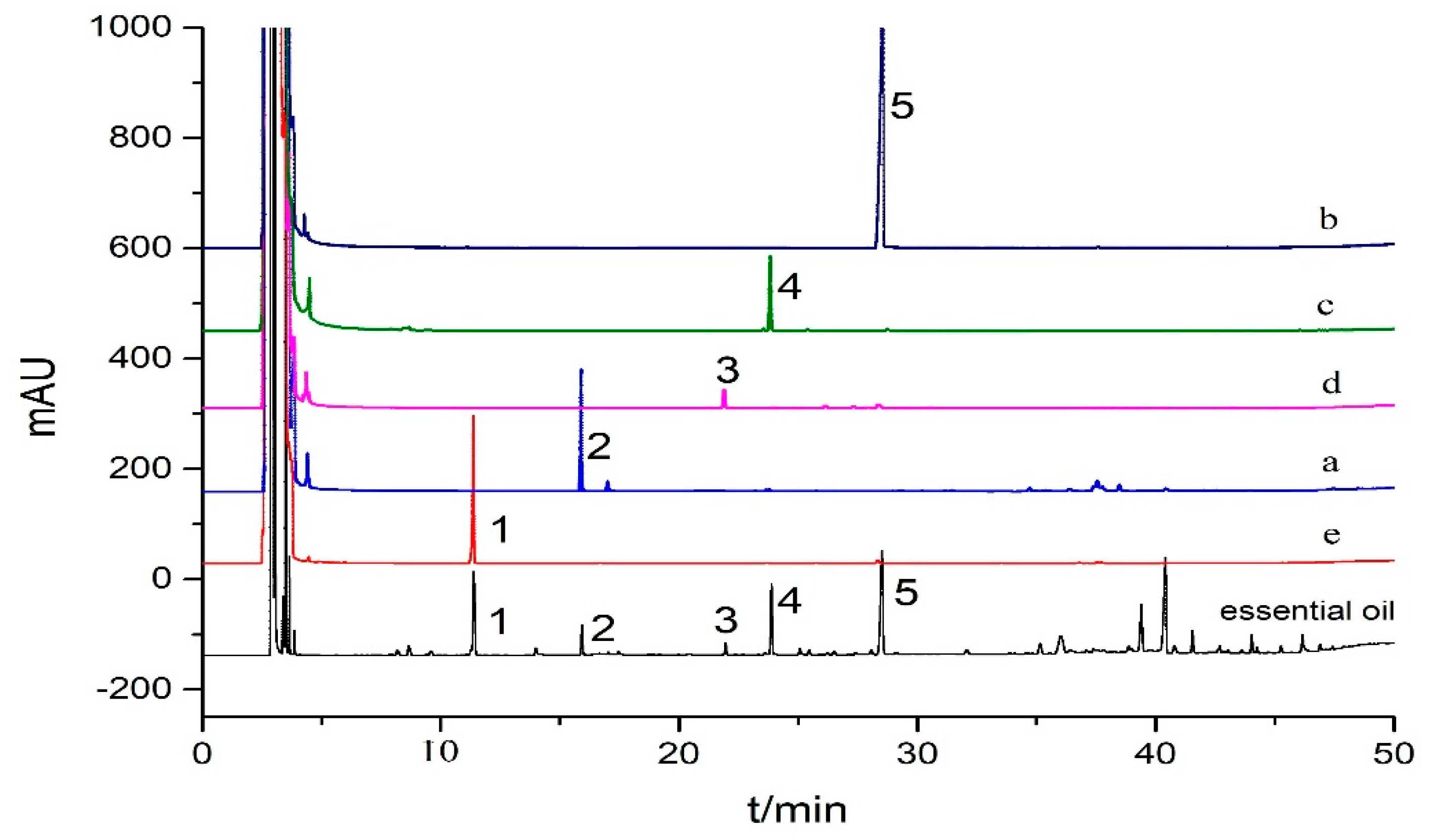
3.2. Screening of Antioxidants in Curcuma wenyujin Y.H. Chen et C. Ling Essential Oil by the DPPH-GC Offline Method
3.3. Determination of Off-Line Experimental Conditions
3.4. Identification of the Antioxidants in Curcuma wenyujin Y.H. Chen et C. Ling Essential Oil by GC-MS
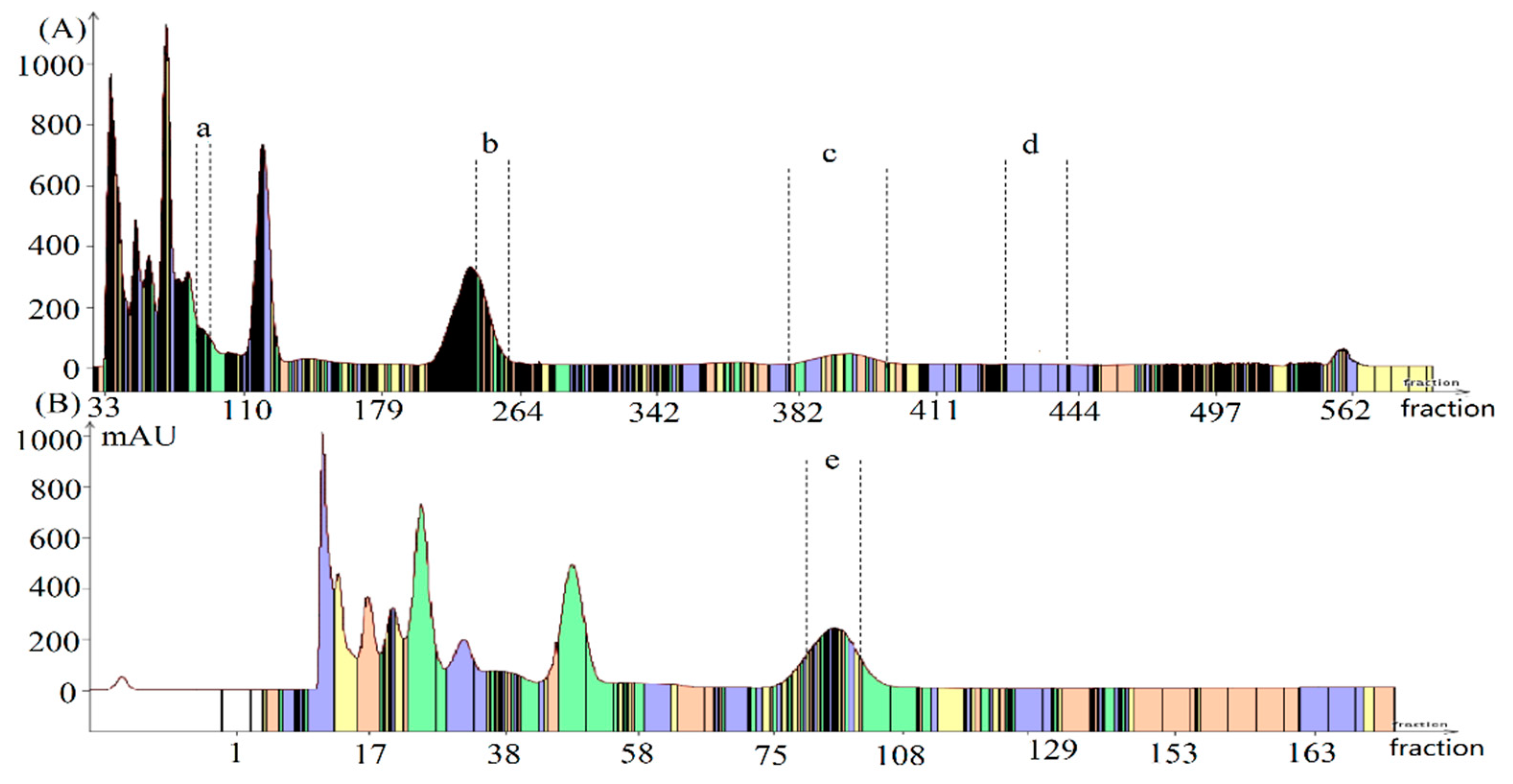
3.5. HSCCC Separation
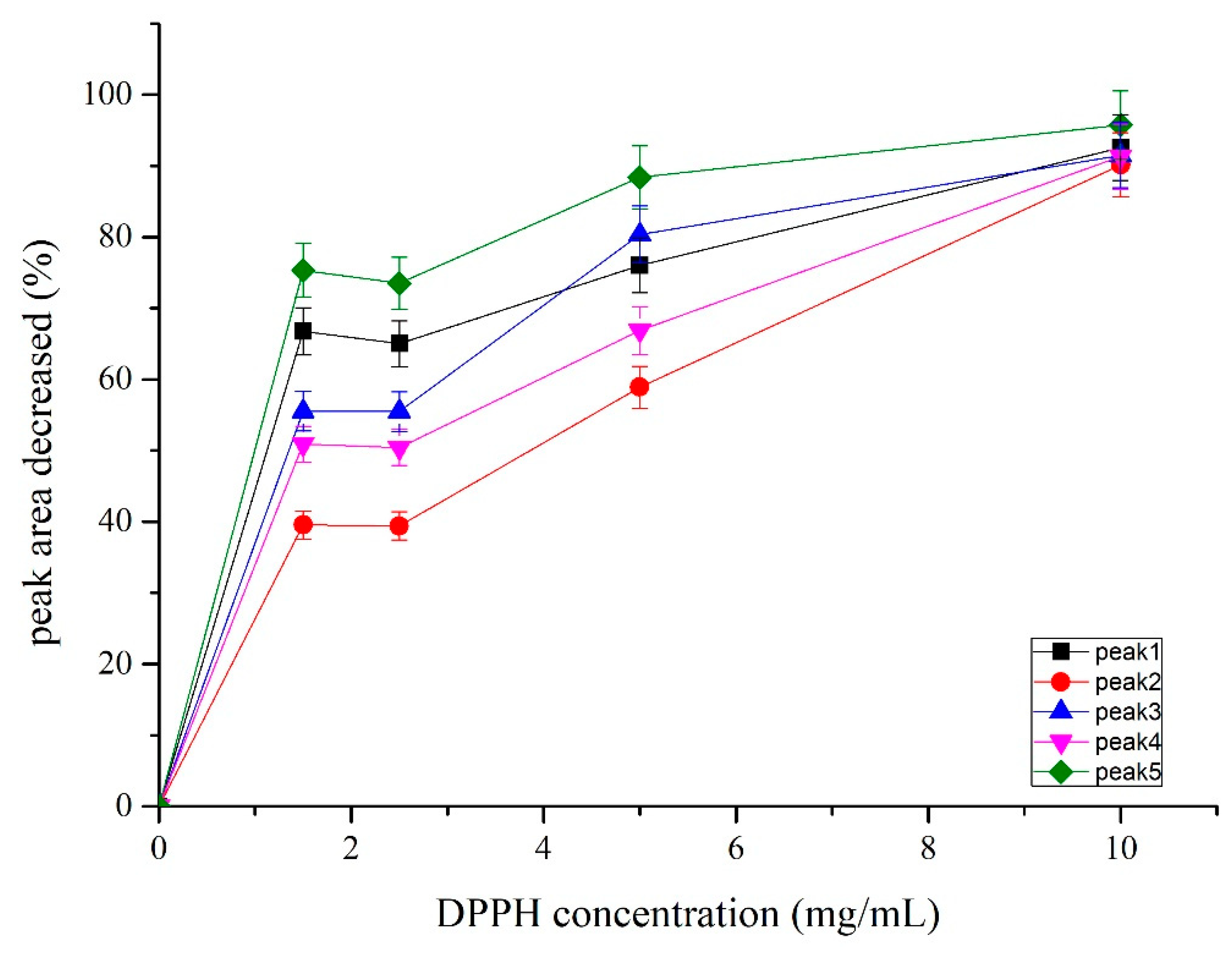
3.6. Antioxidant Activities of the Separated Compounds and Their Potential Synergistic Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valgimigli, L. Essential oils: An overview on origins, chemistry, properties and uses. In Essential Oils as Natural Food Additives; Valgimigli, L., Ed.; Nova Science: New York, NY, USA, 2012; pp. 1–24. [Google Scholar]
- Theanphong, O. Chemical constituents and antioxidant activities of essential oils from roots and rhizomes of Curcuma alismatifolia Gagnap from Thailand. J. Appl. Sci. 2017, 16, 105–111. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, L.; Yang, Z.; Chen, F.; Zheng, X.; Liu, X. Chemical compositions, antioxidative, antimicrobial, anti-inflammatory and antitumor activities of Curcuma aromatica Salisb. essential oils. Ind. Crops Prod. 2017, 108, 6–16. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Yang, Z.W.; Huang, Z.B.; Zhao, M.C.; Li, P.H.; Zhou, W.; Zhang, K.; Zheng, X.; Lin, L.; Tang, J.; et al. Variation in essential oil and bioactive compounds of Curcuma kwangsiensis collected from natural habitats. Chem. Biodivers. 2017, 14, e1700020. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C. Oxidative stability and antioxidant properties of essential oils. In Essential Oils as Natural Food Additives; Valgimigli, L., Ed.; Nova Science: New York, NY, USA, 2012; pp. 75–95. [Google Scholar]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Antonio, A.L.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. A comparative study between natural and synthetic antioxidants: Evaluation of their performance after incorporation into biscuits. Food Chem. 2017, 216, 342–346. [Google Scholar] [CrossRef]
- Simic, M.G.; Karel, M. Natural antioxidants of soybean and other oil-seeds In Autoxidation in Food and Biological Systems; Plenum Press: New York, NY, USA, 1980; pp. 283–292. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food. Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E.A. Review of methods to determine chain breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant activity of essential oils of five spice plants widely used in a Mediterranean diet. Flavour Fragr. J. 2010, 25, 13–19. [Google Scholar] [CrossRef]
- Torres-Martínez, R.; García-Rodríguez, Y.M.; Garciglia, R.S. Antioxidant activity of the essential oil and its major terpenes of satureja macrostema (moc. and sessé ex benth.) briq. Pharmacogn. Mag. 2017, 13, S875–S880. [Google Scholar]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- EI Euch, S.K.; Ciesla, L.; Bouzouita, N. Free radical scavenging fingerprints of selected aromatic and medicinal tunisian plants assessed by means of TLC-DPPH test and image processing. J. AOAC Int. 2014, 97, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Koleva, I.I.; Niederlander, H.A.G.; Beek, T.A. An on-line HPLC method for detection of radical scavenging compounds in complex mixtures. Anal. Chem. 2000, 72, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Y.; Chen, J.L.; Zhou, Q.F.; Lei, H.L.; Luan, L.J.; Liu, X.S.; Wu, Y.J. Indirect identification of antioxidants in Polygalae Radix through their reaction with 2,2-diphenyl-1-picrylhydrazyl and subsequent HPLC–ESI-Q-TOF-MS/MS. Talanta 2015, 144, 830–835. [Google Scholar] [CrossRef]
- Geng, D.D.; Chi, X.F.; Dong, Q.; Hu, F.Z. Antioxidants screening in Limonium aureum by optimized on-line HPLC–DPPH assay. Ind. Crop Prod. 2015, 67, 492–497. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Chen, L.L.; Zhu, Q.J.; Wang, D.J.; Wang, W.L.; Sun., X.; Liu, X.Y.; Du., F.l. Screening natural antioxidants in peanut shell using DPPH–HPLC–DAD–TOF/MS methods. Food Chem 2012, 135, 2366–2371. [Google Scholar] [CrossRef]
- Sikha, A.; Harini, A.; Prakash, H. Pharmacological activities of wild turmeric (Curcuma aromatica Salisb): A review. J. Pharmacogn. Phytochem. 2015, 3, 1–4. [Google Scholar]
- Angel, G.R.; Menon, N.; Vimala, B.; Nambisan, B. Essential oil composition of eight starchy Curcuma species. Ind. Crops Prod. 2014, 60, 233–238. [Google Scholar] [CrossRef]
- Mau, J.; Lai, E.Y.C.; Wang, N.P.; Chen, C.C.; Chang, C.H.; Chyau, C.C. Composition and antioxidant activity of the essential oil from Curcuma zedoaria. Food Chem. 2003, 82, 583–591. [Google Scholar] [CrossRef]
- Wilson, B.; Abraham, G.; Manju, V.S.; Mathew, M.; Vimala, B.; Sundaresan, S.; Nambisan, B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005, 99, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maielti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparativeevaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Nie, J.Y.; Li, R.; Jiang, Z.T.; Wang, Y.; Tan, J.; Tang, S.H.; Zhang, Y. Antioxidant activity screening and chemical constituents of the essential oil from rosemary by ultra-fast GC electronic nose coupled with chemical methodology. J. Sci. Food Agric. 2020, 100, 3481–3487. [Google Scholar] [CrossRef] [PubMed]
- Dosoky, N.S.; Setzer, W.N. Chemical composition and biological activities of essential oils of Curcuma species. Nutrients 2018, 10, 1196. [Google Scholar] [CrossRef]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and comprehensive antioxidant activity of ginger (zingiber officinale) essential oil from Ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [CrossRef]
- Ito, Y.; Bowman, R.L. Countercurrent chromatography: Liquid-liquid partition chromatography without solid support. Science 1970, 3916, 281–283. [Google Scholar] [CrossRef]
- Marques, A.M.; Fingolo, C.E.; Kaplan, M.A.C. HSCCC separation and enantiomeric distribution of key volatile constituents of Piper claussenianum (Miq.) C. DC. (Piperaceae). Food Chem. Toxicol. 2017, 109, 1111–1117. [Google Scholar] [CrossRef]
- Santos, B.C.B.; Silva, J.C.T.; Guerrero, P.G.; Leitão, G.G.; Barata, L.E.S. Isolation of chavibetol from essential oil of Pimenta pseudocaryophyllus leaf by high-speed counter-current chromatography. J. Chromatogr. A 2009, 1216, 4303–4306. [Google Scholar] [CrossRef]
- Hasan, S.M.R.; Hossain, M.M.; Akter, R.; Jamila, M.; Mazumder, M.E.H.; Rahman, S. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. J. Med. Plants Res. 2009, 3, 875–879. [Google Scholar]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; Santos, T.C.; Coube, C.S.; Leitao, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Avanço, G.B.; Ferreira, F.D.; Bomfim, N.S.; dos Santos, P.A.D.R.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu, B.A.; Mikcha, J.M.G.; Machinski, M. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar]
- Kanfer, J.N.; Hakomori, S. Sphingolipid Biochemistry; Plenum Press: New York, NY, USA, 1983; pp. 1–24. [Google Scholar]
- Tütem, E.; Apak, R.; Başkan, K.S. Off-line HPLC integrated to total antioxidant capacity measurement of beverages. Prog. Impact Antioxidants Beve 2014, 265–276. [Google Scholar]
- Seol, G.H.; Kim, K.Y. Eucalyptol and its role in chronic diseases. In Drug Discovery from Mother Nature; Springer: Cham, Germany, 2016; pp. 389–398. [Google Scholar]
- Dang, Y.Y.; Li, X.C.; Zhang, Q.W.; Li, S.P.; Wang, Y.T. Preparative isolation and purification of six volatile compounds from essential oil of Curcuma wenyujin using high-performance centrifugal partition chromatography. J. Sep. Sci. 2010, 33, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.S.; Abadi, K.; Repetto, V.; Vojnov, A.A.; Moreno, S. Synergistic antioxidant and antibacterial activity of rosemary plus butylated derivatives. Food Chem. 2009, 115, 456–461. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
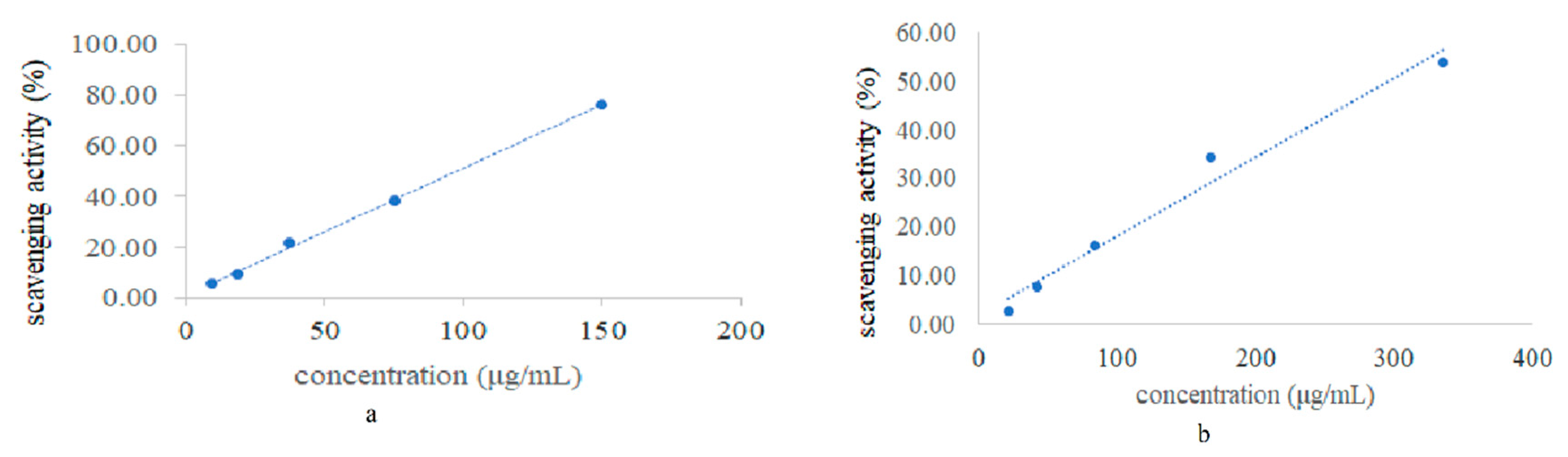

| Sample | IC50 Values (μg/mL) |
|---|---|
| BHT | 97.44 ± 2.63 |
| Essential oil | 292.88 ± 1.69 |
| NO. | Retention Time (min) | Compounds | Formula | Area Ratio (%) |
|---|---|---|---|---|
| 1 | 8.18 | α-Pinene | C10H16 | 0.51 |
| 2 | 8.66 | Camphene | C10H16 | 0.94 |
| 3 | 9.58 | β-Pinene | C10H16 | 0.49 |
| 4 | 11.4 | Eucalyptol (peak 1) | C10H18O | 7.66 |
| 5 | 15.93 | Camphor (peak 2) | C10H16O | 2.34 |
| 6 | 17.04 | Isoborneol | C10H18O | 0.84 |
| 7 | 17.47 | Borneol | C10H18O | 0.22 |
| 8 | 21.97 | δ-Elemene (peak 3) | C15H24 | 1.15 |
| 9 | 23.89 | β-Elemene (peak 4) | C15H24 | 7.1 |
| 10 | 25.08 | Caryophyllene | C15H24 | 0.66 |
| 11 | 25.47 | γ-Elemene | C15H24 | 0.51 |
| 12 | 26.24 | Alloaromadendrene | C15H24 | 0.23 |
| 13 | 26.52 | Humulene | C15H24 | 0.55 |
| 14 | 28.07 | β-Eudesmene | C15H24 | 0.77 |
| 15 | 28.52 | Curzerene (peak 5) | C15H20O | 15.77 |
| 16 | 32.08 | Guaiene | C15H24 | 0.24 |
| 17 | 35.16 | Germacrone | C15H22O | 2.4 |
| 18 | 36.02 | 7a-Isopropenyl-45-dimethyloctahydro-1H-indene-4-carboxylic acid | C15H24O2 | 6.23 |
| 19 | 38.88 | β-Selinenol | C15H26O | 2.85 |
| 20 | 39.41 | Germacron | C15H22O | 9.84 |
| 21 | 40.42 | Curdione | C15H24O2 | 22.94 |
| 22 | 40.79 | Aromadendrene oxide-(2) | C15H24O | 1.67 |
| 23 | 41.56 | Germacr-1(10)-ene-5,8-dione | C15H24O2 | 2.88 |
| 24 | 42.7 | Ledene oxide-(II) | C15H24O | 0.52 |
| 25 | 44.04 | 5,6-azulenedicarboxaldehyde, 1,2,3,3a,8,8a-hexahydro-2,2,8-trimethyl-, (3aα,8α,8aα)-(-)- | C20H26O2 | 1.24 |
| 26 | 44.26 | Norethindrone | C15H20O2 | 0.53 |
| 27 | 45.26 | Nootkaton-11,12-epoxide | C15H22O2 | 2.01 |
| 28 | 46.17 | Deoxysericealactone | C16H20O4 | 1.09 |
| 29 | 47.43 | Dehydrosaussurea lactone | C17H24O4 | 1.07 |
| Total | 95.23 |
| NO. | Solvent System | K-Value | ||||
|---|---|---|---|---|---|---|
| Peak1 | Peak2 | Peak3 | Peak4 | Peak5 | ||
| A | n-Hexane: acetonitrile: ethanol (5:3:2, v/v) | 1.51 | 0.51 | 3.42 | 2.55 | 1.30 |
| B | Petroleum ether: acetonitrile: acetone (4:3:1, v/v) | 3.15 | 0.91 | 7.21 | 4.76 | 1.84 |
| Sample | Concentration (µg/mL) | Eucalyptol/Curzerene Ratio, w/w | ESC (%) |
|---|---|---|---|
| Essential oil | 3.36 | - | 68.98 ± 2.57 c |
| Eucalyptol (1) | 3.36 | - | 47.14 ± 6.62 d |
| Camphor (2) | 10.08 | - | 36.03 ± 7.71 d |
| δ-Elemene (3) | 10.08 | - | 44.57 ± 3.86 d |
| β-Elemene (4) | 10.08 | - | 39.07 ± 5.45 d |
| Curzerene (5) | 3.36 | - | 70.02 ± 5.86 b,c |
| mix 1 | 3.36 | 1:1 | 85.28 ± 3.83 a |
| mix 2 | 3.36 | 3:1 | 85.74 ± 3.70 a |
| mix 3 | 3.36 | 4:1 | 84.29 ± 2.58 a,b |
| mix 4 | 3.36 | 5:1 | 84.65 ± 4.24 a,b |
| mix 5 | 3.36 | 6:1 | 87.33 ± 2.77 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zuo, G.-L.; Wang, C.-Y.; Kim, H.Y.; Lim, S.S.; Tong, S.-Q. An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil. Antioxidants 2020, 9, 702. https://doi.org/10.3390/antiox9080702
Wang X, Zuo G-L, Wang C-Y, Kim HY, Lim SS, Tong S-Q. An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil. Antioxidants. 2020; 9(8):702. https://doi.org/10.3390/antiox9080702
Chicago/Turabian StyleWang, Xiang, Guang-Lei Zuo, Chao-Yue Wang, Hyun Yong Kim, Soon Sung Lim, and Sheng-Qiang Tong. 2020. "An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil" Antioxidants 9, no. 8: 702. https://doi.org/10.3390/antiox9080702
APA StyleWang, X., Zuo, G.-L., Wang, C.-Y., Kim, H. Y., Lim, S. S., & Tong, S.-Q. (2020). An Off-Line DPPH-GC-MS Coupling Countercurrent Chromatography Method for Screening, Identification, and Separation of Antioxidant Compounds in Essential Oil. Antioxidants, 9(8), 702. https://doi.org/10.3390/antiox9080702