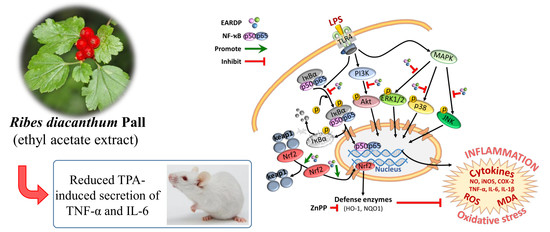
Anti-Inflammatory Effects of Ribes diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of EARDP
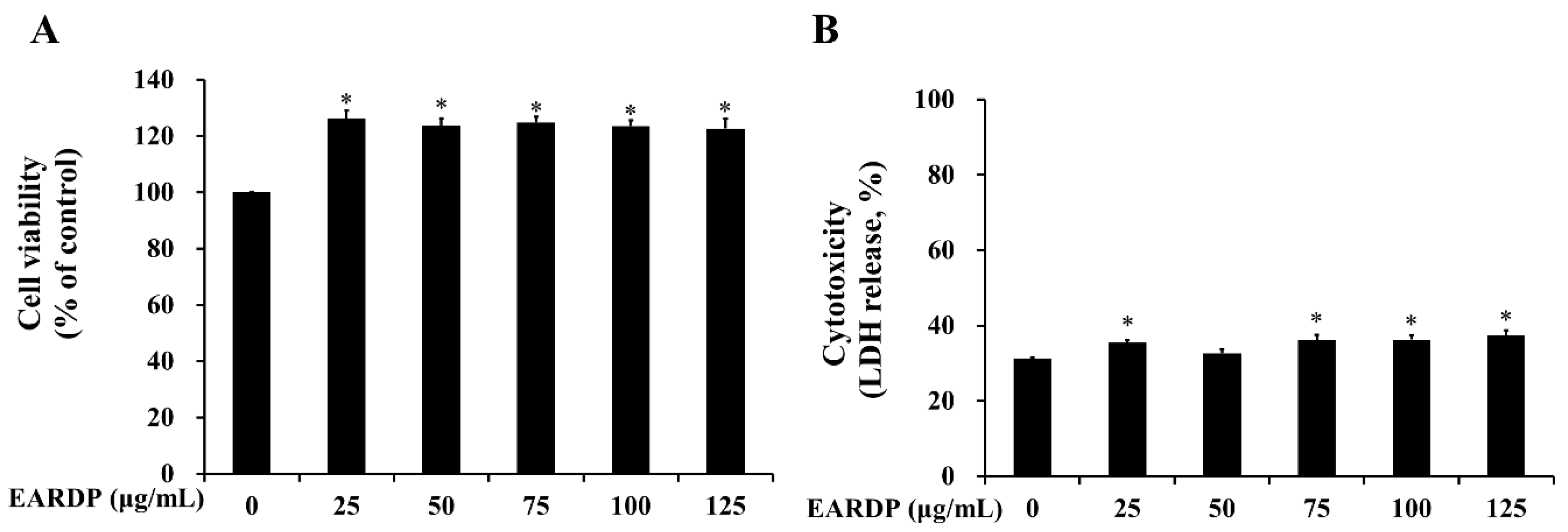
2.3. Cell Culture, Cell Viability, and Cytotoxicity Assays
2.4. Determination of NO Production and Pro-Inflammatory Cytokines (TNF-α, IL-6, and IL-1β) Assays
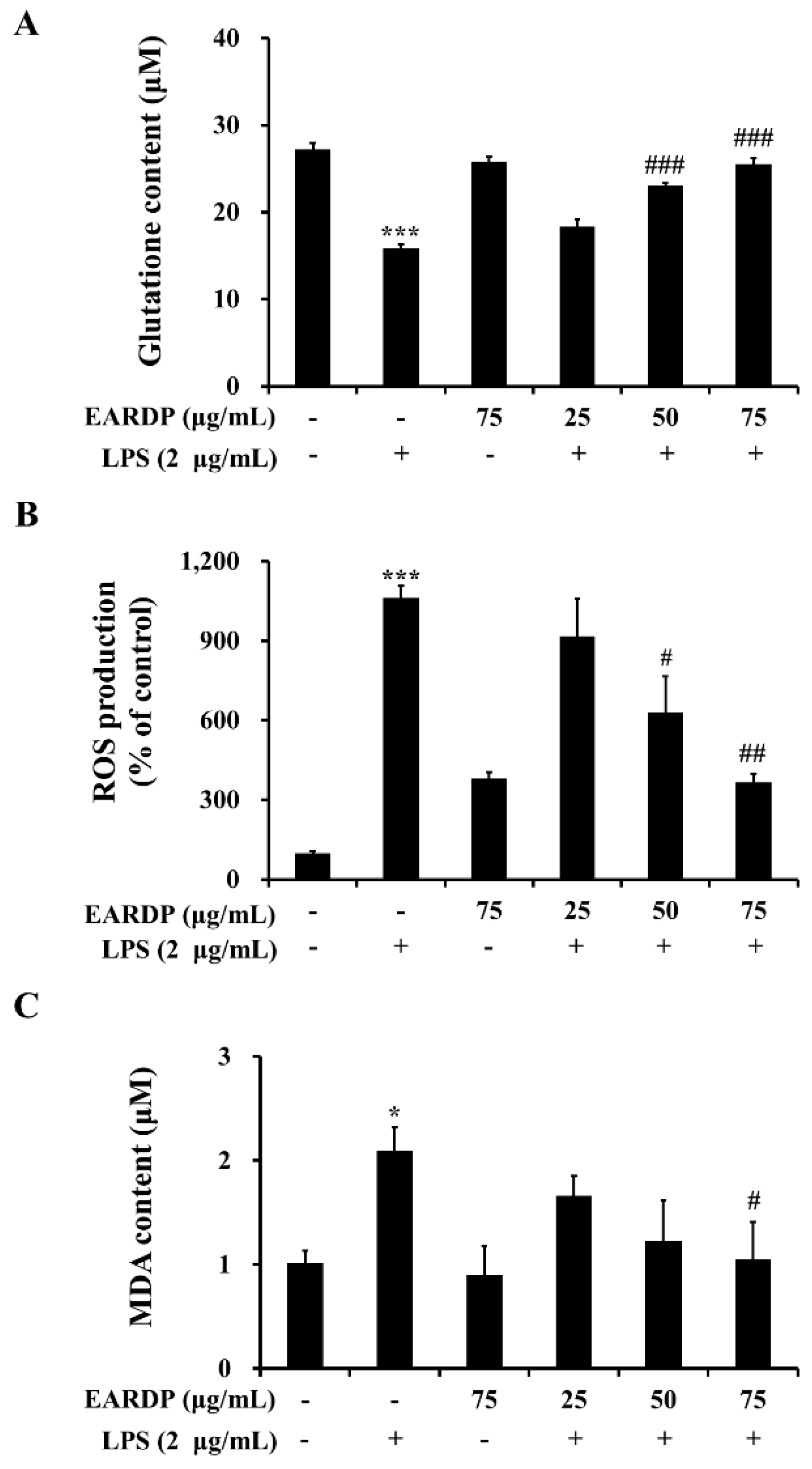
2.5. Measurement of Intracellular Glutathione (GSH), ROS, and MDA Levels
2.6. Preparation of Nuclear and Cytosolic Extraction
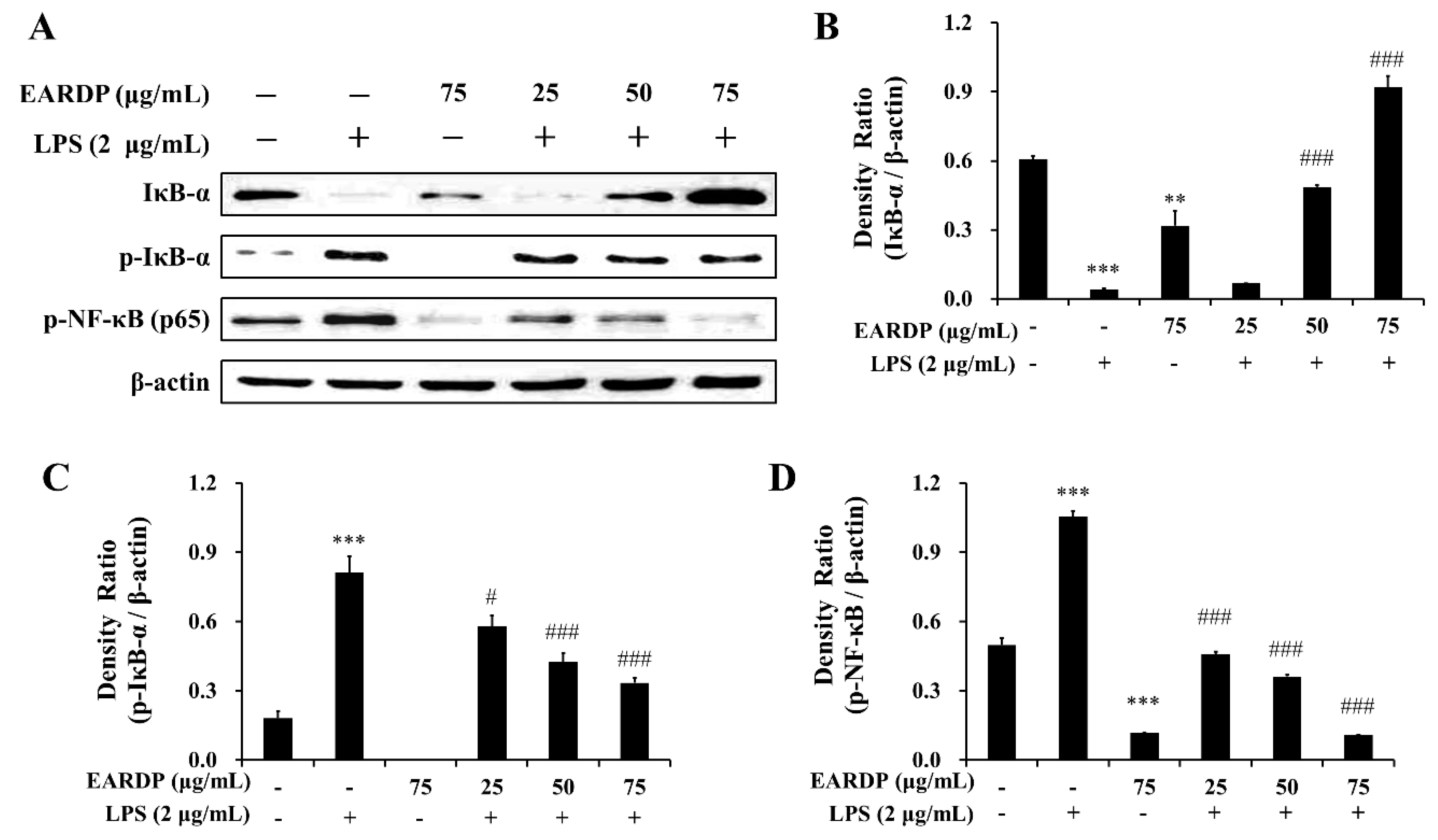
2.7. Western Blotting Analysis
2.8. In Vivo Animal Experiment
2.9. Statistical Analysis
3. Results
3.1. Cytotoxicity of EARDP on RAW 264.7 Macrophages
3.2. Effect of EARDP on Intracellular GSH Level, ROS Generation, and Lipid Peroxidation in LPS-Stimulated RAW 264.7 Macrophages
3.3. Effects of EARDP on Morphological Changes, Production of Nitrite, TNF-α, IL-6 and IL-1β in LPS-Stimulated RAW 264.7 Macrophages
3.4. Effects of EARDP on Expression Levels of IκB-α, p-IκB-α, and p-NF-κB in LPS-Stimulated RAW 264.7 Macrophages
3.5. Effects of EARDP on Nrf2-Mediated HO-1 Induction in RAW 264.7 Macrophages
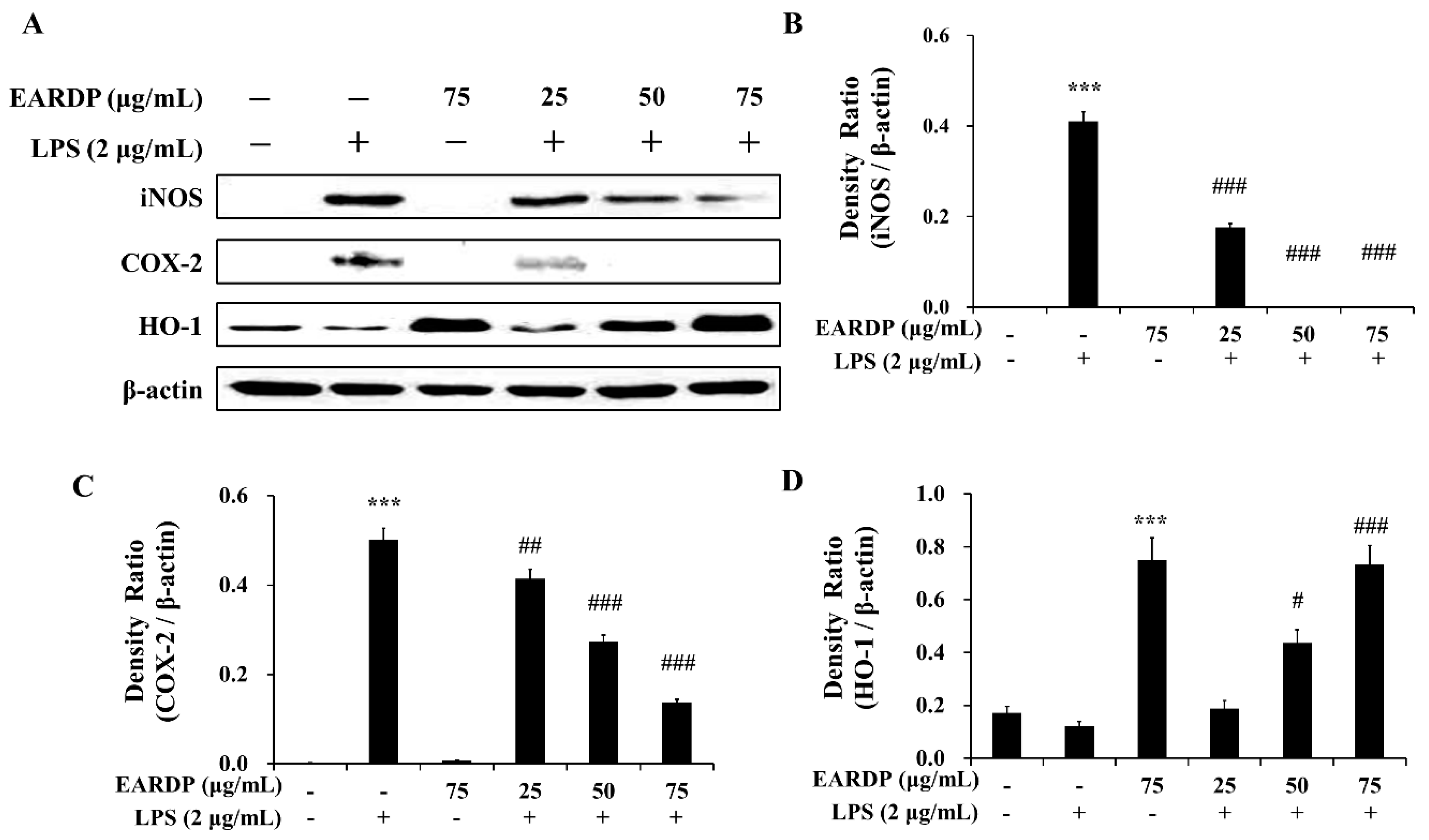
3.6. Effects of EARDP on iNOS, COX-2, and HO-1 Expression in LPS-Stimulated RAW 264.7 Macrophages
3.7. Effects of HO-1 Protein Expression on the Inhibition of NO and TNF-α by EARDP in LPS-Stimulated RAW 264.7 Macrophages
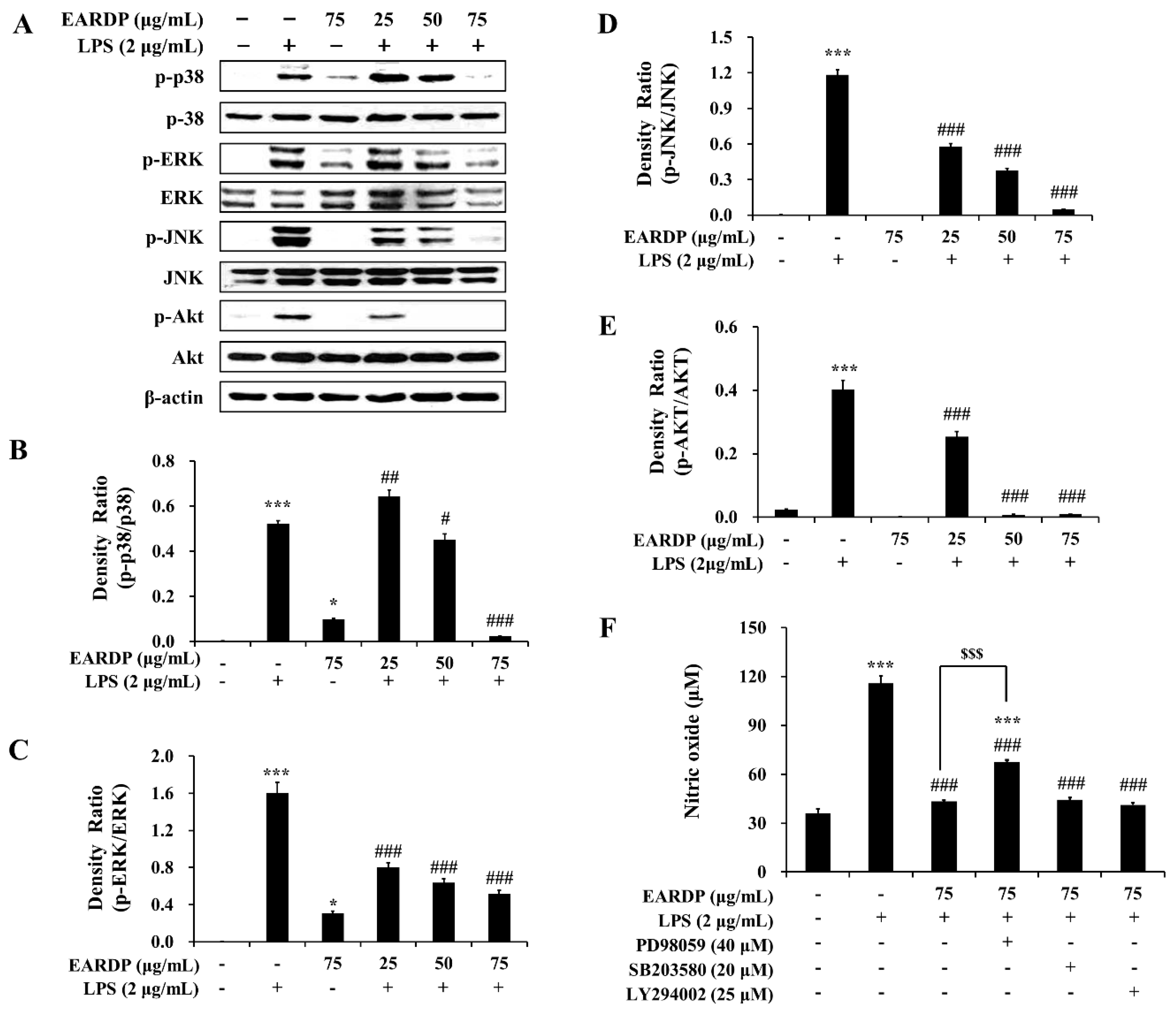
3.8. Role of the MAPK and PI3K/Akt Pathways in the EARDP-Induced HO-1 Expression
3.9. Anti-Inflammatory Effects of EARDP in TPA-Induced Mouse Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldsby, R.A.; Kindt, T.J.; Osborne, B.A. Kuby Immunology, 4th ed.; W. H. Freeman and Company (Sd): New York, NY, USA, 2000; pp. 303–327. [Google Scholar]
- Shao, J.; Li, Y.; Wang, Z.; Xiao, M.; Yin, P.; Lu, Y.; Qian, X.; Xu, Y.; Liu, J. 7b, A novel naphthalimide derivative, exhibited anti-inflammatory effects via targeted-inhibiting TAK1 following down-regulation of ERK1/2- and p38 MAPK-mediated activation of NF-κB in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2013, 17, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I. Free radicals oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Shigrenaga, M.K.; Hagen, T.M. Oxidant, antioxidant and degenerative disease of aging. Proc. Natl. Acad. Sci. USA 1993, 90, 7915–7922. [Google Scholar] [CrossRef]
- Bharti, A.C.; Aggarwal, B.B. Nuclear factor-kappa B and cancer: Its role in prevention and therapy. Biochem. Pharmacol. 2002, 64, 883–888. [Google Scholar] [CrossRef]
- Lampe, J.W. Spicing up a vegetarian diet: Chemopreventive effects of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 579S–583S. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, P.A. Prooxidant status and tumor promotion. Science 1985, 227, 379–381. [Google Scholar] [CrossRef]
- Moskovitz, J.; Yim, K.A.; Choke, P.B. Free radicals and disease. Arch. Biochem. Biophys. 2002, 397, 354–359. [Google Scholar] [CrossRef]
- Pae, H.O.; Kim, E.C.; Chung, H.T. Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J. Clin. Biochem. Nutr. 2008, 42, 197–203. [Google Scholar] [CrossRef]
- Salah, N.; Miller, N.J.; Paganga, G.; Tijburg, L.; Bolwell, G.P.; Rice-Evans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef]
- Silva, B.M.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Seabra, R.M.; Ferreria, M.A. Quince (Cydonia oblonga Miller) fruit (pulp, peel, and seed) and jam: Antioxidant activity. J. Agric. Food Chem. 2004, 52, 4705–4712. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, M.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorizing assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Mazar, D.; Greenberg, L.; Shamir, D.; Meyerstein, D.; Meyerstein, N. Antioxidant properties of bucillamine: Possible mode of action. Biochem. Biophys. Res. Commun. 2006, 349, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Platanias, L.C. Map kinase signaling pathways and hematologic malignancies. Blood 2003, 101, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Ligaa, U.; Davaasuren, B.; Ninjil, N. Medicinal Plants of Mongolia Used in Western and Eastern Medicine; JKC. Printing: Ulaanbaatar, Mongolia, 2006; p. 374. [Google Scholar]
- Birasuren, B.; Oh, H.L.; Kim, C.R.; Kim, N.Y.; Jeon, H.L.; Kim, M.R. Antioxidant Activities of Ribes diacanthum Pall Extracts in the Northern Region of Mongolia. Prev. Nutr. Food Sci. 2012, 17, 261–268. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Ko, Y.K.; Jung, H.J.; Cho, S.W.; Lee, M.; Rhee, C.S. Different anti-allergic mechanism of 2 green tea extracts in murine rhinitis model. J. Allergy Clin. Immunol. 2018, 141, AB75. [Google Scholar] [CrossRef]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M., Jr.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef]
- Floreani, M.; Petrone, M.; Debetto, P.; Palatini, P. A comparison between different methods for determination of reduced and oxidized glutathione in mammalian tissues. Free Radic. Res. 1997, 26, 449–455. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Jeon, H.L.; Yoo, J.M.; Lee, B.D.; Lee, S.J.; Sohn, E.J.; Kim, M.R. Anti-inflammatory and antioxidant actions of N-arachidonoyl serotonin in RAW264.7 cells. Pharmacology 2016, 97, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lee, Y.J.; Yang, H.M.; Han, S.R.; Kim, J.H.; Lee, Y.S.; Kim, C.H.; Han, M.H.; Kim, M.Y.; Lee, J.H.; et al. Inhibitory effect of Sanguisorba officinalis ethanol extract on NO and PGE₂ production is mediated by suppression of NF-κB and AP-1 activation signaling cascade. J. Ethnopharmacol. 2011, 134, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Gu, L.; Wang, Y.; Yang, G.; Tilyek, A.; Zhang, C.; Li, S.; Yu, B.; Chai, C.; Cao, Z. Ribes diacanthum Pall (RDP) ameliorates UUO-induced renal fibrosis via both canonical and non-canonical TGF-β signaling pathways in mice. J. Ethnopharmacol. 2019, 231, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Tilyek, A.; Chai, C.; Hou, X.; Zhou, B.; Zhang, C.; Cao, Z.; Yu, B. The protective effects of Ribes diacanthum Pall on cisplatin-induced nephrotoxicity in mice. J. Ethnopharmacol. 2016, 178, 297–306. [Google Scholar] [CrossRef]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Llesuy, S.F.; Tomaro, M.L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim. Biophys. Acta 1994, 1223, 9–14. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar] [CrossRef]
- Smith, D.; Hansch, H.; Bancroft, G.; Ehlers, S. T-cell-independent granuloma formation in response to Mycobacterium avium: Role of tumour necrosis factor-alpha and interferon-gamma. Immunology 1997, 92, 413–421. [Google Scholar] [CrossRef]
- Kumar, P.P.; Kuttan, G. Vernonia cinerea L. scavenges free radicals and regulates nitric oxide and proinflammatory cytokines profile in carrageenan induced paw edema model. Immunopharmacol. Immunotoxicol. 2009, 31, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Lee, S.H.; Cho, H.S.; Kim, Y.; Yun, Y.P.; Jung, H.Y.; Jung, J.K.; Lee, B.C.; Pyo, H.B.; Hong, J.T. Inhibitory effect of obovatol on nitric oxide production and activation of NF-kappaB/MAP kinases in lipopolysaccharide-treated RAW 264.7cells. Eur. J. Pharmacol. 2007, 556, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Thanos, D.; Maniatis, T. Identification of the rel family members required for virus induction of the human beta interferon gene. Mol. Cell Biol. 1995, 15, 152–164. [Google Scholar] [CrossRef]
- Kim, B.H.; Cho, S.M.; Reddy, A.M.; Kim, Y.S.; Min, K.R.; Kim, Y. Down-regulatory effect of quercitrin gallate on nuclear factor-kappa B-dependent inducible nitric oxide synthase expression in lipopolysaccharide-stimulated macrophages RAW 264.7. Biochem. Pharmacol. 2005, 69, 1577–1583. [Google Scholar] [CrossRef]
- Kim, H.G.; Shrestha, B.; Lim, S.Y.; Yoon, D.H.; Chang, W.C.; Shin, D.J.; Han, S.K.; Park, S.M.; Park, J.H.; Park, H.I.; et al. Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW264.7 macrophage cells. Eur. J. Pharmacol. 2006, 545, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Francisco, V.; Figueirinha, A.; Neves, B.M.; García-Rodríguez, C.; Lopes, M.C.; Cruz, M.T.; Maria Teresa Batista, M.T. Cymbopogon citratus as source of new and safe anti-inflammatory drugs: Bio-guided assay using lipopolysaccharide-stimulated macrophages. J. Ethnopharmacol. 2011, 133, 818–827. [Google Scholar] [CrossRef]
- Balogun, E.; Hoque, M.; Gong, P.; Killeen, E.; Green, C.J.; Foresti, R.; Alam, J.; Motterlini, R. Curcumin activates the heme oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003, 371, 887–895. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Wu, L.; Wang, R. Carbon monoxide: Endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005, 57, 585–630. [Google Scholar] [CrossRef] [PubMed]
- Motterlini, R.; Foresti, R. Heme oxygenase-1 as a target for drug discovery. Antioxid. Redox Signal. 2014, 20, 1810–1826. [Google Scholar] [CrossRef] [PubMed]
- Morse, D.; Pischke, S.E.; Zhou, Z.; Davis, R.J.; Flavell, R.A.; Loop, T.; Otterbein, S.L.; Otterbein, L.E.; Choi, A.M. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J. Biol. Chem. 2003, 278, 36993–36998. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.Y.; Jin, Y.; Yi, A.K.; Wang, X.M.; Choi, A.M. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am. J. Respir. Cell Mol. Biol. 2006, 35, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Rushworth, S.A.; Ogborne, R.M.; Charalambos, C.A.; O’Connell, M.A. Role of protein kinase C delta in curcumin-induced antioxidant response element-mediated gene expression in human monocytes. Biochem. Biophys. Res. Commun. 2006, 341, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Jang, J.H.; Li, M.H.; Surh, Y.J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochem. Biophys. Res. Commun. 2005, 331, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Jeong, G.S.; Li, B.; Park, H.; Kim, Y.C. Anti-inflammatory effects of sulfuretin from Rhus verniciflua Stokes via the induction of heme oxygenase-1 expression in murine macrophages. Int. Immunopharmacol. 2010, 10, 850–858. [Google Scholar] [CrossRef]
- Jeong, G.S.; Lee, D.S.; Kim, Y.C. Cudratricusxanthone A from Cudrania tricuspidata suppresses pro-inflammatory mediators through expression of anti-inflammatory heme oxygenase-1 in RAW264.7 macrophages. Int. Immunopharmacol. 2009, 9, 241–246. [Google Scholar] [CrossRef]
- Yao, P.; Nussler, A.; Liu, L.; Hao, L.; Song, F.; Schirmeier, A.; Nussler, N. Quercetin protects human hepatocytes from ethanol-derived oxidative stress by inducing heme oxygenase-1 via the MAPK/Nrf2 pathways. J. Hepatol. 2007, 47, 253–261. [Google Scholar] [CrossRef]
- Ji, R.R.; Gereau, R.W.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Haque, M.A.; Jantan, I.; Harikrishnan, H. Zerumbone suppresses the activation of inflammatory mediators in LPS-stimulated U937 macrophages through MyD88-dependent NF-κB/MAPK/PI3K-Akt signaling pathways. Int. Immunopharmacol. 2018, 55, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.Y.; Kim, J.H.; Kim, H.Y.; Lee, Y.J.; Park, S.Y.; Lee, S.J.; Kim, Y.H. Achyranthes japonica exhibits anti-inflammatory effect via NF-κB suppression and HO-1 induction in macrophages. J. Ethnopharmacol. 2012, 144, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Park, S.D.; Koh, Y.J.; Kim, D.I.; Lee, J.H. Aqueous extract of Dipsacus asperoides suppresses lipopolysaccharide-stimulated inflammatory responses by inhibiting the ERK1/2 signaling pathway in RAW 264.7 macrophages. J. Ethnopharmacol. 2019, 231, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Guan, H.; Liu, D.; Wu, X.; Fan, M.; Han, J. Flavonoids from sea buckthorn inhibit the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through the MAPK and NF-κB pathways. Food Funct. 2017, 8, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Yoo, J.M.; Baek, S.Y.; Kim, M.R. Anti-inflammatory effect of aged black garlic on 12-O-tetradecanoylphorbol-13-acetate-induced dermatitis in mice. Nutr. Res. Pract. 2019, 13, 189–195. [Google Scholar] [CrossRef]
- Rocha, F.G.; Brandenburg, M.M.; Pawloski, P.L.; Soley, B.D.S.; Costa, S.C.A.; Meinerz, C.C.; Baretta, I.P.; Otuki, M.F.; Cabrini, D.A. Preclinical study of the topical anti-inflammatory activity of Cyperus rotundus L. extract (Cyperaceae) in models of skin inflammation. J. Ethnopharmacol. 2020, 254, 112709. [Google Scholar] [CrossRef]
- Rauh, L.K.; Horinouchi, C.D.; Loddi, A.M.; Pietrovski, E.F.; Neris, R.; Souza-Fonseca-Guimarães, F.; Buchi, D.F.; Biavatti, M.W.; Otuki, M.F.; Cabrini, D.A. Effectiveness of Vernonia scorpioides ethanolic extract against skin inflammatory processes. J. Ethnopharmacol. 2011, 138, 390–397. [Google Scholar] [CrossRef]
- Domínguez, M.; Avila, J.G.; Nieto, A.; Céspedes, C.L. Anti-inflammatory activity of Penstemon gentianoides and Penstemon campanulatus. Pharm. Biol. 2011, 49, 118–124. [Google Scholar] [CrossRef][Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, N.Y.; Cheong, S.H.; Lee, K.J.; Sok, D.-E.; Kim, M.R. Anti-Inflammatory Effects of Ribes diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model. Antioxidants 2020, 9, 622. https://doi.org/10.3390/antiox9070622
Kim NY, Cheong SH, Lee KJ, Sok D-E, Kim MR. Anti-Inflammatory Effects of Ribes diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model. Antioxidants. 2020; 9(7):622. https://doi.org/10.3390/antiox9070622
Chicago/Turabian StyleKim, Na Yeon, Sun Hee Cheong, Kun Jong Lee, Dai-Eun Sok, and Mee Ree Kim. 2020. "Anti-Inflammatory Effects of Ribes diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model" Antioxidants 9, no. 7: 622. https://doi.org/10.3390/antiox9070622
APA StyleKim, N. Y., Cheong, S. H., Lee, K. J., Sok, D.-E., & Kim, M. R. (2020). Anti-Inflammatory Effects of Ribes diacanthum Pall Mediated via Regulation of Nrf2/HO-1 and NF-κB Signaling Pathways in LPS-Stimulated RAW 264.7 Macrophages and a TPA-Induced Dermatitis Animal Model. Antioxidants, 9(7), 622. https://doi.org/10.3390/antiox9070622