Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Preparation of Platelet-Rich Fibrin
2.3. Preparation of Blood Fractions
2.4. Cell Viability Assay
2.5. Trypan Blue Staining and Live-Dead Staining
2.6. Visualizing Bubble Assay and Bubble Microscopic Screening
2.7. Western Blot Analysis
2.8. Reverse Transcription Quantitative Polymerase Chain Reaction
2.9. Statistical Analysis
3. Results
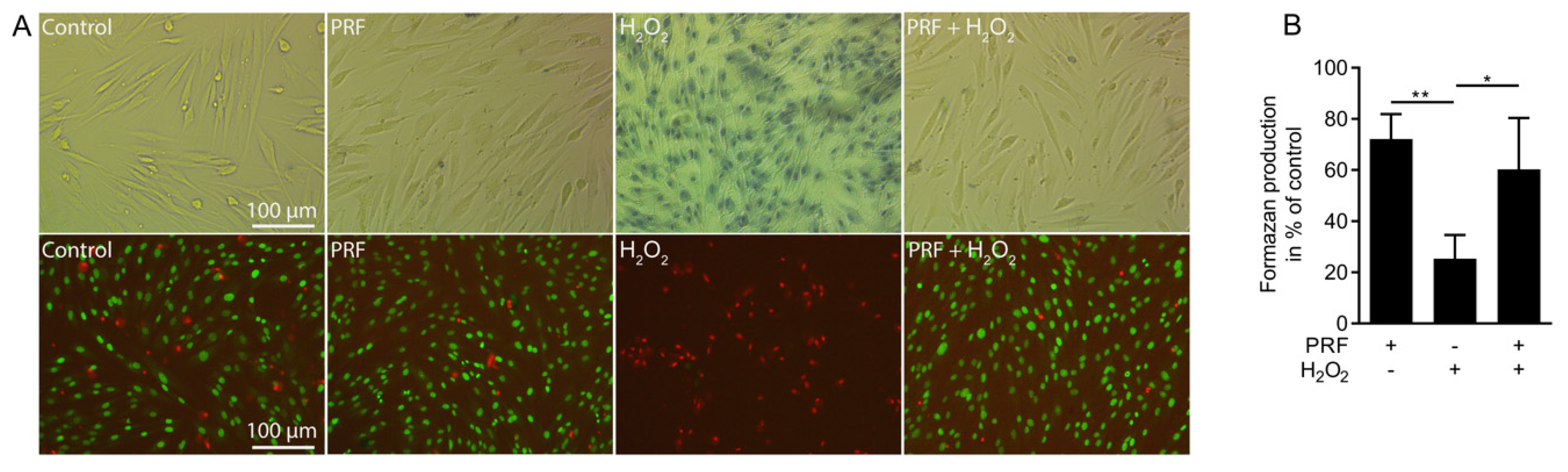
3.1. PRF Lysates Rescue Cells from Acute Hydrogen Peroxide Toxicity
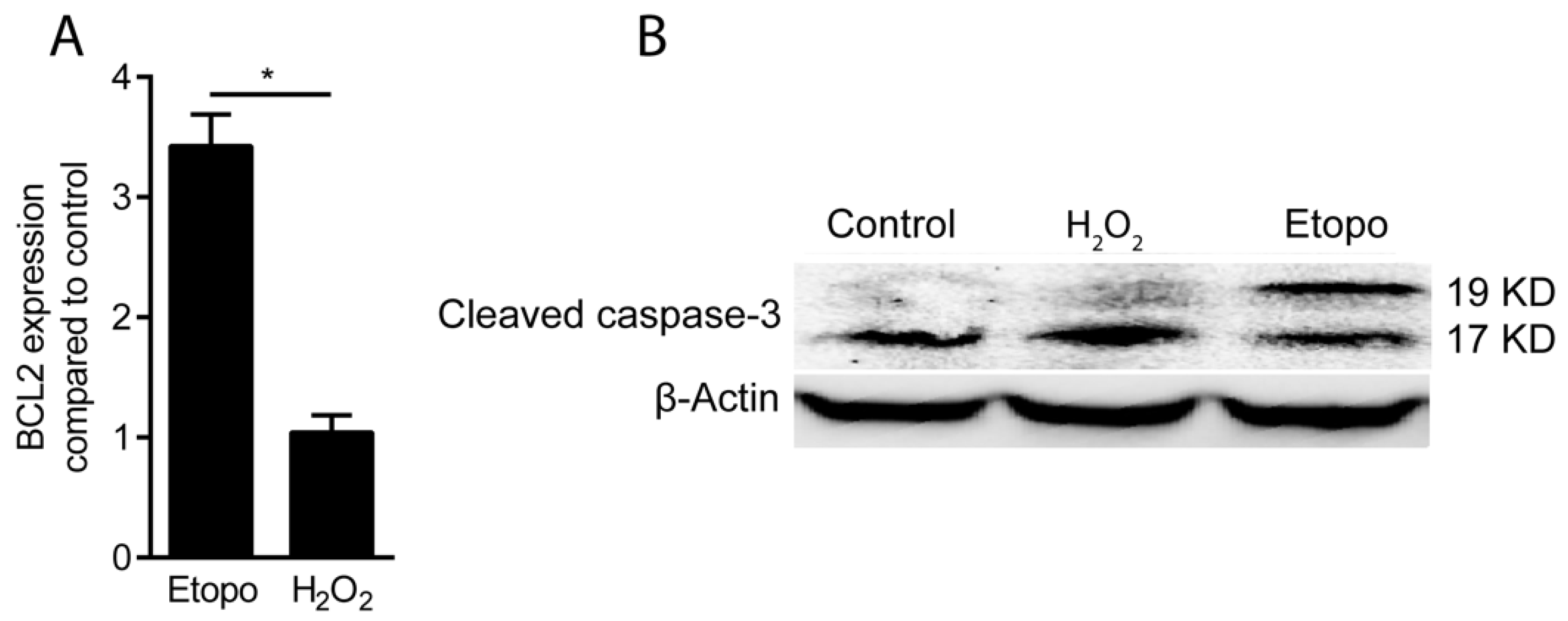
3.2. Hydrogen Peroxide Toxicity Occurs by Necrosis but Not by Apoptosis
3.3. PRF Conditioned Medium Protects Cells from Necrotic Cell Death
3.4. Blocking Catalase Attenuates the Activity of PRF Lysate in Preventing the Cells from Cell Death
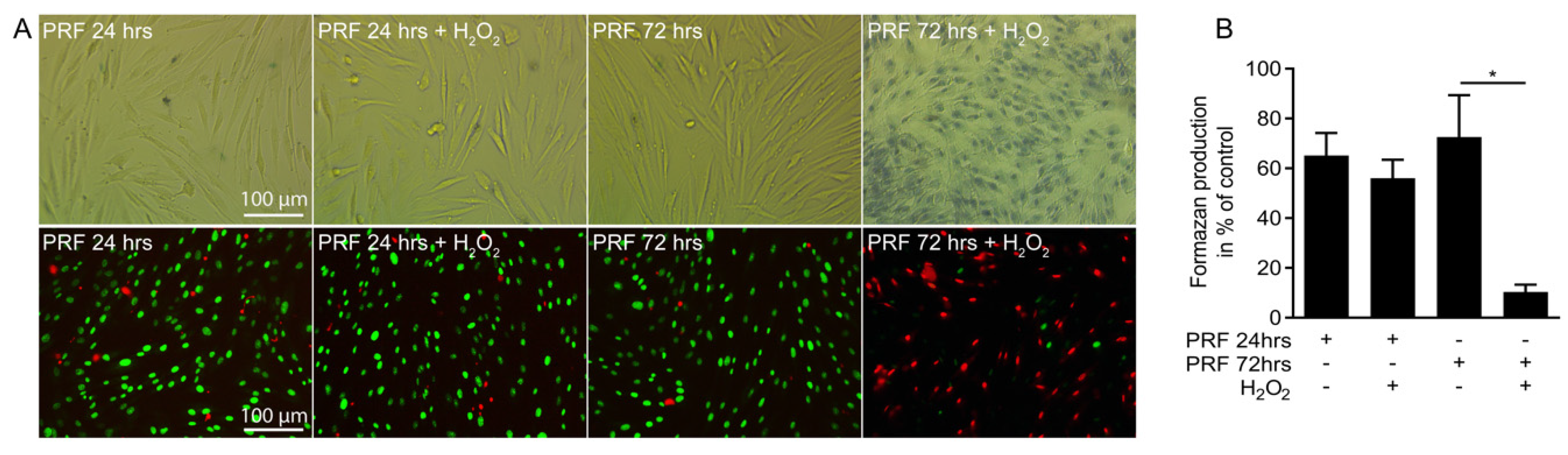
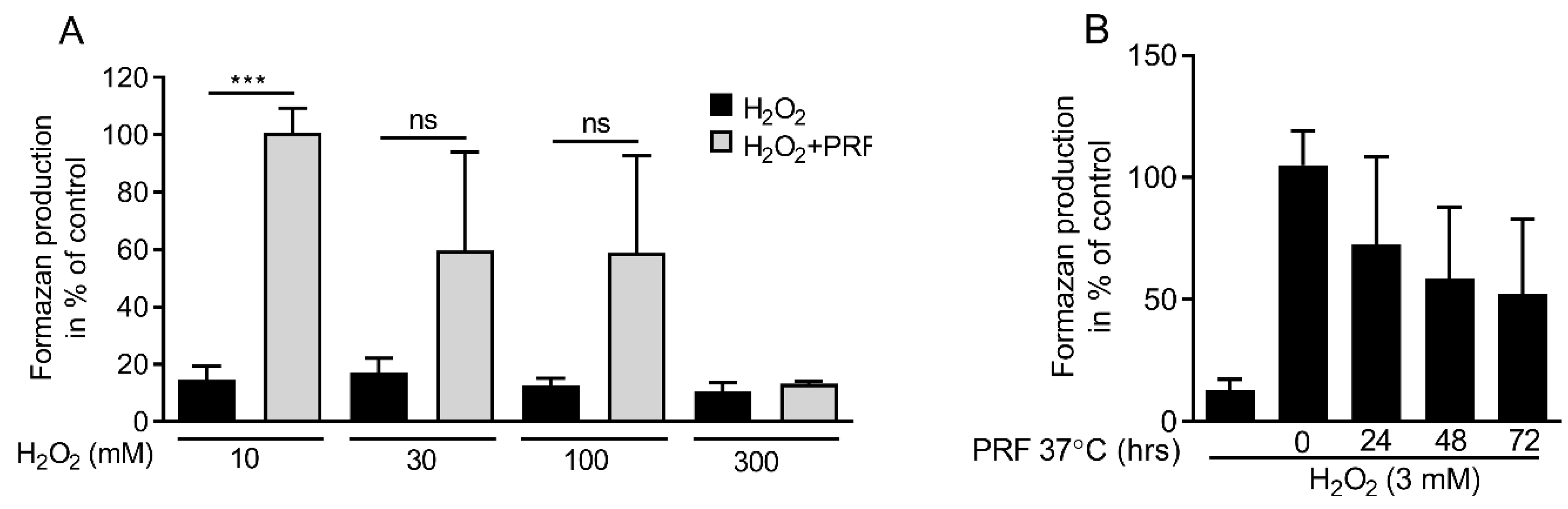
3.5. PRF Capacity in Neutralizing Hydrogen Peroxide Is Limited
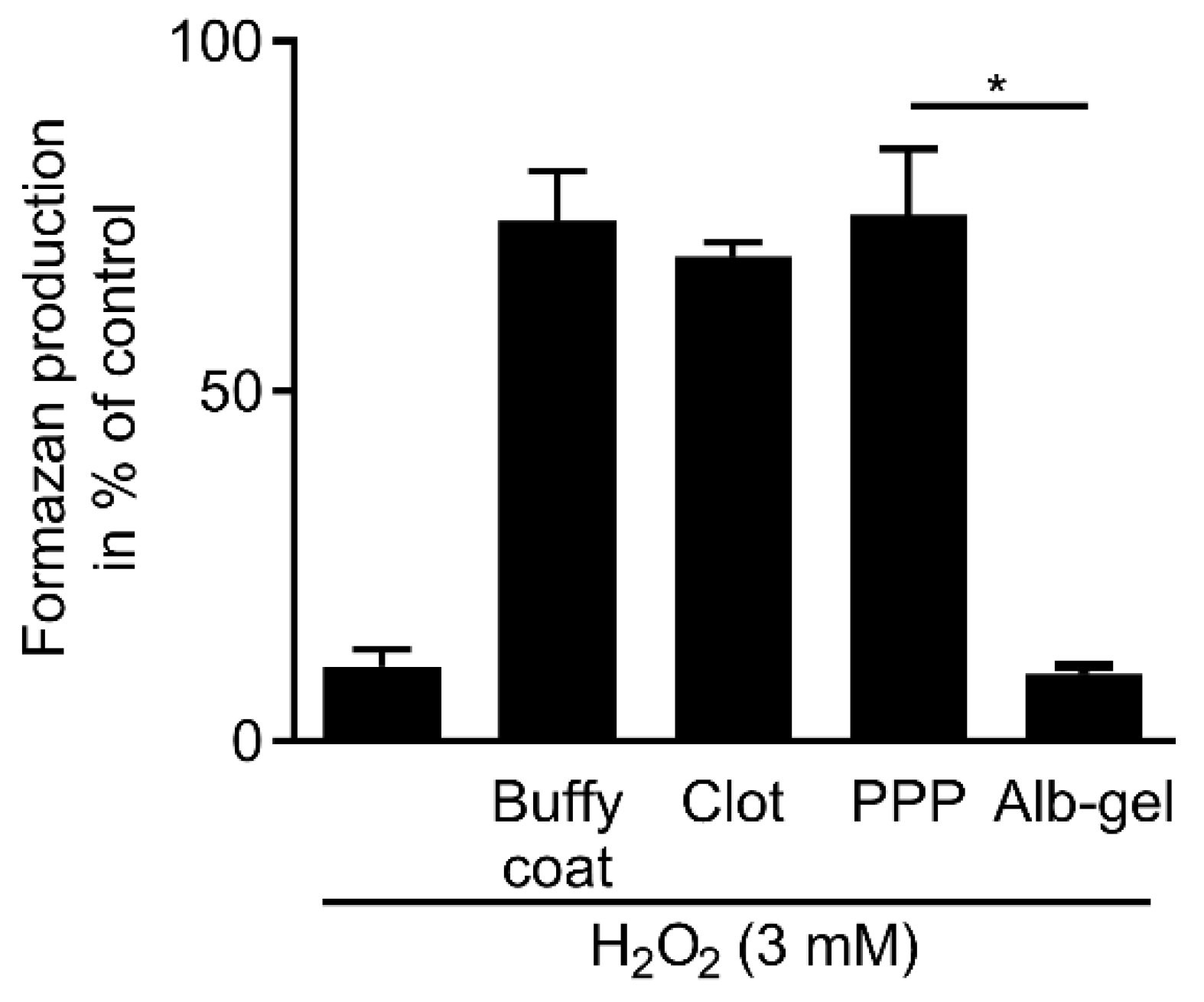
3.6. Regular PPP, Buffy Coat and Red Blood Clot but Not Albumin Gel Neutralize Hydrogen Peroxide
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral. Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, A.; Vandessel, J.; Castro, A.; Jacobs, R.; Teughels, W.; Pinto, N.; Quirynen, M. The use of leucocyte and platelet-rich fibrin in socket management and ridge preservation: A split-mouth, randomized, controlled clinical trial. J. Clin. Periodontol. 2016, 43, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Temmerman, A.; Cleeren, G.J.; Castro, A.B.; Teughels, W.; Quirynen, M. L-PRF for increasing the width of keratinized mucosa around implants: A split-mouth, randomized, controlled pilot clinical trial. J. Periodontal. Res. 2018, 53, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hamzacebi, B.; Oduncuoglu, B.; Alaaddinoglu, E.E. Treatment of Peri-implant Bone Defects with Platelet-Rich Fibrin. Int. J. Periodontics Restor. Dent. 2015, 35, 415–422. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stahli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral. Implant. Res. 2018, 29 (Suppl. 18), 6–19. [Google Scholar] [CrossRef]
- Pinto, N.R.; Ubilla, M.; Zamora, Y.; Del Rio, V.; Dohan Ehrenfest, D.M.; Quirynen, M. Leucocyte- and platelet-rich fibrin (L-PRF) as a regenerative medicine strategy for the treatment of refractory leg ulcers: A prospective cohort study. Platelets 2018, 29, 468–475. [Google Scholar] [CrossRef]
- Andreone, A.; den Hollander, D. A Retrospective Study on the Use of Dermis Micrografts in Platelet-Rich Fibrin for the Resurfacing of Massive and Chronic Full-Thickness Burns. Stem. Cells Int. 2019, 2019, 8636079. [Google Scholar] [CrossRef]
- Strauss, F.J.; Nasirzade, J.; Kargarpoor, Z.; Stahli, A.; Gruber, R. Effect of platelet-rich fibrin on cell proliferation, migration, differentiation, inflammation, and osteoclastogenesis: A systematic review of in vitro studies. Clin. Oral. Investig. 2020, 24, 569–584. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fluckiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin. Oral. Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Castro, A.B.; Herrero, E.R.; Slomka, V.; Pinto, N.; Teughels, W.; Quirynen, M. Antimicrobial capacity of Leucocyte-and Platelet Rich Fibrin against periodontal pathogens. Sci. Rep. 2019, 9, 8188. [Google Scholar] [CrossRef]
- Nasirzade, J.; Kargarpour, Z.; Hasannia, S.; Strauss, F.J.; Gruber, R. Platelet-rich fibrin elicits an anti-inflammatory response in macrophages in vitro. J. Periodontol. 2020, 91, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kargarpour, Z.; Nasirzade, J.; Strauss, F.J.; Di Summa, F.; Hasannia, S.; Muller, H.D.; Gruber, R. Platelet-rich fibrin suppresses in vitro osteoclastogenesis. J. Periodontol. 2019, 91, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L., Jr. Reactive oxygen species: A breath of life or death? Clin. Cancer. Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166 (12 Pt 2), S4–S8. [Google Scholar] [CrossRef]
- Doni, M.G.; Avventi, G.L.; Bonadiman, L.; Bonaccorso, G. Glutathione peroxidase, selenium, and prostaglandin synthesis in platelets. Am. J. Physiol. 1981, 240, H800–H803. [Google Scholar] [CrossRef]
- Pandey, N.R.; Kaur, G.; Chandra, M.; Sanwal, G.G.; Misra, M.K. Enzymatic oxidant and antioxidants of human blood platelets in unstable angina and myocardial infarction. Int. J. Cardiol. 2000, 76, 33–38. [Google Scholar] [CrossRef]
- Kandler, B.; Maitz, P.; Fischer, M.B.; Watzek, G.; Gruber, R. Platelets can neutralize hydrogen peroxide in an acute toxicity model with cells involved in granulation tissue formation. Bone 2005, 36, 671–677. [Google Scholar] [CrossRef]
- Agar, N.S.; Sadrzadeh, S.M.; Hallaway, P.E.; Eaton, J.W. Erythrocyte catalase. A somatic oxidant defense? J. Clin. Investig. 1986, 77, 319–321. [Google Scholar] [CrossRef]
- Dallegri, F.; Ballestrero, A.; Ottonello, L.; Patrone, F. Platelets as inhibitory cells in neutrophil-mediated cytolysis. J. Lab. Clin. Med. 1989, 114, 502–509. [Google Scholar]
- Gruber, R.; Karreth, F.; Kandler, B.; Fuerst, G.; Rot, A.; Fischer, M.B.; Watzek, G. Platelet-released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow-derived mesenchymal progenitor cells under in vitro conditions. Platelets 2004, 15, 29–35. [Google Scholar] [CrossRef]
- Guild, W.R.; Van Tubergen, R.P. Heat Inactivation of Catalase in Deuterium Oxide. Science 1957, 125, 939. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.Y.; Kim, H.Y.; Oh, H.J.; Choi, E.; Cho, M.S.; Kim, H.S. Feasibility of autologous plasma gel for tonsil-derived stem cell therapeutics in hypoparathyroidism. Sci. Rep. 2018, 8, 11896. [Google Scholar] [CrossRef] [PubMed]
- Doghaim, N.N.; El-Tatawy, R.A.; Neinaa, Y.M.E. Assessment of the efficacy and safety of platelet poor plasma gel as autologous dermal filler for facial rejuvenation. J. Cosmet. Dermatol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Schaller, B.; Mourao, C.; Zhang, Y.; Sculean, A.; Miron, R.J. Biological characterization of an injectable platelet-rich fibrin mixture consisting of autologous albumin gel and liquid platelet-rich fibrin (Alb-PRF). Platelets 2020, 1–8. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Miron, R.J.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Choukroun, J. Optimized Platelet-Rich Fibrin With the Low-Speed Concept: Growth Factor Release, Biocompatibility, and Cellular Response. J. Periodontol. 2017, 88, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Tajima, A.; Sugimoto, S.; Okuda, K.; Hironaka, I.; Kamata, Y.; Takada, K.; Mizunoe, Y. A simple assay for measuring catalase activity: A visual approach. Sci. Rep. 2013, 3, 3081. [Google Scholar] [CrossRef]
- Andrews, G.P.; Martin, S.E. Heat inactivation of catalase from Staphylococcus aureus MF-31. Appl. Environ. Microbiol. 1979, 37, 1180–1185. [Google Scholar] [CrossRef]
- Gentile, R.D. Easy Platelet-Rich Fibrin (Injectable/Topical) for Post-resurfacing and Microneedle Therapy. Facial. Plast. Surg. Clin. North. Am. 2020, 28, 127–134. [Google Scholar] [CrossRef]
- Davies, C.; Miron, R.J. PRF in Facial Esthetics; Quintessence Publishing Co Inc.: Batavia, IL, USA, 2020. [Google Scholar]
- De Angelis, P.; De Angelis, S.; Passarelli, P.C.; Liguori, M.G.; Manicone, P.F.; D’Addona, A. Hard and Soft Tissue Evaluation of Different Socket Preservation Procedures Using Leukocyte and Platelet-Rich Fibrin: A Retrospective Clinical and Volumetric Analysis. J. Oral. Maxillofac. Surg. 2019, 77, 1807–1815. [Google Scholar] [CrossRef]
- Sepasi Tehrani, H.; Moosavi-Movahedi, A.A. Catalase and its mysteries. Prog. Biophys. Mol. Biol. 2018, 140, 5–12. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Boutonnet, M.; Svensson, B.; Butler, E.; Lood, R.; Blom, K.; Vallejo, B.; Anderson, C.; Engblom, J.; Ruzgas, T.; et al. New concepts for transdermal delivery of oxygen based on catalase biochemical reactions studied by oxygen electrode amperometry. J. Control. Release 2019, 306, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Moore, J.; Wood, J.M.; Beazley, W.D.; Gaze, D.C.; Tobin, D.J.; Marshall, H.S.; Panske, A.; Panzig, E.; Hibberts, N.A. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J. Investig. Dermatol. Symp. Proc. 1999, 4, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Ma, Y.; Mu, Z. Association of total oxidant status, total antioxidant status, and malondialdehyde and catalase levels with psoriasis: A systematic review and meta-analysis. Clin. Rheumatol. 2019, 38, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Doctrow, S.R.; Lopez, A.; Schock, A.M.; Duncan, N.E.; Jourdan, M.M.; Olasz, E.B.; Moulder, J.E.; Fish, B.L.; Mader, M.; Lazar, J.; et al. A synthetic superoxide dismutase/catalase mimetic EUK-207 mitigates radiation dermatitis and promotes wound healing in irradiated rat skin. J. Investig. Dermatol. 2013, 133, 1088–1096. [Google Scholar] [CrossRef]
- Kantarci, A.; Oyaizu, K.; Van Dyke, T.E. Neutrophil-mediated tissue injury in periodontal disease pathogenesis: Findings from localized aggressive periodontitis. J. Periodontol. 2003, 74, 66–75. [Google Scholar] [CrossRef]
- Chapple, I.L. Reactive oxygen species and antioxidants in inflammatory diseases. J. Clin. Periodontol. 1997, 24, 287–296. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, J.K.; Kim, S.I.; Na, H.J.; Jun, S.Y.; Lee, S.J.; Choi, M.E. TGF-{beta}1 protects against mesangial cell apoptosis via induction of autophagy. J. Biol. Chem. 2010, 285, 37909–37919. [Google Scholar] [CrossRef]
- Abdel-Mageed, H.M.; Fahmy, A.S.; Shaker, D.S.; Mohamed, S.A. Development of novel delivery system for nanoencapsulation of catalase: Formulation, characterization, and in vivo evaluation using oxidative skin injury model. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 1), 362–371. [Google Scholar] [CrossRef]
- Roos, D.; Weening, R.S.; Wyss, S.R.; Aebi, H.E. Protection of human neutrophils by endogenous catalase: Studies with cells from catalase-deficient individuals. J. Clin. Investig. 1980, 65, 1515–1522. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zheng, S.; Feng, M.; Sculean, A.; Zhang, Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J. Biomed. Mater. Res. A 2019, 107, 2257–2271. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kargarpour, Z.; Nasirzade, J.; Di Summa, F.; Panahipour, L.; Miron, R.J.; Gruber, R. Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts. Antioxidants 2020, 9, 560. https://doi.org/10.3390/antiox9060560
Kargarpour Z, Nasirzade J, Di Summa F, Panahipour L, Miron RJ, Gruber R. Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts. Antioxidants. 2020; 9(6):560. https://doi.org/10.3390/antiox9060560
Chicago/Turabian StyleKargarpour, Zahra, Jila Nasirzade, Francesca Di Summa, Layla Panahipour, Richard J. Miron, and Reinhard Gruber. 2020. "Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts" Antioxidants 9, no. 6: 560. https://doi.org/10.3390/antiox9060560
APA StyleKargarpour, Z., Nasirzade, J., Di Summa, F., Panahipour, L., Miron, R. J., & Gruber, R. (2020). Platelet-Rich Fibrin Can Neutralize Hydrogen Peroxide-Induced Cell Death in Gingival Fibroblasts. Antioxidants, 9(6), 560. https://doi.org/10.3390/antiox9060560






